Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- ALT
alanine aminotransferase
- ARFI
acoustic radiation force impulse
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- CI
confidence interval
- CK18
caspase‐cleaved cytokeratin 18
- ELF
enhanced liver fibrosis
- FIB‐4
Fibrosis‐4
- GGT
gamma‐glutamyl transpeptidase
- HA
hyaluronic acid
- HCC
hepatocellular carcinoma
- MRE
magnetic resonance elastography
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD fibrosis score
- PNFS
pediatric NFS
- TE
transient elastography
- VCTE
vibration‐controlled transient elastography
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the obesity and metabolic syndrome epidemics. It now affects up to 45% of adults and 10% of children in the United States.1, 2 Recent studies have shown clearly that the stage of fibrosis in adults with NAFLD is the most important histological feature in predicting long‐term outcomes and the development of liver‐related complications.3, 4 Despite the paucity of data regarding the natural history of pediatric NAFLD, its progression to cirrhosis and end‐stage liver disease requiring liver transplantation in children and young adults is well documented.5 Several studies have clearly shown that children with NAFLD may have advanced fibrosis (stage 3‐4) on liver biopsy, making the identification of this high‐risk group a top priority. Given the high prevalence of NAFLD in children and adults, there is an urgent need to find safe and cost‐effective alternatives to biopsy to determine the stage of liver fibrosis. In this review, we will briefly discuss the noninvasive diagnosis of liver fibrosis in adults and provide details on the current status of noninvasive testing in children and adolescents.
Noninvasive Diagnosis of Liver Fibrosis in Adult NAFLD
Multiple noninvasive tests have been developed and validated in the adult NAFLD population to predict the stage of fibrosis.6 These tests are being widely used by gastroenterologists and hepatologists to risk‐stratify patients with NAFLD without the need for liver biopsy. These tests can be divided into one of three categories: simple fibrosis scores that can be calculated from readily available clinical variables, complex fibrosis scores that rely on measuring serum biomarkers of fibrosis and extracellular matrix turnover, and imaging studies that are based on measuring liver stiffness as an indirect way to determine fibrosis stage.
The two most validated simple fibrosis scores in adults are the Fibrosis‐4 (FIB‐4) index (includes aspartate aminotransferase [AST], alanine aminotransferase [ALT], platelet count, and age; http://gihep.com/calculators/hepatology/fibrosis‐4‐score/) and the NAFLD fibrosis score (NFS; age, impaired fasting glucose/diabetes, body mass index, platelets, albumin, and AST/ALT ratio; http://nafldscore.com/). NFS has two cutoff values: < −1.455 to predict the absence of advanced fibrosis (F0‐F2) and >0.675 to predict the presence of advanced fibrosis (F3‐F4). Complex fibrosis scores include the European liver fibrosis panel (includes three fibrosis biomarkers: hyaluronic acid [HA], tissue inhibitor of metalloproteinase 1, and aminoterminal peptide of procollagen III) and the FibroTest (includes five biomarkers: haptoglobin, α2‐macroglobulin, apolipoprotein A1, total bilirubin, and gamma‐glutamyl transpeptidase [GGT]). Liver stiffness measurement by vibration‐controlled transient elastography (VCTE) or FibroScan (Echosens, Paris, France) is one of the most commonly used imaging studies to stage fibrosis in hepatology clinics around the United States. A special probe (XL) was developed for obese patients with NAFLD. Other imaging modalities include acoustic radiation force impulse (ARFI) and magnetic resonance elastography (MRE).
Noninvasive Diagnosis of Liver Fibrosis in Pediatric NAFLD
Significant progress has been made in the field of noninvasive diagnosis of fibrosis in pediatric hepatology. Table 1 provides an overview of the use and accuracy of different noninvasive tests to diagnose liver fibrosis in pediatric NAFLD. However, it is important to realize that, at the present time, liver biopsy remains the only reliable method to stage fibrosis in children with NAFLD.
Table 1.
