Abstract
Background
Persistence of bacterial pathogens in the airways has profound consequences on the course and pathogenesis of chronic obstructive pulmonary disease (COPD). Patients with COPD continuously acquire and clear strains of Moraxella catarrhalis, a major pathogen in COPD. Some strains are cleared quickly and some persist for months to years. The mechanism of the variability in duration of persistence is unknown.
Methods
Guided by genome sequences of selected strains, we studied the expression of Hag/MID, hag/mid gene sequences, adherence to human cells, and autoaggregation in longitudinally collected strains of M. catarrhalis from adults with COPD.
Results
Twenty-eight of 30 cleared strains of M. catarrhalis expressed Hag/MID whereas 17 of 30 persistent strains expressed Hag/MID upon acquisition by patients. All persistent strains ceased expression of Hag/MID during persistence. Expression of Hag/MID in human airways was regulated by slipped-strand mispairing. Virulence-associated phenotypes (adherence to human respiratory epithelial cells and autoaggregation) paralleled Hag/MID expression in airway isolates.
Conclusions
Most strains of M. catarrhalis express Hag/MID upon acquisition by adults with COPD and all persistent strains shut off expression during persistence. These observations suggest that Hag/MID is important for initial colonization by M. catarrhalis and that cessation of expression facilitates persistence in COPD airways.
Keywords: Moraxella catarrhalis, chronic obstructive pulmonary disease, persistent infection, bacterial pathogenesis, slipped-strand mispairing, respiratory tract infection, bacterial genome
Moraxella catarrhalis persists in COPD with profound clinical and pathogenetic consequences. Most strains express the major surface antigen Hag/MID on acquisition and then shut off expression, suggesting that Hag/MID is critical for initial colonization and cessation of expression facilitates persistence.
Chronic obstructive pulmonary disease (COPD) is a debilitating disease that is the fourth most common cause of death in the world. While death rates from heart disease and stroke are declining, the death rate from COPD has doubled since 1970 and is projected to be the third most common cause of death globally by 2030 [1, 2]. Bacterial infection plays a critical role in the course and pathogenesis of COPD, causing acute exacerbations and chronic lower airway infection, which results in host inflammatory responses that increase symptoms and accelerate the progressive lung dysfunction and destruction that are hallmarks of COPD [3–5].
Moraxella catarrhalis and nontypeable Haemophilus influenzae are the 2 most common bacterial pathogens in COPD based on 2 longitudinal studies performed on 2 continents [6–8]. Indeed, M. catarrhalis, the focus of the present study, is estimated to cause 2 to 4 million exacerbations of COPD annually in the United States [6].
Adults with COPD continuously acquire and clear strains of M. catarrhalis from the respiratory tract. In our 20-year prospective study of COPD, the longer a patient was followed in the study, the more likely they were to acquire M. catarrhalis, suggesting that all patients with COPD are susceptible to infection by the pathogen [6]. Marked variability in the duration of carriage of M. catarrhalis is observed, with some strains cleared quickly and others persisting for 2 years or longer [6, 7]. The cause of this variability in duration of carriage of M. catarrhalis is unknown. Previous observations are consistent with the concept that both host and pathogen factors could account for whether a newly acquired strain is cleared or persists in the respiratory tract. Patients with COPD make systemic and mucosal immune responses to M. catarrhalis and appear to develop strain-specific protection [6, 9, 10]. Thus, a patient’s prior exposure to strains of M. catarrhalis may affect whether a new strain is cleared or persists. The M. catarrhalis genome shows modest diversity [11] and several known virulence determinants show extensive antigenic and sequence diversity among strains, consistent with the idea that different virulence determinants among strains influence the duration of carriage of individual strains [11–13].
In the present study, we hypothesized that strains of M. catarrhalis that persist in the respiratory tract of adults with COPD alter selected virulence determinants to enable persistence in the hostile environment of the COPD airways. Analysis of genome sequences of persistent COPD strains revealed changes in simple sequence repeats in Hag/MID, a major adhesin, during persistence in the airways, suggesting regulation by slipped-strand mispairing. This observation guided an analysis of hemagglutinin/Moraxella IgD binding protein (Hag/MID) expression in cleared strains and serial isolates of persistent strains. We show that most strains of M. catarrhalis express Hag/MID upon acquisition by the patient and persistent strains shut off expression of Hag/MID during persistence. These observations suggest that Hag/MID is important for initial colonization and that cessation of expression facilitates persistence in COPD airways.
