Abstract
Bumped kinase inhibitors (BKIs) have been shown to be potent inhibitors of Toxoplasma gondii calcium-dependent protein kinase 1. Pyrazolopyrimidine and 5-aminopyrazole-4-carboxamide scaffold-based BKIs are effective in acute and chronic experimental models of toxoplasmosis. Through further exploration of these 2 scaffolds and a new pyrrolopyrimidine scaffold, additional compounds have been identified that are extremely effective against acute experimental toxoplasmosis. The in vivo efficacy of these BKIs demonstrates that the cyclopropyloxynaphthyl, cyclopropyloxyquinoline, and 2-ethoxyquinolin-6-yl substituents are associated with efficacy across scaffolds. In addition, a broad range of plasma concentrations after oral dosing resulted from small structural changes to the BKIs. These select BKIs include anti-Toxoplasma compounds that are effective against acute experimental toxoplasmosis and are not toxic in human cell assays, nor to mice when administered for therapy. The BKIs described here are promising late leads for improving anti-Toxoplasma therapy.
Keywords: Toxoplasma gondii, toxoplasmosis treatment, bumped kinase inhibitors, calcium-dependent protein kinase 1
This manuscript describes new bumped kinase inhibitors with pyrazolopyrimidine, 5-aminopyrazole-4-carboxamide, and pyrrolopyrimidine scaffolds that are highly effective against toxoplasmosis in systemic and brain infection models, and possess diverse pharmacokinetic properties that allow for once-daily oral administration.
Toxoplasma gondii is an apicomplexan parasite that is estimated to be living in billions of people. Severe infection in the brain and eyes or systemic infection develops when T. gondii reactivates during immunosuppression. Additionally, Toxoplasma ocular disease occurs in normal hosts and primary Toxoplasma infection in pregnant women can cause fetal death or brain damage.
Current medicines for toxoplasmosis are limited by adverse events and do not eradicate infection from the host, due to their inactivity against bradyzoite cysts. Pyrimethamine-sulfadiazine requires an extended treatment time and has been associated with allergic, hematologic, and nephrotoxic side effects, and teratogenicity [1, 2]. Spiramycin can be used during pregnancy to decrease vertical transmission, but does not cross placental barriers to treat infections already established in the fetus [3]. Other therapies, such as clindamycin and atovaquone, are less efficacious. Moreover, none of these treatments are effective at eradicating tissue cysts in the brain that can reactivate and cause encephalitis in immunocompromised persons. More effective therapies that have fewer side effects are needed for treatment of toxoplasmosis.
The bumped kinase inhibitor (BKI) class of compounds has broad activity against apicomplexan pathogens including Toxoplasma, Cryptosporidium, Neospora, Sarcocystis, Babesia, and Plasmodium [4]. BKIs inhibit the apicomplexan calcium-dependent protein kinase 1 (CDPK1) selectively due to the small gatekeeper residue in the CDPK1 ATP binding site that allows the BKI access, while larger residues in mammalian kinases block BKIs from binding [5]. In T. gondii, CDPK1 regulates the calcium-dependent pathway of microneme secretion and is required for gliding motility, cell invasion, and egress [6]. T. gondii CDPK1 (TgCDPK1) inhibitor scaffolds include imidazo[1,2-b]pyridazines [7], biphenylimidazoazines [8], benzoylbenzimidazoles [9], pyrazolopyrimidines (PP) [5, 10, 11], pyrrolopyrimidine (PrP) [12], and 5-aminopyrazole-4-carboxamides (AC) [13, 14] (Figure 1). Several AC and PP compounds have been previously identified that reduce acute systemic T. gondii burden more than a million-fold when given orally at 20 mg/kg, and BKI 1553 also reduced latent T. gondii brain tissue bradyzoite cyst burden by 89% when given orally at 30 mg/kg in mouse models [11, 13] (Figure 2). These compounds share similar R1 groups that consist of a cyclopropyloxynaphthyl or cyclopropyloxyquinoline moiety. The potency of these BKIs is related to hydrophobic interactions between the distal 2-cyclopropyloxy group and the N-terminal lobe of the TgCDPK1 hydrophobic pocket [11]. In addition, previous BKIs, 1294 and 1597, which possess a 2-ethoxyquinolin-6-yl R1 group, were found to be potent TgCDPK1 inhibitors [10, 13]. Prior R2 substituent optimization for selectivity and pharmacokinetic properties resulted in the selection of a t-butyl group for AC compounds and a 2-methylpropan-2-ol for PP compounds. While initial compounds such as BKI 1294 possess human Ether-à-go-go Related Gene (hERG) cardiac liabilities, further synthesis and in vitro tests identified new AC and PrP compounds that do not [15]. The in vivo pharmacokinetic properties and efficacy of these new compounds are examined here to explore their potential for the treatment of toxoplasmosis.
