Abstract
Background
The aim of this study was to assess the effects and mechanisms of allicin in a sepsis-induced lung injury in vivo study.
Material/Methods
The rats (n=54) were divided into 6 groups: Normal, DMSO, LPS, LPS+LD, LPS+MD, and LPS+HD groups. After being treated by different methods, we collected the lung tissues of different groups and evaluated the pathology by HE staining and positive apoptosis cells by TUNEL. We assessed the W/D ratio, inflammatory cytokines (TNF-α, IL-6 and IL-1β), and relative protein expressions (TLR4, MyD88, NF-κB, caspase-3, and caspase-9) by IHC assay.
Results
Compared with LPS group, the lung injury and positive cell number of allicin treated groups were significantly improved with dose-dependent (P<0.05, respectively) and the W/D ratio and TNF-α, IL-6 and IL-1β concentration were significantly down-regulation compared with those of LPS group with dose-dependent (P<0.05, respectively). By IHC, the TLR4, MyD88, NF-κB, caspase-3 and caspase-9 protein activities of allicin treated groups were significantly suppressed compared with those of LPS group (P<0.05, respectively) in lung tissues.
Conclusions
This in vivo study shows that allicin improved sepsis-induced lung injury by regulation of TLR4/MyD88/NF-κB.
MeSH Keywords: Acute Lung Injury, Gallic Acid, Myeloid Differentiation Factor 88, Receptor Activator of Nuclear Factor-kappa B, Toll-Like Receptor 4
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common critical diseases that are associated with high mortality [1]. The incidence of ALI/ARDS varies by cause. According to studies of ICU patients in different areas, the incidence of ALI/ARDS is estimated to be between 20% and 50% [2,3], and the mortality rate of ALI/ARDS is 15% to 72% [4–6]. Overall, the mortality rate of ARDS is still high. The pathogenesis of ALI/ARDS is complex [7], and the lungs are most susceptible. Systemic immune inflammation induced by severe injury may lead to decreased lung capacity and lung compliance, as well as progressive hypoxemia and respiratory distress resulting from imbalance of ventilation/blood flow ratio, which eventually becomes life-threatening.
The specific cellular and molecular mechanisms of ALI/ARDS remain unclear. Prior evidence has shown that the pathogenesis of ALI mainly includes imbalances of cytokines, oxidative stress injury, and apoptosis [8]. Cytokine imbalance refers to the massive release of inflammatory factors by activation of many monocytes and macrophages in the pathogenesis of ALI. The imbalance between inflammatory factors and anti-inflammatory factors can induce lung and systemic inflammatory response. Activation of neutrophils produces and releases a large amount of reactive oxygen species (ROS) in ALI. ROS can damage pulmonary parenchymal cells directly through oxidized cell membrane lipids, and can also damage interstitial components such as capillary basement membrane to cause lung injury. Furthermore, inflammation and oxidative damage together contribute to the increase in capillary permeability, tissue edema, and further aggravation in tissue damage. Apoptosis increases remarkably in the course of ALI, which can occur in pulmonary vascular endothelial cells in the early stage of ALI, and also participates in the reconstruction and functional recovery of lung tissue in the recovery stage of ALI.
Garlic is a medicine and food dual-use food in Asian countries such as China. Allicin is a volatile sulfide extracted from garlic bulbs. In recent decades, increasing attention has been paid to research on and application of allicin. Studies have shown that garlic has anti-tumor, antibacterial, and antiviral effects, as well as improving immunity [9,10]. However, there is still no report on the role and mechanism of allicin in improving ALI. In this study, a rat model of ALI was constructed by LPS to explore the therapeutic effect of allicin on ALI by intragastric administration and to determine the mechanism by detecting the related signaling pathways.
Material and Methods
Experimental animals
We obtained 40 male SD rats weighing 200–220 g purchased from the Laboratory Animal Center of Nanjing Medical University. Before experiments, animals were kept at 20–22°C for 1–2 weeks in an SPF animal room to adapt to the environment, with free access to food and water. All experimental procedures were approved by the Laboratory Animal Management Ethics Committee of Wenzhou Medical University. Animal processing and experimental operations were in accordance with the ethical requirements for laboratory animals.