MATERIALS AND METHODS
The study was approved by the Institutional Review Boards of the University at Buffalo and the Western New York Veterans Affairs Healthcare System. Written, informed consent was provided by participants.
COPD Study Clinic
The COPD study clinic, described previously [6, 14], is a prospective study conducted from 1994 to 2014. Adults with COPD were seen monthly and at suspected exacerbations. Rigorous clinical criteria determined whether patients were experiencing an exacerbation. At each visit clinical data and expectorated sputum samples were collected. An exacerbation caused by M. catarrhalis was defined by the onset of clinical symptoms simultaneous with the acquisition of a new strain [6, 14].
Bacterial Strains
The identity of an isolate as M. catarrhalis was confirmed by colony morphology and the presence of butyrate esterase. All isolates were subjected to molecular typing by pulsed field gel electrophoresis; multilocus sequence types were determined on selected isolates [15]. Strains that were isolated at a single monthly clinic visit and were not isolated again at subsequent monthly clinic visits were classified as “cleared”. Strains that were isolated from a study participant at more than 1 monthly clinic visit and that were the same strain by molecular typing were classified as “persistent”. Duration of persistence was estimated as the number of days between the date when the strain was first detected and date when the strain was last detected in approximately monthly sputum cultures, which provides a minimum estimate of the duration of persistence.
Strains were grown on brain heart infusion (BHI) agar plates at 37°C in 5% CO2 or in BHI broth at 37°C with shaking.
Genome Sequencing, Assembly, and Analysis
Genomic DNA was extracted from low-passage M. catarrhalis strains (3 passages from the original isolation) using the gram-negative bacteria protocol of the Qiagen DNeasy Blood and Tissue kit. Samples from 6 isolates were subjected Pacific Biosciences (Pac-Bio) Sequel sequencing to obtain finished (gap-free) genomes at the Institute for Genome Sciences. PacBio barcoded large insert libraries (approximately 8 kb) with BluePippin insert size selection were constructed for each genome. Five libraries were multiplexed per run of the PacBio Sequel platform using the v2 chemistry (6-hour movie). Finished genomes were assembled with CANU v1.4 software [16] and annotated using the CloVR Microbe pipeline [17]. Samples from 2 isolates were subjected to 150-bp paired-end sequencing on the Illumina HiSeq 2500 in the Next-Generation and Expression Analysis Core at the University at Buffalo. Illumina reads were assembled with SPAdes v3.9.0 using default parameters. All genome sequencing data were submitted to the NCBI SRA and WGS repositories (accession numbers pending).
Antisera to Hag/MID
Mouse antisera specific for the aminoterminal region, central region, and carboxyterminal regions of Hag/MID were described previously [18].
Adherence to Respiratory Epithelial Cells
Quantitative adherence assays were performed with the NCI-H292 human respiratory epithelial cell line in RPMI 1640 plus l-glutamine base medium (Life Technologies) supplemented with fetal bovine serum (10% final concentration), HEPES (10 mM final concentration), and sodium pyruvate (1 mM final concentration), as previously described [19, 20]. H292 cells used for experiments were passaged no more than 10 times and were tested for mycoplasma using the Mycosensor polymerase chain reaction (PCR) assay kit (Agilent). Briefly, 2 × 105 H292 cells were seeded into each well of a 24-well tissue culture plate and incubated for approximately 48 hours when cells showed confluent growth. Cells were inoculated with broth-grown log-phase bacteria (multiplicity of infection = 1), and the plates were centrifuged at 170g for 5 minutes at room temperature to facilitate contact between bacteria and H292 cells. Plates were incubated for 3 hours at 37°C. Nonadherent cells were removed by gently washing the wells 3 times with phosphate-buffered saline. To quantify adherent cells, 200 μL of trypsin (0.25%) was added to each well and plates were incubated at 37°C for 10 minutes to remove adherent cells. A 300-μL volume of 1% saponin was applied to each well, and contents were pipetted into microcentrifuge tubes and after vigorous vortexing were plated in duplicate to perform bacterial cell counts. “Percent adherence” was calculated by dividing the number of adherent cells by the total number of cells in the well and multiplied by 100.
Aggregation Assay
Cultures were grown overnight in BHI broth at 37°C with shaking. Broth cultures were placed on the bench top at room temperature and aliquots were removed from the top of the liquid culture at 15-minute intervals and the optical density (OD600) was measured and plotted over time.