Figure 1.
Bumped kinase inhibitor central scaffolds.
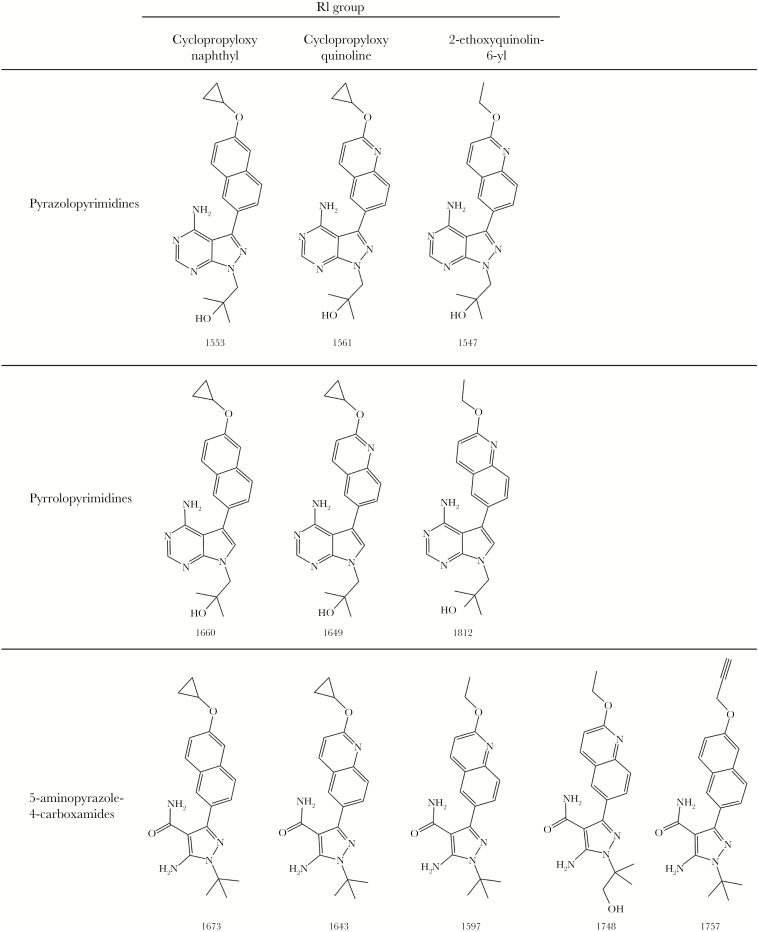
Figure 2.
Chemical structures of pyrazolopyrimidines 1553, 1561, and 1547, pyrrolopyrimidines 1660, 1649, and 1812, and 5-aminopyrazole-4-carboxamides 1673, 1643, 1597, 1748, and 1757.
METHODS
BKI Synthesis
Synthesis of BKIs 1517 [11, 13], 1553 [11], 1561 [11], 1547 [11], 1660 [12], 1649 [16], 1812 [12], 1673 [17], 1643 [13], and 1597 [13] have been previously described. Synthesis of BKIs 1748 and 1757 followed previously reported procedures [12, 16]. Characterization of BKIs reported for the first time are included in the Supplementary Materials (Supplementary Figure 1).
In Vitro Assays
Methods for in vitro T. gondii inhibition [18], TgCDPK1 assays [5], Src kinase assays [5], cytotoxicity assays in CRL-8155 and HepG2 human cells [19], hERG IC50 assays [20], and plasma protein binding assays [19] were all previously described. Details of and variations from the published procedures can be found in the Supplementary Materials and Methods.