Major experimental reagents
We used the following reagents: Allitride Injection (Shanghai Hefeng Pharmaceutical Co.; specification: 5 mL/60 mg); endotoxin (LPS, SIGMA, 5 mg/kg, Escherichia coliB55: 5, iv); ELISA kit for tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β) and interleukin 6 (IL-6) in rats, and DMSO (R&D); TUNEL kit (ROCHE); mouse anti-rat TLR4, MyD88, NF-κB, and caspase-3 and caspase-9 antibodies (CST Company).
Experimental methods
We randomly divided 54 healthy adult male SD rats into 6 groups (n=9): normal group (Normal), DMSO gavage group (DMSO), LPS group, LPS model rat low-dose allicin group (25 μg/mL) (LPS+LD), LPS model rat middle-dose allicin group (50 μg/mL) (LPS+MD), and LPS model rat high-dose allicin group (100 μg/mL) (LPS+HD). Rats were fasted for 12 h before the experiment, with free access to drinking water. Rats in LPS, LPS+LD, LPS+MD, and LPS+HD groups were injected intraperitoneally with the corresponding dose of endotoxin (20 mg/kg) to establish the ALI rat model. Rats in LPS+LD, LPS+MD, and LPS+HD groups were injected with 25 μg/mL, 50 μg/mL or 100 μg/mL allicin (injection volume of 20 mL/kg) at the same time as LPS injection, once every 12 h. Furthermore, rats in the DMSO group were given intraperitoneal injection of DMSO and normal saline at the volume of 20 mL/kg once every 12 h. Rats in the Normal and LPS groups received intraperitoneal injection of 20 mL/kg of saline once every 12 h. The rats in each group were killed by excessive anesthesia with chloral hydrate after 24 h of corresponding treatment, after which the lung tissue and alveolar lavage fluid were removed to test the corresponding indicators.
Main outcome measures
Wet/dry mass ratio (W/D) of lung tissue
After 24 h after modeling, 5 rats in each group were killed via excessive anesthesia with 10% chloral hydrate. The left lung was removed after thoracotomy, followed by quick removal of water and blood on the lung surface with filter paper. After weighing and recording the mass, the collected tissues were baked in an oven at 80°C for 48 h to reach a constant mass. With another weighing of tissues via electronic balance, the W/D values of lung tissues were calculated in each group (n=5)
Hematoxylin-eosin (HE) staining of lung tissue
The upper lobe of the right lung was collected in the rats for the measurement of W/D. The collected tissues were fixed with 40 g/L polyformaldehyde for 12 h, followed by embedding in paraffin for routine dehydration treatment, and preparation of 5-μm slices. After dewaxing and staining with hematoxylin-eosin staining, pathological changes in lung tissue were observed under an optical microscope (Olympus, Japan) (n=5).
Detection of levels of inflammatory factors TNF-α, IL-1β, and IL-6 in bronchoalveolar lavage fluid by ELISA
After 24 h of endotoxin treatment, rats were anesthetized with chloral hydrate in 10% volume fraction. The bronchoalveolar lavage fluid of each group was collected according to the above methods. The mass concentrations of TNF-α, IL-1β, and IL-6 in bronchoalveolar lavage fluid were detected by use of an ELISA kit (R&D Company) according to the instruction manual. The absorbance value was measured at 450 nm using an ELx800 Microplate Reader (BioTek). Mass concentrations of TNF-α, IL-1β, and IL-6 in bronchoalveolar lavage fluid samples were calculated according to the standard curve.
Detection of apoptosis in lung tissues via TUNEL assay
Rats were anesthetized with chloral hydrate in 10% volume fraction after endotoxin treatment for 24 h. After cardiac perfusion with pre-cooled 0.01 mol/L PBS in anesthetized rats, the right middle lobe was removed quickly and fixed with 40 g/L polyformaldehyde for 12 h. Following immobilization, dehydration, and paraffin embedding, the apoptosis of lung tissue was detected in strict accordance with the procedure of TUNEL staining. The staining of lung tissue was observed under a light microscope, in which the positive apoptotic cells showed yellow staining in the nuclei. With the selection of over 10 fields of vision (high magnification of ×400), the number of apoptotic cells and the total number of cells were observed by Image-Pro Plus 6.0 software to calculate the number of apoptotic cells in lung tissues.