Summary of Different Serological and Imaging Tests to Diagnose Fibrosis in Children with NAFLD
| Marker | Interpretation | Accuracy | Cost |
|---|---|---|---|
| PNFS |
≥26% gives specificity of 92% for predicting advanced fibrosis ≤8% gives sensitivity of 97% for ruling out advanced fibrosis |
AUROC of 0.74 for advanced fibrosis | + |
| HA |
≥1200 ng/mL: absence of fibrosis was unlikely, 7% (95% CI: 1%‐14%) ≥2100 ng/mL: made F2‐F4 likely, 89% (95% CI: 75%‐100%). |
AUROC of 0.95 | ++ |
| ELF |
≥9.28 = presence of any fibrosis ≥10.18 = presence of significant fibrosis ≥10.51 = presence of advanced fibrosis |
AUROC of 0.92 for detecting any fibrosis AUROC of 0.98 for detecting significant fibrosis AUROC of 0.99 for detecting advanced fibrosis |
++ |
| TE |
5‐7 kPa: F1‐F4 7‐9 kPa: F2‐F4 >9 kPa: F3‐F4 |
AUROC of 0.977 for detecting any fibrosis AUROC of 0.992 for detecting significant fibrosis AUROC of 1.000 for detecting advanced fibrosis |
+++ |
| MRE | Liver stiffness value of 2.71 kPa gives sensitivity of 88% and specificity of 85% for ≥ F2 fibrosis | AUROC of 0.92 for detecting significant fibrosis | +++ |
| ARFI | ARFI cutoff of > 2.0 m/s for ≥ F3 fibrosis | 100% sensitivity, 39% specificity | +++ |
Abbreviations: CI, confidence interval; TE, transient elastography.
Fibrosis Scores
In a study that included pediatric patients with biopsy‐proven NAFLD, our group has clearly shown that simple fibrosis scores that were developed and validated in adult patients with NAFLD (including FIB‐4 index and NFS) are not accurate in predicting fibrosis stage in children.7 Indeed, there was no significant difference in FIB‐4 index and NFS values in children with and without advanced fibrosis. Therefore, by using a large cohort of children (n = 242) with NAFLD, we developed the pediatric NFS (PNFS), which includes ALT, alkaline phosphatase, platelet count, and GGT and can be calculated using the following online calculator:
http://www.r‐calc.com/calculator.aspx?calculator_id=JYAVKOWT. However, it is critical to understand the limitations of this score, including the relatively small number of patients with advanced fibrosis (36/242) and the lack of external validation to date.8
Fibrosis Biomarkers
Caspase‐cleaved cytokeratin 18 (CK18) fragments are considered a serum marker for hepatocyte apoptosis, a process that can activate the hepatic stellate cells to produce liver fibrosis. Fitzpatrick et al.9 analyzed CK18 levels in 45 children with biopsy‐proven NAFLD and found it to be a good marker to predict the presence of significant fibrosis. Similar results were replicated by Lebensztejn et al.10 in a cohort of 52 children with NAFLD showing higher CK18 levels in those with fibrosis; however, the accuracy of CK18 to predict the presence of fibrosis was modest with an area under the receiver operating characteristic curve (AUROC) of 0.666. Our group conducted the largest study (n = 201) to assess the use of CK18 as a biomarker of liver fibrosis in children with NAFLD.11 CK18 fragment levels were significantly higher in children with any fibrosis compared with those without fibrosis (304.6 versus 210.4; P < 0.001). CK18 level demonstrated good accuracy for prediction of any fibrosis (F1‐F3) with an AUROC of 0.75. On multivariate logistic regression analysis, the combination of CK18 and waist circumference percentile generated an AUROC of 0.842 for prediction of any fibrosis.
w?>HA is an extracellular matrix glycosaminoglycan that is produced by activated hepatic stellate cells and is considered a direct biomarker of liver fibrosis. Conflicting results have been presented on the role of HA in predicting fibrosis in pediatric NAFLD, and more studies are needed to establish its role as a noninvasive biomarker.