Pharmacokinetic Analysis in Mice
Procedures for mouse oral pharmacokinetic (PK) studies (performed under University of Washington IACUC protocol number 2145-01) were previously described [19]. Three female BALB/c mice (10 to 12 weeks old) were used in each group. Each group received a test compound at a single dose of 2, 10, or 25 mg/kg body weight in 3% ethanol/7% Tween 80/90% normal saline by oral gavage. Blood samples were taken at designated time points by tail bleeding, centrifuged to obtain plasma, and stored at −20°C. The test compounds were extracted from the plasma samples using acetonitrile/0.1% formic acid containing an internal standard. A standard mix of all test compounds in control plasma was prepared for comparison and quantification. The compounds were quantified by liquid chromatography-mass spectrometry/mass spectrometry analysis with a Acquity ultra performance liquid chromatography system in tandem with a Xevo TQ-S mass spectrometer (Waters, Milford, MA). PK calculations of maximum concentration (Cmax), time at maximum concentration (Tmax), area under the curve (AUC), and oral clearance were performed using Phoenix WinNonlin software (Certara, USA Inc., Princeton, NJ). Nonparametric superposition with Phoenix WinNonlin software was used to simulate plasma total and fractional unbound (fu) plasma concentrations for multiple dose regimens using the results from single-dose PK for each compound. Analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA).
Efficacy Against Systemic Toxoplasmosis in Mice
Infection and drug administration were performed as previously described [10]. Mice were infected with type I RH strain T. gondii (RH strain) expressing a yellow fluorescent protein. T. gondii were harvested from human foreskin fibroblasts, passed through a 3-μm filter, and 105 tachyzoites were inoculated in 100 μL of phosphate-buffered saline (PBS) intraperitoneally into 4- to 5-week-old, 25-g female CF-1 mice. The compounds were dissolved in polyethylene glycol (PEG) 400 and administered once daily for 5 days by oral gavage 48 hours after inoculation. The control group received vehicle only. Groups consisted of 4 mice. After mice were euthanized on the eighth day, peritoneal lavage was performed with 3 mL of PBS (pH 7.4) and brains were collected for quantitative real-time polymerase chain reaction (PCR) using methods that we previously published [21]. In brief, brain and spleen were collected from infected and noninfected mice and homogenized in PBS using a hand-held homogenizer. DNA was isolated with a DNA purification kit (Qiagen, Germantown, MD). Three hundred nanograms of total DNA from the brain homogenate and 300 ng of total DNA from the spleen homogenate were analyzed per mouse. A standard curve was generated from DNA purified from T. gondii tachyzoites in 10-fold dilutions from 160 ng to 1.6 fg of DNA. Quantitative real-time PCR was performed in duplicate using a 7300 real-time PCR system (Applied Biosystems, Grand Island, NY) with iTaq SYBR GREEN PCR Supermix (Biorad) and primers for the T. gondii 529-bp repeat element (sense 5′-AGG AGA GAT ATC AGG ACT GTA G-3′ and antisense 5′-GCG TCG TCT CGT CTA GAT CG-3′). Results were quantified as T. gondii DNA per total DNA. Analysis of differences of the tissue burden of T. gondii infection were performed using an unpaired t test. GraphPad Prism 7.0 software was used for statistical analysis.
Efficacy Against T. gondii Brain Infection in Mice
Mice were infected with type II Prugniaud strain T. gondii. T. gondii were harvested from human foreskin fibroblasts, passed through a 3-μm pore filter, and 500 tachyzoites were inoculated, in a volume of 100 μL of PBS, intraperitoneally into 4- to 5-week-old female CBA/J mice. The compounds were dissolved in PEG 400 and administered for 5 days once daily by oral gavage beginning 9 days after infection. The control group received vehicle only. Groups consisted of 5 mice with the exception of the BKI 1812 group, which was 4 mice. Mice were euthanized the day after treatment was complete, and brain and spleens were collected for analysis using quantitative PCR as described above. Statistical analysis was performed with GraphPad Prism software.
Animal Ethics Statement
All animal experiments conducted at the University of Washington and the Portland Veterans Administration Medical Center were approved by the Institutional Animal Care and Use Committees. All animals used in these studies were handled in strict accordance with practices made to minimize suffering.