Immunohistochemical staining
Rat lung tissue blocks (0.5×0.5 cm) were placed in 4% neutral paraformaldehyde for 24 h, followed by routine paraffin embedding, preparation of 4-μm slices, baking at 60°C for 90 min, routine dewaxing, washing, and high-pressure antigen repair (pH 6.0). After adding normal serum for blocking, TLR4, MyD88, NF-κB, caspase-9, and caspase-3 (1: 200) were dripped, respectively, followed by the addition of biotinylated sheep anti-rabbit antibody. Then, 3,3′-diaminobenzidine (DAB) was applied for development, followed by re-staining with hematoxylin, dehydration, transparentizing, and sealing. The phosphate buffer instead of the primary antibody was applied in the Normal group. The expression of related proteins was measured using the Alpha Innotech (USA) image analysis system.
Statistical analysis
Graph Pad Prism 5.0 software was used for mapping and SPSS 21.0 (Chicago, USA) statistical software was used for data analysis. Measurement data are expressed as mean ±SD, and comparisons between groups were made using one-way analysis of variance (ANOVA). P<0.05 meant that the difference was significant.
Results
HE staining in lung tissue
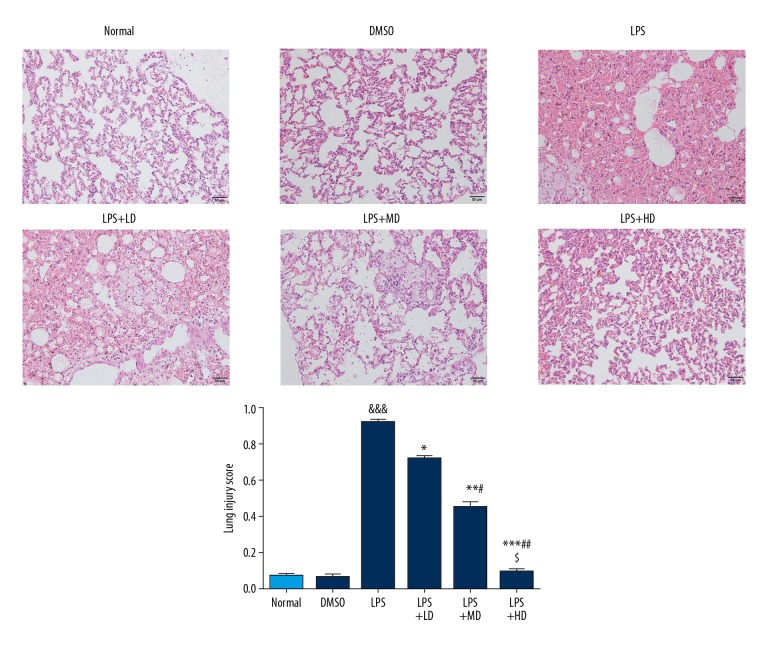
After endotoxin treatment for 1 h, rats began to exhibit reduced activity, poor spirits, and dysentery. Compared with the LPS group, rats in the LPS+LD, LPS+MD, and LPS+HD groups showed alleviation in the above symptoms, and the activity and mental state of rats was acceptable, showing a dose-effect relationship. Histological examination showed that the surface of lung tissue in the Normal group and DMSO group was smooth and pink, with no fluid exudation or bleeding points. Meanwhile, observation under the optical microscope revealed that the lung tissue was clear, the alveolar wall was thin, and there was no liquid exudation in the alveoli (Figure 1). The lung tissue of LPS-treated rats showed congestion and edema, rough surface, and patchy necrosis. Furthermore, there were pulmonary interstitial edema, diffuse hemorrhage, extensive infiltration of inflammatory cells, thickening of alveolar wall, and a large amount of fluid exudation as revealed by optical microscopy (Figure 1). The degree of pulmonary edema was improved in LPS+LD, LPS+MD, and LPS+HD groups, with alveolar hemorrhage and inflammatory cell infiltration observed under an optical microscope. Pulmonary edema, alveolar hemorrhage, and inflammatory cell infiltration were milder than in the ALI group, and the improvement of symptoms showed a dose-effect relationship. Comparison in the pathological score showed that the degree of lung injury in LPS+LD, LPS+MD and LPS+HD groups was significantly lower than that in LPS group (P<0.05, P<0.01 or P<0.001, Figure 1), with a remarkable dose-effect relationship (P<0.05, respectively).
Figure 1.