RESULTS
Identification of Phase Variation of Hag/MID During Persistence
Analysis of the hag/mid gene in the first and last isolates of 3 persistent strains of M. catarrhalis from 3 different patients revealed nucleotide changes during persistence within the open reading frame in 2 of the 3 strains. A 6-G mononucleotide repeat was present immediately downstream of the start codon in strain 5P47B2 (initial acquisition isolate) while the final isolate (5P54B2) had a 7-G repeat, placing the hag/mid gene out of frame in the latter isolate. The final isolate of strain 173P31B1 had an in-frame stop codon within the open reading frame compared to the isolate of the same strain upon acquisition by the patient (173P27B1). These observations suggested that Hag/MID underwent phase variation during persistence in the human COPD airways. To test this hypothesis, we assessed expression of Hag/MID in serial isolates of 30 persistent strains (mean duration 161 ± 138 days) and 30 strains that were promptly cleared from the respiratory tract (ie, isolated only once from a patient in monthly sputum cultures).
Expression of Hag/MID in COPD
To assess expression of the Hag/MID adhesin by strains of M. catarrhalis, 60 strains that were initial acquisition isolates based on monthly cultures (ie, a strain that was not previously isolated from that patient) were subjected to immunoblot assay with antiserum to Hag/MID. The strains included 30 cleared strains and 30 persistent strains (ie, isolated 2 or more times in monthly sputum cultures). A total of 28 of the 30 cleared strains expressed Hag/MID compared to 17 of the 30 initial acquisition isolates of persistent strains.
To assess changes in expression of Hag/MID during persistence in the human respiratory tract, the final isolate of each of the 30 persistent strains (mean duration 161 ± 138 days) was subjected to immunoblot assays with antiserum to Hag/MID. None of the 30 final isolates of persistent strains expressed Hag/MID, indicating that M. catarrhalis altered regulation of expression of the Hag/MID adhesin in the human airways.
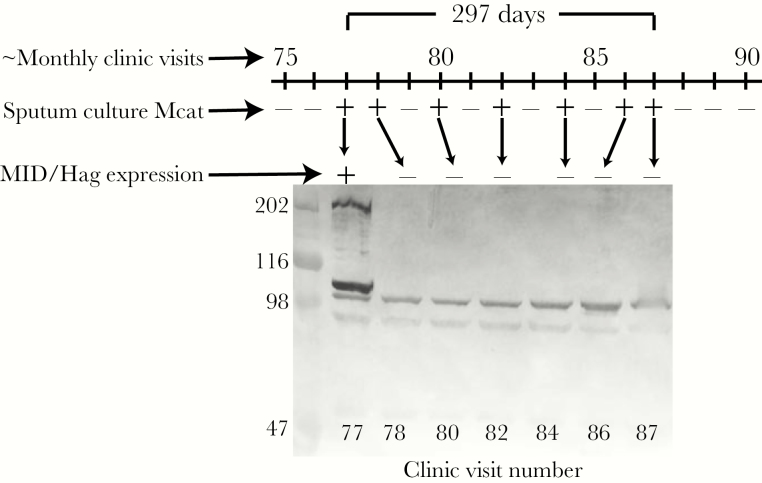
To further explore the dynamics of expression of Hag/MID during persistence in COPD airways, sequential isolates of each of the 30 persistent strains were assessed for expression. Figure 1 is a timeline of patient 142 showing culture results and Hag/MID expression of 7 sequential isolates of a single strain that persisted for 296 days. The strain expressed Hag/MID in the first isolate following acquisition; each subsequent isolate of the same strain expressed no Hag/MID.
Figure 1.
Timeline showing monthly visits 75 through 90 of patient 142 in a prospective study of chronic obstructive pulmonary disease. Sputum culture indicates results of culture for Moraxella catarrhalis (Mcat) where “+” indicates Mcat present and “−” indicates Mcat absent by culture. All isolates are multilocus sequence type 224. Immunoblot assay shows whole-cell lysates of each isolate probed with antiserum to Hag/MID. The isolate recovered at visit 77 expresses Hag/MID whereas subsequent isolates have no detectable expression. The 2 bands near the 98 kDa standard are background rabbit antibodies that are present in all isolates. Molecular mass markers are noted on the left in kDa.