RESULTS
In Vitro Toxoplasma gondii Assays
All BKIs tested were potent inhibitors of TgCDPK1 at a range of 1 to 11 nM and inhibited T. gondii proliferation in vitro at 50% effective concentrations (EC50s) ranging from 45 nM to 271 nM (Table 1). Src inhibition was used as a counter screen for specificity because it has one of the smallest gatekeeper residues for mammalian protein kinases, that is threonine, and hence would be a likely off-target mammalian protein kinase for BKIs [5]. Tested compounds did not inhibit the human Src protein kinase at concentrations up to 10 µM. Compounds were generally not toxic to human cell lines up to 40 µM, and unlike the previously described BKI 1294 [15], did not inhibit hERG up to 20 µM (Table 1). Plasma protein binding in mouse plasma was variable among these compounds, ranging from 99% for BKI 1660, to 80% for BKIs 1597 and 1748.
Table 1.
In Vitro and In Vivo Properties of Bumped Kinase Inhibitors
| T. gondii EC50 | T. gondii EC90 | TgCDPK1 IC50 | Src Kinase IC50 | CRL8155 CC50 | HepG2 CC50 | hERG IC50 | Protein Binding (%) | PK Oral Dose Concentration | Cmax | AUC | Tmax | Oral Clearance | Brain/Plasma Concentration Ratio at 60 min Postdose | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central Scaffold | BKI | (nM) | (nM) | (nM) | (µM) | (µM) | (µM) | (µM) | Mouse | Human | (mg/kg) | (µM) | (µM*min) | (min) | (mL/min) | |
| PP | 1553a | 60 | 2076 | 1 | >10 | 39.5 | >40 | >30 | 90 | 91 | 10 | 12.8 | 13700 | 320 | 0.2 | 0.33 |
| PP | 1561a | 162 | 1925 | 2 | >10 | >40 | >40 | >30 | 88 | ND | 10 | 7.8 | 16600 | 560 | 0.3 | 1.65 |
| PP | 1547a | 173 | 1014 | 3 | >10 | >80 | >80 | >30 | ND | 99.9 | 10 | 5.2 | 850 | 50 | 0.2 | ND |
| PrP | 1660 | 271 | 4472 | 9 | >10 | >40 | >40 | 30 | 99 | 98 | 10 | 24.9 | 27867 | 480 | 0.01 | 0.17 |
| PrP | 1649 | 84 | 1168 | 3 | >10 | >40 | >40 | >30 | 94 | 56 | 2 | 4.9 (24.5) c | 6416 (32080)c | 400 | 0.01 | 0.02 |
| PrP | 1812 | 114 | 456 | 11 | >10 | 80 | >80 | >44 | 85 | 99.9 | 25 | 28 (11.2)c | 8495 (3398)c | 50 | 0.2 | ND |
| AC | 1673 | 116 | 1294 | 3 | >10 | >40 | >40 | 27 | 95 | 82 | 10 | 12.9 | 9098 | 180 | 0.5 | 0.04 |
| AC | 1643b | 45 | 849 | 5 | >10 | >40 | >40 | >30 | 88 | ND | 10 | 3.2 | 2679 | 160 | 0.2 | 0.43 |
| AC | 1597 | 201 | 1050 | 6 | >10 | >40 | >40 | >30 | 80 | 89 | 10 | 5.33 | 1694 | 80 | 0.4 | 0.34 |
| AC | 1748 | 76 | 596 | 7 | >10 | >40 | >40 | >21.5 | 80 | 84 | 25 | 38.6 (15.4)c | 8530 (3412)c | 70 | 0.4 | ND |
| AC | 1757 | 68 | 975 | 2 | >10 | >80 | >80 | >22.6 | 89 | 89 | 25 | 16.9 (6.8)c | 11170 (4468)c | 160 | 0.1 | ND |
Abbreviations: AC, 5-aminopyrazole-4-carboxamides; AUC, area under the curve; BKI, bumped kinase inhibitor; CC50, 50% cytotoxicity concentration; Cmax, maximum concentration; EC50, 50% effective concentration; EC90, 90% effective concentration; IC50, 50% inhibitory concentration; ND, not determined; PK, pharmacokinetic; PP, pyrazolopyrimidines; PrP, pyrrolopyrimidines; TgCDPK1, Toxoplasma gondii calcium-dependent protein kinase 1; Tmax, time at maximum concentration.
aBKIs and some associated data previously reported [11] as compounds 31, 32, and 33.
bBKI and some associated data previously reported [13] as compound 35.
cValues in parenthesis are dose normalized to 10 mg/kg for direct comparison.