Pathological in different groups by HE staining (200×). Normal: The rats were injected with normal saline; DMSO: The rats were injected with DMSO; LPS: The rats were injected with LPS; LPS+LD: The rats were injected with LPS and low-dose allicin (25 μg/mL); LPS+MD: The rats were injected with LPS and Middle-dose allicin (50 μg/mL); LPS+HD: The rats were injected with LPS and High-dose allicin (100 μg/mL). &&& P<0.001 vs. normal; * P<0.05; ** P<0.01; *** P<0.001 vs. LPS group; # P<0.05; P<0.01 vs. LPS+LD group; $ P<0.05 vs. LPS+MD group.
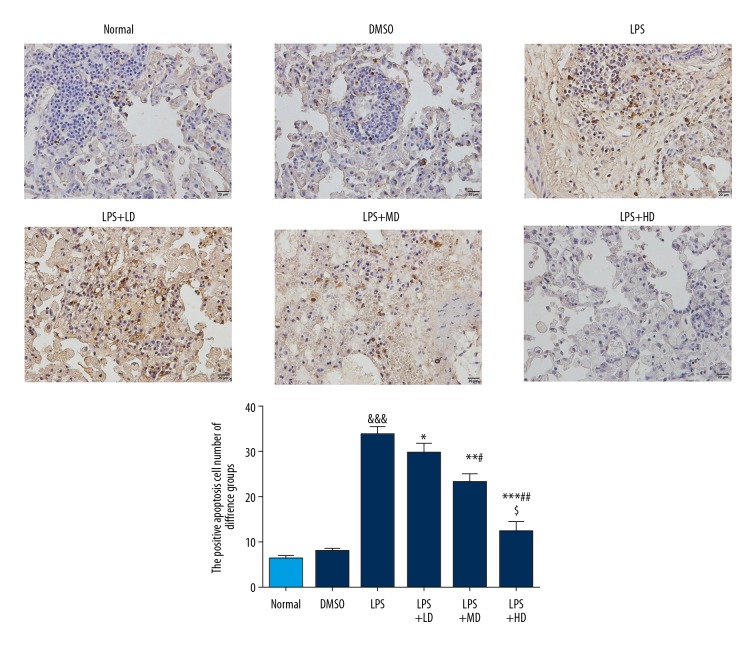
Effect of allicin on apoptosis of lung tissue in rats with ALI
As shown in Figure 2, the nuclei of normal cells were blue-green in TUNEL staining, while those of apoptotic cells were stained yellowish-brown. TUNEL staining of lung tissue showed that there were yellow-brown and brownish-brown apoptotic nuclei in all groups. The presence of yellow-brown was extremely low in Normal and DMSO groups, with relatively fewer apoptotic cells, indicating no significant difference between the 2 groups (P>0.05). Compared with the Normal group, the number of yellow-brown apoptotic cells increased significantly in the LPS group (P<0.001). However, compared with the LPS group, the number of yellow-brown apoptotic cells in the LPS+LD, LPS+MD, and LPS+HD groups decreased significantly after the administration of different doses of allicin (P<0.05, P<0.01 or P<0.001, respectively).
Figure 2.
The number of positive cells in different groups by TUNEL (400×). Normal: The rats were injected with normal saline; DMSO: The rats were injected with DMSO; LPS: The rats were injected with LPS; LPS+LD: The rats were injected with LPS and low-dose allicin (25 μg/mL); LPS+MD: The rats were injected with LPS and Middle-dose allicin (50 μg/mL); LPS+HD: The rats were injected with LPS and High-dose allicin (100 μg/mL). &&& P<0.001 vs. normal; * P<0.05; ** P<0.01; *** P<0.001 vs. LPS group; # P<0.05; P<0.01 vs. LPS+LD group; $ P<0.05 vs. LPS+MD group.