Sequential isolates of each of the 17 persistent strains (n = 94 isolates) that expressed Hag/MID upon acquisition were studied in the same way to determine expression during persistence. Figure 2 shows that M. catarrhalis strains expressed Hag/MID upon acquisition and ceased expression shortly thereafter. All 17 strains expressed Hag/MID on the first isolate cultured following acquisition; 6 strains expressed for 2 sequential monthly visits and 1 strain expressed Hag/MID for 3 sequential monthly visits. All subsequent isolates did not express Hag/MID, indicating that expression of the protein ceases after acquisition by the patient.
Figure 2.
A, Expression of Hag/MID of sequential isolates of persistent strains cultured from sputum. Gray bars indicate isolates that express Hag/MID at a monthly clinic visit (x-axis) and Black bars indicate no expression of Hag/MID. For example, of the 30 visit 1 isolates, 17 expressed Hag/MID; of the 30 visit 2 isolates, 6 expressed Hag/MID, and so forth. B, Expression of Hag/MID of the single isolate of each of the cleared strains of Moraxella catarrhalis.
Mechanism of Phase Variation of Hag/MID
To explore the mechanism that accounts for changes in expression of Hag/MID, sequences of the genes in isolates of 5 persistent strains were aligned and compared. In each case, the hag/mid gene in isolates that expressed Hag/MID was in-frame whereas the gene was out of frame in the nonexpressing isolates of persistent strains. Changes in the region of the polynucleotide repeat of Gs located immediately downstream of the hag/mid start codon accounted for phase variation of Hag/MID in 4 of the 5 strains (Table 1) [21]. The final isolate of strain 173P31B1 acquired an in-frame stop codon within the open reading frame during persistence due to a single nucleotide polymorphism (C to T), accounting for loss of expression of Hag/MID that was expressed by the isolate upon acquisition by the patient (173P27B1). We conclude that phase variation of Hag/MID is regulated by slipped-strand mispairing due to changes in a polynucleotide repeat near the start codon in the open reading frame. These repeats accounted for loss of expression of Hag/MID during persistence of M. catarrhalis in the airways of adults with COPD.
Table 1.
Analysis of the hag/mid Gene in Persistent Strains of Moraxella catarrhalis
| Strain (Initial Isolate/ Final Isolate) |
Duration of Persistence (Days) | Hag/MID Expression | Sequence Immediately Downstream of Start Codon | ||
|---|---|---|---|---|---|
| Initial Isolate |
Final Isolate |
Initial Isolate |
Final Isolate |
||
| 5P47B2/ 5P54B2 |
260 | + | − | 6Gs in frame | 7Gs out of frame |
| 142P77B1a/ 142P87B1 |
296 | + | − | 6Gs in frame | 7Gs out of frame |
| 173P27B1b/ 173P31B1b |
134 | + | − | 6Gs in frame | 6Gs in frame (downstream in-frame stop codon) |
| 74P50B1/ 74P58B1 |
253 | − | − | 1Ac+9Gs out of frame | 1Ac + 9Gs out of frame |
| 46P58B1/ 46P73B1a |
435 | − | − | 1Ac+9Gs out of frame | 1Ac + 9Gs out of frame |
aMultilocus sequence type and Hag/MID sequence determined by polymerase chain reaction.
bGenome sequence determined on Illumina platform. Remaining isolates determined on Pacific Biosciences (PacBio) Sequel platform.
cAn additional A was present immediately upstream of the polynucleotide G repeat.