Similar to adult studies, the enhanced liver fibrosis (ELF) panel showed excellent accuracy for predicting fibrosis in a cohort of 112 children with NAFLD (AUROC of 0.92 for any fibrosis); however, these results need further external validation.12
Imaging Studies
The same imaging modalities used routinely in adults to assess for liver fibrosis are being studied in the pediatric age group. Nobili et al.13 measured liver stiffness by VCTE in 52 children with NAFLD and showed excellent accuracy for predicting advanced fibrosis (F3‐F4) with an AUROC of 1.000. It is important to note that the study included only five children with advanced fibrosis. MRE was recently studied in a pilot project that included 35 children with different liver disease including NAFLD with promising results.14 ARFI and other ultrasound‐based methods to determine liver stiffness are being validated in the pediatric population. We need to establish specific cutoff values for children of different ages and different liver diseases before the wide use of these imaging techniques can be recommended in routine clinical practice.
Conclusion
Significant progress has been made in noninvasive diagnosis of hepatic fibrosis in adults with NAFLD, and the majority of patients can be risk‐stratified without the need for liver biopsy. In our practice, we determine the NFS and liver stiffness measurement by VCTE in each patient with NAFLD with the results being consistent with one of three scenarios as shown in Figure 1: 1 both tests indicate the absence of advanced fibrosis → advanced fibrosis excluded, repeat testing in 2 to 3 years; 2 both tests indicate the presence of advanced fibrosis → advanced fibrosis confirmed, start screening for hepatocellular carcinoma (HCC) and consider screening for varices; or 3 the tests are discordant → perform liver biopsy to stage fibrosis.
Figure 1.
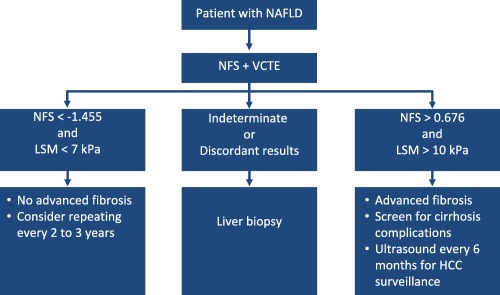
Algorithm used to determine the presence of advanced fibrosis in adults with NAFLD.
The situation is different in children with NAFLD, for whom liver biopsy remains the gold standard for staging fibrosis. Adult fibrosis scores are not useful in children with NAFLD. Fibrosis biomarkers need further validation in different and larger cohorts. Imaging studies are promising, but pediatric‐specific cutoffs need to be established. In conclusion, the validation of noninvasive markers and imaging studies in children with NAFLD is urgently needed to stage the severity of fibrosis and determine response to new therapeutic agents.
Potential conflict of interest: Nothing to report.
References
- 1. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 2. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 5. Alkhouri N, Hanouneh IA, Zein NN, Lopez R, Kelly D, Eghtesad B, Fung JJ. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int 2016;29:418‐424. [DOI] [PubMed] [Google Scholar]
- 6. Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016;65:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansoor S, Yerian L, Kohli R, Xanthakos S, Angulo P, Ling S, et al. The evaluation of hepatic fibrosis scores in children with nonalcoholic fatty liver disease. Dig Dis Sci 2015;60:1440‐1447. [DOI] [PubMed] [Google Scholar]
- 8. Alkhouri N. The development of the pediatric NAFLD fibrosis score (PNFS) to predict the presence of advanced fibrosis in children with nonalcoholic fatty liver disease. Curr Pediatr Rev 2014;9:e104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr 2010;51:500‐506. [DOI] [PubMed] [Google Scholar]
- 10. Lebensztejn DM, Wierzbicka A, Socha P, Pronicki M, Skiba E, Werpachowska I, Kaczmarski M. Cytokeratin‐18 and hyaluronic acid levels predict liver fibrosis in children with non‐alcoholic fatty liver disease. Acta Biochim Pol 2011;58:563‐566. [PubMed] [Google Scholar]
- 11. Mandelia C, Collyer E, Mansoor S, Lopez R, Lappe S, Nobili V, Alkhouri N. Plasma cytokeratin‐18 level as a novel biomarker for liver fibrosis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2016;63:181‐187. [DOI] [PubMed] [Google Scholar]
- 12. Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non‐alcoholic fatty liver disease. Gastroenterology 2009;136:160‐167. [DOI] [PubMed] [Google Scholar]
- 13. Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 2008;48:442‐448. [DOI] [PubMed] [Google Scholar]
- 14. Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, Kohli R. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr 2014;164:186‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]