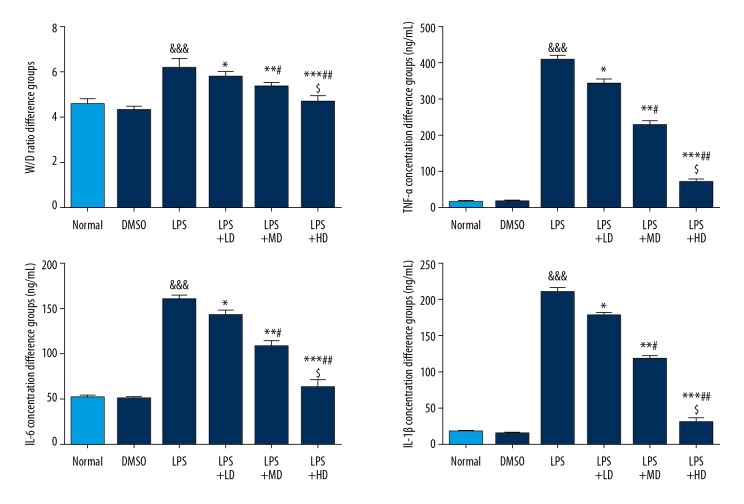
Allicin affected W/D ratio and related inflammatory cytokines
Compared with the Normal group, the W/D ratio, as well as TNF-α, IL-6, and IL-1β concentrations, of the LPS group were significantly increased (P<0.001 all). With allicin supplementation, the W/D ratio and TNF-α, IL-6, and IL-1β concentrations of allicin-treated groups were significantly lower compared with those of the LPS group, and the effect was dose-dependent (P<0.05 all) (Figure 3).
Figure 3.
The W/D ratio and relative inflammatory cytokines. Normal: The rats were injected with normal saline; DMSO: The rats were injected with DMSO; LPS: The rats were injected with LPS; LPS+LD: The rats were injected with LPS and low-dose allicin (25 μg/mL); LPS+MD: The rats were injected with LPS and Middle-dose allicin (50 μg/mL); LPS+HD: The rats were injected with LPS and High-dose allicin (100 μg/mL). &&& P<0.001 vs. normal; * P<0.05; ** P<0.01; *** P<0.001 vs. LPS group; # P<0.05; P<0.01 vs. LPS+LD group; $ P<0.05 vs. LPS+MD group.
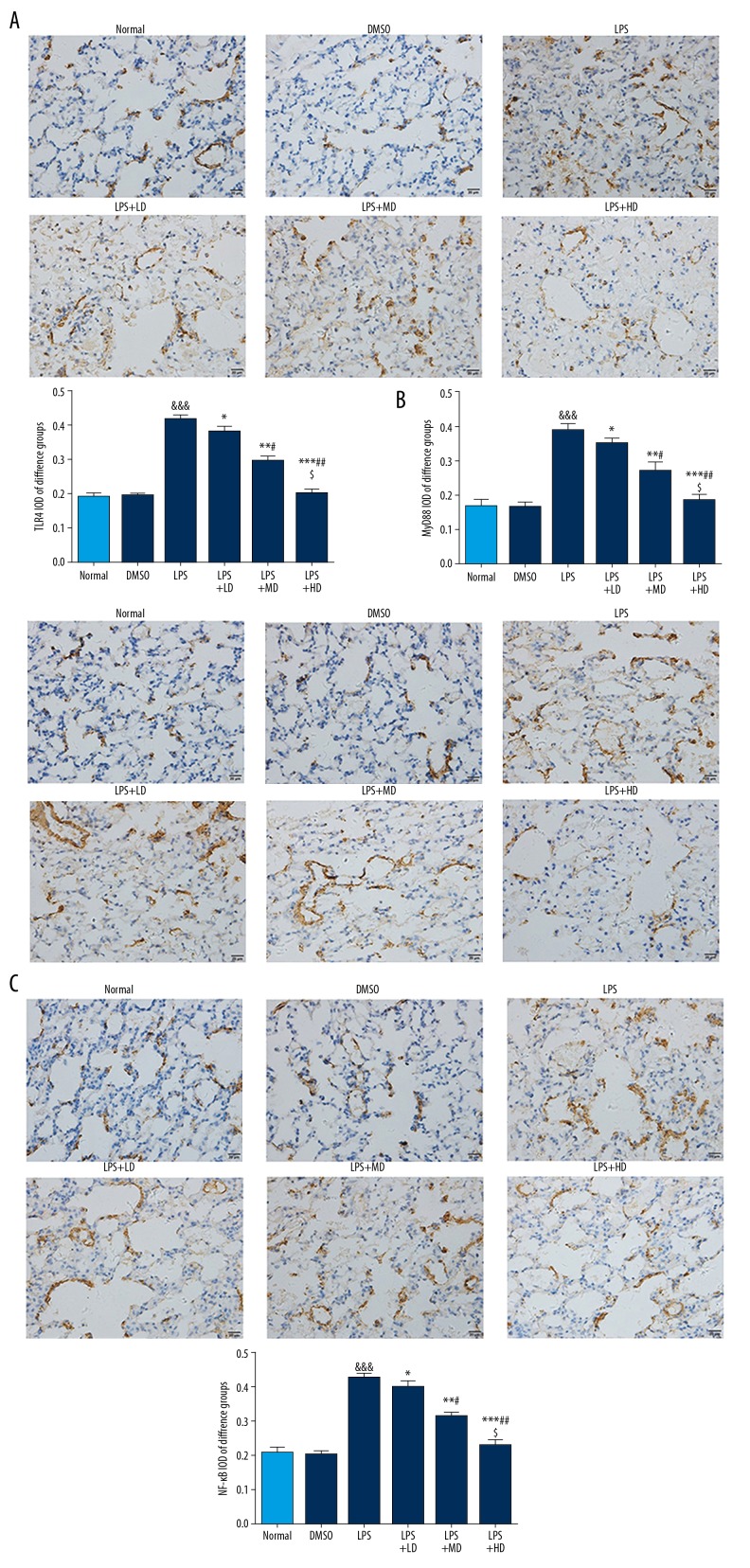
Allicin affected inflammatory-related proteins expression by IHC