Impact of Hag/MID Expression on Virulence-Associated Phenotypes
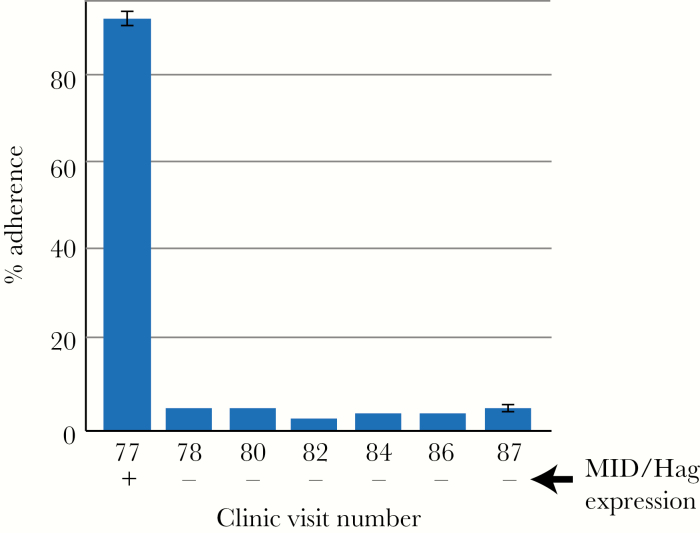
We next assessed the role of changes in expression of Hag/MID on 2 virulence-associated phenotypes in strains of M. catarrhalis isolated from adults with COPD: adherence to human respiratory epithelial cells and autoaggregation. Previous work has established that Hag/MID mediates adherence of M. catarrhalis to respiratory epithelial cells [22]. To assess the effect of changing expression during human airway persistence on adherence, sequential isolates of the same strain cultured from the same patient were evaluated for adherence to human respiratory epithelial cells. Figure 3 shows that an initial isolate (142P77B1), which expressed Hag/MID, showed 95% adherence. Six subsequent sequential isolates, which did not express Hag/MID, showed adherence between 3% and 5%. We conclude that cessation of expression of Hag/MID by M. catarrhalis during persistence in COPD airways causes a marked decrease in adherence to human respiratory epithelial cells.
Figure 3.
Results of assays showing adherence of 7 isolates of a persistent strain of Moraxella catarrhalis (strain 142P77B1) to H292 human respiratory epithelial cells. The y-axis shows percent adherence calculated by dividing the number of adherent cells by the total number of cells in the well and multiplied by 100. The x-axis shows the clinic visit number of sequential isolates collected over 296 days. Expression of Hag/MID is noted at the bottom. Error bars show standard deviation of triplicate independent experiments.
We next assessed autoaggregation of serial isolates of a strain of M. catarrhalis that stopped expression of Hag/MID during persistence. Aggregation is a virulence phenotype for several bacterial pathogens and previous work established that Hag/MID mediates self-aggregation [23–26]. A quantitative aggregation assay demonstrated that the initial Hag/MID-positive acquisition isolate (142P77B1) showed prominent aggregation, whereas the subsequent 6 Hag/MID-negative isolates showed a marked loss in aggregation (Figure 4). We conclude that clinical isolates that shut off expression of Hag/MID during persistence in COPD airways show reduced aggregation compared to the initial Hag/MID-expressing isolate.
Figure 4.
Results of aggregation assays on 7 sequential isolates of a persistent strain of Moraxella catarrhalis (strain 142P77B1). The y-axis is optical density of the broth culture of each isolate and the x-axis is time in hours. Three independent assays showed the same result.
DISCUSSION
The course of COPD is characterized by exacerbations that result in enormous mortality, morbidity, and health care costs [4, 27, 28]. A second, equally important role of bacteria in the pathogenesis of COPD is chronic persistence of bacterial pathogens in the lower airways, which has profound clinical and pathogenetic consequences [3, 5]. Patients have increased airway inflammation and experience increased daily symptoms during times when pathogens are present compared to pathogen-free periods, accelerating the progressive loss of pulmonary function in COPD [4, 29–32]. In view of the central role of bacterial pathogen persistence in the course and pathogenesis of COPD, understanding the mechanisms by which pathogens persist is critical.
In the present study, determination and analysis of the genome sequence of the first and last isolates of 3 persistent strains of M. catarrhalis suggested that expression of the autotransporter, Hag/MID, was changing during persistence in the airways of adults with COPD. Guided by this observation, we performed further studies showing that 28 of 30 strains that were cleared upon acquisition by patients expressed Hag/MID whereas only 17 of 30 strains that persisted in the airways (mean duration 161 ± 138 days) expressed Hag/MID. Analysis of a total of 94 serial isolates of these strains showed the intriguing observation that each of the 17 persistent strains shut off expression of Hag/MID once the strain was acquired, suggesting selective pressure against expression during persistence in COPD airways. We further demonstrate that expression of 2 virulence-associated phenotypes (adherence to human respiratory epithelial cells and autoaggregation) in sequential isolates in 1 persistent strain closely parallels the expression of Hag/MID in isolates from COPD airways.
Hag/MID is a 200-kilodalton autotransporter of M. catarrhalis with multiple virulence-related functions. This major surface antigen binds IgD, is an adhesin for human respiratory epithelial cells, binds collagen, and mediates both autoaggregation and hemagglutination [18, 22, 23, 33–38]. Immunization with a recombinant fragment of Hag/MID induces protective responses in the mouse pulmonary clearance model [39]. Hag/MID is a target of both systemic and mucosal immune responses following clearance of M. catarrhalis in adults with COPD [9, 10]. These antibody responses are directed at an immunodominant region of the Hag/MID molecule, which overlaps with several biologically relevant domains, including epithelial cell adherence, IgD binding, collagen binding, and hemagglutination [40].
To explore the mechanism of regulation of expression of Hag/MID during persistence in COPD airways, we analyzed gene sequences of paired isolates of 5 strains that ceased expression during persistence. Four strains underwent a change in polynucleotide G repeats in the 5′ region of the hag/mid gene and 1 strain acquired an in-frame stop codon resulting in cessation of expression, consistent with the observation of Möllenkvist et al [21] who studied 91 strains of M. catarrhalis isolated at a single point in time. They demonstrated that all strains had the hag/mid gene and that 84% of the strains expressed Hag/MID. The present study advances our understanding of the potential relevance of the regulation of expression Hag/MID by studying longitudinally collected sequential isolates in persistent human infection. A striking contrast is observed between the proportion of initial isolates that express Hag/MID upon acquisition by COPD patients (47 of 60) compared to expression of Hag/MID in isolates after persistence (0 of 30). These observations suggest that Hag/MID is important for the initial establishment of colonization and that the absence of expression of the antigen facilitates persistence.
If Hag/MID is important for initial colonization of M. catarrhalis by adults with COPD, why did 13 of 30 persistent strains not show expression of the protein in the first isolate collected? Patients were seen approximately monthly in the study. However, missed appointments by patients periodically resulted in longer intervals between visits during which sputum samples were collected, accounting for some of the absence of expression by the initial isolate. In addition, M. catarrhalis appears to have the capacity to shut off expression in less than 1 month. In the case of 1 patient, the initial isolate expressed Hag/MID and the second isolate of the same strain cultured 15 days later showed absent expression. Thus, we speculate that some of the 13 persistent strains with absent expression in fact expressed Hag/MID initially but our periodic cultures missed the initial isolate. An alternative explanation is that other adhesins played a more important role than Hag/MID for initial colonization in the 13 strains that did not express Hag/MID in the initial isolate.
Expression of 4 other genes of M. catarrhalis, in addition to Hag/MID, are regulated by slipped-strand mispairing, including the adhesins UspA1 and UspA2, lipooligosaccharide synthesis genes, and a restriction modification system that influences the expression of multiple genes [41–45]. The present study reports novel observations related to the regulation of Hag/MID during the course of human infection in the clinical setting of COPD. In view of the robust antibody response to Hag/MID by patients with COPD [9, 10, 40], we speculate that immune selective pressure may account for the reduced expression of during persistence in the airways.
Regulation of virulence factors by slipped-strand mispairing during persistence in COPD airways is reminiscent of recent observations involving nontypeable H. influenzae, which shares an ecological niche with M. catarrhalis (ie, the human respiratory tract). Analysis of genomes of cleared and persistent strains of nontypeable H. influenzae from the same prospective study showed that slipped-strand mispairing in multiple genes during persistence regulates expression of critical virulence functions, including adherence, nutrient uptake, and modification of surface molecules, and is a major mechanism for survival of nontypeable H. influenzae in the hostile environment of the human airways [46]. Cholon et al [47] previously showed that expression of the major adhesins HMW 1 and HMW 2 decreased with persistence due to graded phase variation controlled by the number of repeats upstream of the structural genes.
UspA1 is another major adhesin of M. catarrhalis that, like Hag/MID, mediates adherence to respiratory epithelial cells and autoaggregation [48, 49]. Expression of UspA1 is regulated by slipped-strand mispairing by a poly-G tract in the promoter region. LaFontaine et al [44] showed that M. catarrhalis isolates with 10 G residues in their uspA1 poly-G tracts expressed 2- to 3-fold more uspA1 mRNA than did isolates that had 9 G residues in their poly-G tracts. Analysis of this region upstream of the uspA1 gene in the 5 strains in Table 1 showed that the poly-G tracts were unchanged in the first and last isolates of 3 persistent strains. The poly-G tracts in 2 strains (74P50B1/74P58B1 and 142P77B1/142P87B) changed from 10 Gs in the initial isolate to 9 Gs in the last isolate. Based on these observations, reduced expression of both Hag/MID and UspA1 during persistence may both contribute to the observations in Figures 3 and 4 that show reduced adherence to respiratory epithelial cells and reduced autoagglutination during persistence in human airways following acquisition of strain 142P77B1.