Compared with the Normal group, the TLR4, MyD88, and NF-κB proteins expressions of the LPS group were significantly stimulated (P<0.001 all); however, the TLR4, MyD88, and NF-κB proteins expressions were significantly depressed in allicin-treated groups compared with those of the LPS group, and the effect was dose-dependent (P<0.05, respectively) (Figure 4).
Figure 4.
(A–C) Inflammatory relative proteins expression by IHC (400×). Normal: The rats were injected with normal saline; DMSO: The rats were injected with DMSO; LPS: The rats were injected with LPS; LPS+LD: The rats were injected with LPS and low-dose allicin (25 μg/mL); LPS+MD: The rats were injected with LPS and Middle-dose allicin (50 μg/mL); LPS+HD: The rats were injected with LPS and High-dose allicin (100 μg/mL). &&& P<0.001 vs. normal; * P<0.05; ** P<0.01; *** P<0.001 vs. LPS group; # P<0.05; P<0.01 vs. LPS+LD group; $ P<0.05 vs. LPS+MD group.
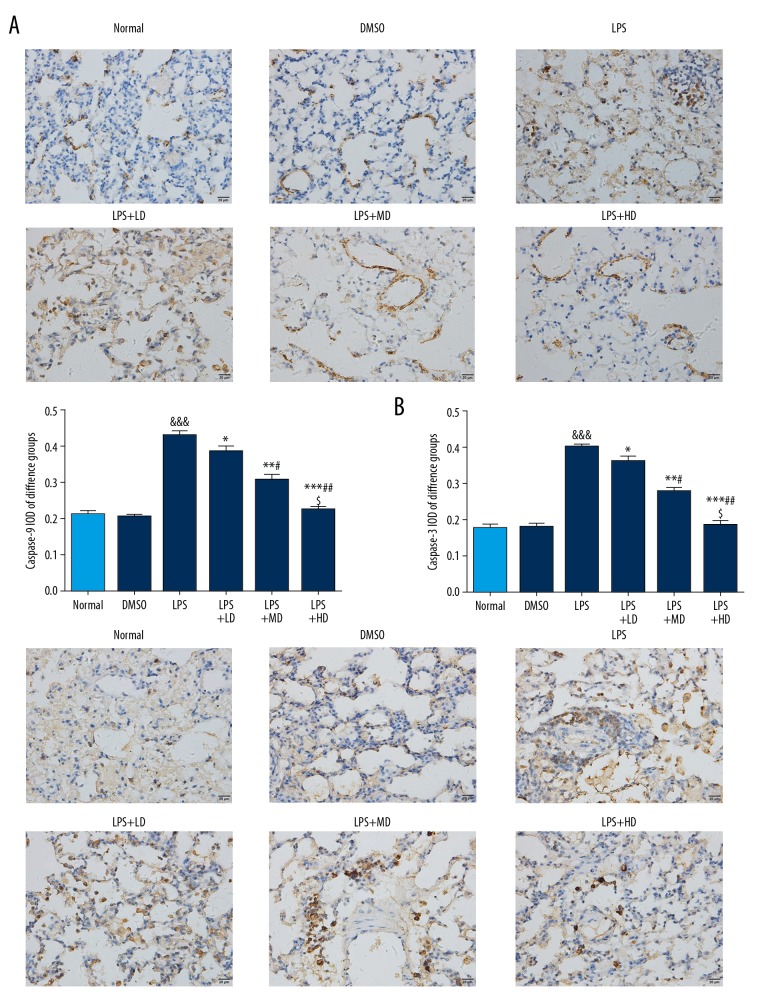
Allicin affected cell apoptosis-related proteins expression by IHC
Compared with the Normal group, the caspase-9 and caspase-3 proteins expressions of the LPS group were significantly stimulated (P<0.001, respectively); however, the caspase-9 and caspase-3 proteins expressions were significantly depressed in allicin-treated groups compared with those of the LPS group, and the effect was dose-dependent (P<0.05 all) (Figure 5).