In contrast to the airway microbiome of healthy people, the airway microbiome in adults with COPD includes bacterial pathogens for months at a time during clinically stable periods [34, 50]. Persistence of pathogens in the airways has substantial clinical and pathogenetic impact through induction of inflammatory host responses, increased symptoms, and acceleration of the progressive decline in lung function in COPD. The present study reports the novel observation that the autotransporter Hag/MID, which mediates key virulence phenotypes, undergoes regulation of expression during human infection, facilitating persistence of M. catarrhalis in COPD airways. Understanding how pathogens persist in the airways is critical to developing new therapeutic approaches. Such approaches may target key molecules that mediate colonization and persistence, leading to the development of interventions that will enable pathogen-specific eradication, sparing the normal microbiome.
Notes
Acknowledgment. The authors thank Charmaine Kirkham and Antoinette Johnson for expert technical assistance.
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases (grant number R01 AI19641 to T. F. M., M. M. P., and H. T.) and National Institute of Deafness and Other Communication Disorders (grant number R01 DC012200 to T. F. M.); and by the National Center for Advancing Translational Sciences (grant number UL1 TR001412 to the University at Buffalo).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379:1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Chronic respiratory diseases. Burden of COPD http://www.who.int/respiratory/copd/burden/en/. Accessed 10 November 2018.
- 3. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–65. [DOI] [PubMed] [Google Scholar]
- 4. Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11:303–9. [DOI] [PubMed] [Google Scholar]
- 5. Ahearn CP, Gallo MC, Murphy TF. Insights on persistent airway infection by nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease. Pathog Dis 2017; 75:ftx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 2005; 172:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis 2009; 49:124–31. [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson TMA, Aris E, Bourne S, et al. ; AERIS Study Group A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017; 72:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy TF, Brauer AL, Aebi C, Sethi S. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect Immun 2005; 73:3471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy TF, Brauer AL, Aebi C, Sethi S. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect Immun 2005; 73:8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davie JJ, Earl J, de Vries SP, et al. Comparative analysis and supragenome modeling of twelve Moraxella catarrhalis clinical isolates. BMC Genomics 2011; 12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su YC, Hallström BM, Bernhard S, Singh B, Riesbeck K. Impact of sequence diversity in the Moraxella catarrhalis UspA2/UspA2H head domain on vitronectin binding and antigenic variation. Microbes Infect 2013; 15:375–87. [DOI] [PubMed] [Google Scholar]
- 13. Blakeway LV, Tan A, Peak IRA, Seib KL. Virulence determinants of Moraxella catarrhalis: distribution and considerations for vaccine development. Microbiology 2017; 163:1371–84. [DOI] [PubMed] [Google Scholar]
- 14. Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347:465–71. [DOI] [PubMed] [Google Scholar]
- 15. Du Y, Zhou H, Wang F, et al. Multilocus sequence typing-based analysis of Moraxella catarrhalis population structure reveals clonal spreading of drug-resistant strains isolated from childhood pneumonia. Infect Genet Evol 2017; 56:117–24. [DOI] [PubMed] [Google Scholar]
- 16. Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 2017; 27:722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angiuoli SV, Matalka M, Gussman A, et al. CloVR: a virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinformatics 2011; 12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bullard B, Lipski S, Lafontaine ER. Regions important for the adhesin activity of Moraxella catarrhalis Hag. BMC Microbiol 2007; 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clementi CF, Håkansson AP, Murphy TF. Internalization and trafficking of nontypeable Haemophilus influenzae in human respiratory epithelial cells and roles of IgA1 proteases for optimal invasion and persistence. Infect Immun 2014; 82:433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy TF, Kirkham C, Gallo MC, Yang Y, Wilding GE, Pettigrew MM. Immunoglobulin A protease variants facilitate intracellular survival in epithelial cells by nontypeable Haemophilus influenzae that persist in the human respiratory tract in chronic obstructive pulmonary disease. J Infect Dis 2017; 216:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Möllenkvist A, Nordström T, Halldén C, Christensen JJ, Forsgren A, Riesbeck K. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J Bacteriol 2003; 185:2285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balder R, Krunkosky TM, Nguyen CQ, Feezel L, Lafontaine ER. Hag mediates adherence of Moraxella catarrhalis to ciliated human airway cells. Infect Immun 2009; 77:4597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearson MM, Lafontaine ER, Wagner NJ, St Geme JW 3rd, Hansen EJ. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect Immun 2002; 70:4523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabón W, Horswill AR. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog 2016; 12:e1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farnsworth CW, Schott EM, Jensen SE, et al. Adaptive upregulation of clumping factor A (ClfA) by Staphylococcus aureus in the obese, type 2 diabetic host mediates increased virulence. Infect Immun 2017; 85:e01005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Travier L, Guadagnini S, Gouin E, et al. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog 2013; 9:e1003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics 2005; 23:345–63. [DOI] [PubMed] [Google Scholar]
- 28. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–65. [DOI] [PubMed] [Google Scholar]
- 29. Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parameswaran GI, Wrona CT, Murphy TF, Sethi S. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis 2009; 9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005; 128:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170:400–7. [DOI] [PubMed] [Google Scholar]
- 33. Forsgren A, Brant M, Möllenkvist A, et al. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J Immunol 2001; 167:2112–20. [DOI] [PubMed] [Google Scholar]
- 34. Forsgren A, Brant M, Karamehmedovic M, Riesbeck K. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect Immun 2003; 71:3302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bullard B, Lipski SL, Lafontaine ER. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect Immun 2005; 73:5127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hallström T, Müller SA, Mörgelin M, Möllenkvist A, Forsgren A, Riesbeck K. The Moraxella IgD-binding protein MID/Hag is an oligomeric autotransporter. Microbes Infect 2008; 10:374–81. [DOI] [PubMed] [Google Scholar]
- 37. Riesbeck K, Nordstrom T. Structure and immunological action of the human pathogen Moraxella catarrhalis IgD-binding protein. Crit Rev Immunol 2006; 26:353–76. [DOI] [PubMed] [Google Scholar]
- 38. Holm MM, Vanlerberg SL, Sledjeski DD, Lafontaine ER. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect Immun 2003; 71:4977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forsgren A, Brant M, Riesbeck K. Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J Infect Dis 2004; 190:352–5. [DOI] [PubMed] [Google Scholar]
- 40. LaFontaine ER, Snipes LE, Bullard B, Brauer AL, Sethi S, Murphy TF. Identification of domains of the Hag/MID surface protein recognized by systemic and mucosal antibodies in adults with chronic obstructive pulmonary disease following clearance of Moraxella catarrhalis. Clin Vaccine Immunol 2009; 16:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Attia AS, Hansen EJ. A conserved tetranucleotide repeat is necessary for wild-type expression of the Moraxella catarrhalis UspA2 protein. J Bacteriol 2006; 188:7840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seib KL, Peak IR, Jennings MP. Phase variable restriction-modification systems in Moraxella catarrhalis. FEMS Immunol Med Microbiol 2002; 32:159–65. [DOI] [PubMed] [Google Scholar]
- 43. Blakeway LV, Power PM, Jen FE, et al. ModM DNA methyltransferase methylome analysis reveals a potential role for Moraxella catarrhalis phasevarions in otitis media. FASEB J 2014; 28:5197–207. [DOI] [PubMed] [Google Scholar]
- 44. LaFontaine ER, Wagner NJ, Hansen EJ. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J Bacteriol 2001; 183:1540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peak IR, Jennings MP, Hood DW, Bisercic M, Moxon ER. Tetrameric repeat units associated with virulence factor phase variation in Haemophilus also occur in Neisseria spp. and Moraxella catarrhalis. FEMS Microbiol Lett 1996; 137:109–14. [DOI] [PubMed] [Google Scholar]
- 46. Pettigrew MM, Ahearn CP, Gent JF, et al. Haemophilus influenzae genome evolution during persistence in the human airways in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A 2018; 115:E3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cholon DM, Cutter D, Richardson SK, et al. Serial isolates of persistent Haemophilus influenzae in patients with chronic obstructive pulmonary disease express diminishing quantities of the HMW1 and HMW2 adhesins. Infect Immun 2008; 76:4463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. LaFontaine ER, Cope LD, Aebi C, Latimer JL, McCracken GH Jr, Hansen EJ. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol 2000; 182:1364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol 2003; 48:117–29. [DOI] [PubMed] [Google Scholar]
- 50. Mammen MJ, Sethi S. COPD and the microbiome. Respirology 2016; 21:590–9. [DOI] [PubMed] [Google Scholar]