Figure 5.
(A, B) The apoptosis relative proteins expressions by IHC (400×). Normal: The rats were injected with normal saline; DMSO: The rats were injected with DMSO; LPS: The rats were injected with LPS; LPS+LD: The rats were injected with LPS and low-dose allicin (25 μg/mL); LPS+MD: The rats were injected with LPS and Middle-dose allicin (50 μg/mL); LPS+HD: The rats were injected with LPS and High-dose allicin (100μg/mL). &&& P<0.001 vs. Normal; * P<0.05; ** P<0.01; *** P<0.001 vs. LPS group; # P<0.05; P<0.01 vs. LPS+LD group; $ P<0.05 vs. LPS+MD group.
Discussion
The present study is the first to demonstrate the protective effect of allicin against endotoxin-induced acute lung injury in rats via intraperitoneal injection. Intraperitoneal injection of allicin obviously reduced the level of inflammatory factors in bronchoalveolar lavage fluid of rats and alleviated pulmonary inflammation caused by endotoxin. For cell apoptosis of lung tissue, allicin can inhibit the activation of caspase-3 and caspase-9 in lung tissue, decrease the occurrence of cell apoptosis, and then reduce lung tissue injury. In summary, allicin can play a protective role in endotoxin-induced ALI from different aspects, such as anti-inflammation and reducing apoptosis in the early stage.
ALI and ARDS are common syndromes with high mortality and characterized by progressive dyspnea and intractable hypoxemia due to various causes inside and outside the lung [11]. Sepsis is one of the most important causes of ALI, and sepsis is a common clinical critical disease, which can cause damage to all organs of the body, of which the lung is the most vulnerable. Lung injury occurs early and severely in sepsis with common manifestation of ALI or ARDS, which is an important cause of death [12]. Endotoxin is the main component of the cell wall of gram-negative bacteria. ALI caused by sepsis is closely related to the endotoxin level of the cell wall component of gram-negative bacteria in plasma, of which endotoxin is recognized as the primary substance causing sepsis-induced ALI. Therefore, the animal model of ALI was established by intraperitoneal injection of endotoxin (20 mg/kg) in rats [13]. Our study revealed that animals treated with endotoxin showed significant lung damage and dysfunction, such as increased breathing and decreased activity. HE staining and observation by electron microscopy showed significant alveolar damage, including thinning of the alveolar wall, infiltration of inflammatory cells, fluid exudation, obvious increase in W/D ratio of lung tissue, increased alveolar lavage fluid cells, and remarkable increase in the level of inflammatory factors, which was consistent with a previous study [14].
The pathogenesis of ALI is extensive injury of pulmonary vessels caused by pulmonary inflammation, oxidative stress, and disorder of cell apoptosis, leading to lung ventilation dysfunction and multiorgan dysfunction [15,16]. Therefore, inhibition of inflammatory response, oxidative stress, and cell apoptosis are effective approaches to alleviate ALI. In ALI, inflammatory cells in lung tissues are activated to produce a cascade immune response, which stimulates the release of inflammatory factors, chemokines, adhesion molecules, reactive oxygen species, and other injury mediators. Studies have shown that inflammatory factors such as TNF-α, IL-6, and IL-1β play an important role in the early inflammatory response of ALI [17]. Inflammation is the central link of endotoxin-induced ALI. The intensity of inflammation in lung tissues can be accurately reflected by detecting the contents and morphological changes of cells in bronchoalveolar lavage fluid and the level of important inflammatory factors.
TLR4 is one of the most important TLRs sub-types to identify pathogenic bacteria and play a key role in the innate immune system. The Toll/IL-1 receptor (TIR) domain in the cytoplasmic region binds to TIR at the carboxyl end of the intracytoplasmic adaptor protein MyD88 to form a complex. It recruits and binds to IL-1 receptor-related kinase (IRAK) and phosphorylates itself. After binding to tumor necrosis factor receptor-related factor 6 (TRAF6), it activates IKB kinase (IKKs), cause ubiquitination and degradation of IKB, and activates NF-κB. It also transfers cytoplasm to the nucleus, initiates or enhances the transcription and translation of many genes related to inflammation and immunity, exerts early immune response, and mediates hepatic inflammation [18,19]. The cytokines induced by NF-κB not only mediate inflammation, but also further up-regulate the activity of NF-κB to form a positive feedback regulation mechanism, which can produce cascade amplification of inflammation in organisms [20]. In this study, following LPS induction, there was significant up-regulation of TLR4 expression, synchronous changes in adaptor protein MyD88 and NF-κB with TLR4 expression, up-regulation of expressions in downstream inflammatory cytokines IL-1β, TNF-α, and IL-6, and increased expression of apoptosis-related proteins caspase-9 and caspase-3, suggesting that LPS-induced lung injury is associated with TLR4/MyD88/NF-κB signaling pathway activation induced by LPS. After intraperitoneal injection of allicin, the TLR4/MyD88/NF-κB signaling pathway was also significantly inhibited, which appears to be related to the improvement of LPS-induced lung injury by allicin.
Conclusions
Allicin can improve lung injury induced by LPS-induced inflammatory response by regulating the TLR4/MyD88/NF-κB signaling pathway, providing a potential reference for future research on the application of allicin.
Footnotes
Source of support: Departmental sources
References
- 1.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: A hospital-based registry study. BMJ. 2015;351:h3646. doi: 10.1136/bmj.h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu WJ, Wan YD, Tie HT, et al. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: A meta-analysis. PLoS One. 2014;9(2):e90426. doi: 10.1371/journal.pone.0090426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Londhe C, Ganeriwal A, deSouza R. Study of clinical profile of acute respiratory distress syndrome and acute lung injury in Plasmodium vivax malaria. J Vector Borne Dis. 2014;51(4):339–42. [PubMed] [Google Scholar]
- 4.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. Am J Respir Crit Care Med. 1999;159(6):1849–61. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 5.Irish Critical Care Trials Group. Acute lung injury and the acute respiratory distress syndrome in Ireland: A prospective audit of epidemiology and management. Crit Care. 2008;12(1):R30. doi: 10.1186/cc6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewandowski K, Metz J, Deutschmann C, et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151(4):1121–25. doi: 10.1164/ajrccm.151.4.7697241. [DOI] [PubMed] [Google Scholar]
- 7.Johnson ER, Matthay MA. Acute lung injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–52. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: From bacterial pathogenesis to host response. J Intensive Care. 2014;2(1):10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Sun Y, Li W, et al. The antibacterial mode of action of allitridi for its potential use as a therapeutic agent against Helicobacter pylori infection. FEMS Microbiol Lett. 2010;303(2):183–89. doi: 10.1111/j.1574-6968.2009.01877.x. [DOI] [PubMed] [Google Scholar]
- 10.Song B, Shu Y, Cui T, et al. Allicin inhibits human renal clear cell carcinoma progression via suppressing HIF pathway. Int J Clin Exp Med. 2015;8(11):20573–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Pettitt SJ, Guo G, et al. Isolation of homozygous mutant mouse embryonic stem cells using a dual selection system. Nucleic Acids Res. 2012;40(3):e21. doi: 10.1093/nar/gkr908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aulakh GK, Suri SS, Singh B. Angiostatin inhibits acute lung injury in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2014;306(1):L58–68. doi: 10.1152/ajplung.00368.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie K, Yu Y, Huang Y, et al. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37(5):548–55. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Yu X, Yu S, et al. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol. 2015;29(2):937–46. doi: 10.1016/j.intimp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Ward PA, Grailer JJ. Acute lung injury and the role of histones. Transl Respir Med. 2014;2:1. doi: 10.1186/2213-0802-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–56. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 18.Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;3(6):377–85. doi: 10.3978/j.issn.2304-3881.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin H, Huang L, Ouyang T, et al. Baicalein improve liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int Immunopharmacol. 2018;55:55–62. doi: 10.1016/j.intimp.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Mehal MZ. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig Dis. 2014;32(5):507–15. doi: 10.1159/000360495. [DOI] [PubMed] [Google Scholar]