Pharmacokinetic Properties
The Cmax, AUC, and oral clearances varied widely for the tested BKIs (Table 1). At a dose of 10 mg/kg, BKI 1660 achieved the highest Cmax and AUC, and the lowest oral clearance at 0.01 mL/min. BKI 1649 also had a low oral clearance, equivalent to 1660’s value of 0.01 mL/min, and had a similarly high Cmax and AUC if dose normalized to 10 mg/kg. The remaining BKIs tested showed an oral clearance over 10-fold higher than this, with 1673 having the highest oral clearance at 0.5 mL/min. For Cmax and AUC, the only statistically significant results showed the cyclopropyloxynaphthyl and cyclopropyloxyquinoline R1 substituents having a significantly higher Cmax (P < .05) than the 2-ethoxyquinolin-6-yl substituent. No other significant determinations concerning structure activity relationship for Cmax or AUC from this limited data set could be made when looking at either the R1 substituent or the central scaffold alone, as these properties seemed to change with the different combinations of R1 and scaffold. The Cmax and AUC of cyclopropyloxynapthyl bearing BKI 1660 and cyclopropylquinoline bearing 1649 (PrP scaffold) were approximately twice those of BKIs 1553 and 1561 (PP scaffold). Similarly, for the 2-ethoxyquinolin-6-yl bearing compounds, and the Cmax and AUC of BKI 1812 (PrP scaffold) are over 2 times and 7 times higher, respectively, than 1547 (PP scaffold). This suggests that the PrP scaffold may allow for greater oral absorption and/or lower intrinsic clearance than the PP scaffold when comparing compounds with identical substituent groups. Also, the cyclopropyloxynaphthyl and cyclopropyloxyquinoline substituents showed a >10-fold decrease in oral clearance, to 0.01 mL/min, when associated with the PrP scaffold over the PP or AC scaffolds. No matter the R1 substituent or central scaffold, all other oral clearance rates ranged from 0.1 to 0.5 mL/min. The wide range of plasma binding and pharmacokinetic properties led to wide ranges in the simulated BKI total and fractional unbound (fu) concentrations during in vivo administration for efficacy (Table 2).
Table 2.
Modeled Pharmacokinetic Data and Efficacy Against Acute Toxoplasmosis in Mice
| Administered Oral Dose | Simulated AUC | Simulated Cavg | Simulated fu Cavg | Simulated Cmax | Simulated fu Cmax | Simulated Fraction of Time Above EC50 | Simulated Fraction of Time Above EC90 | Reduction Over Controls for Peritoneal Infection for Type I RH Strain | Reduction Over Controls for Brain Infection for Type I RH Strain | Reduction Over Controls for Spleen Infection for Type I RH Strain | Reduction Over Controls for Brain Infection for Type II Prugniaud strain | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central Scaffold | BKI | (starting on Day 2 postinfection) | (µM*h) | (µM) | (µM) | (µM) | (µM) | Total | fu | Total | fu | (%) | (%) | (%) | (%) |
| PP | 1553a | 2 mg/kg QD × 5 days | 408 | 1.2 | 0.1 | 4.3 | 0.4 | 0.81 | 0.57 | 0.38 | 0 | 71 | 34 | 46 | ND |
| 10 mg/kg × 1 dose | 408 | 1.2 | 0.1 | 12.3 | 1.2 | 0.80 | 0.37 | 0.29 | 0 | 65 | 57 | 41 | ND | ||
| 20 mg/kg loading dose on day 2 + 5 mg/kg QD on days 3–6 | 1630 | 4.9 | 0.5 | 24.6 | 2.5 | 0.74 | 0.55 | 0.44 | 0.03 | 99.9 | 79 | 97 | ND | ||
| 20 mg/kg QD × 5 days | 4076 | 12.1 | 1.2 | 43.0 | 4.3 | 0.85 | 0.66 | 0.55 | 0.30 | 100c | 95 | 99 | ND | ||
| PP | 1561a | 2 mg/kg QD × 5 days | 319 | 1.5 | 0.2 | 3.8 | 0.5 | 0.68 | 0.51 | 0.40 | 0 | 80 | 95 | 97 | ND |
| 10 mg/kg QD × 1 dose | 319 | 1.5 | 0.2 | 15.2 | 1.8 | 0.78 | 0.45 | 0.40 | 0 | 97 | 99 | 99 | ND | ||
| 20 mg/kg loading dose on day 2 + 5 mg/kg QD on days 3–6 | 1276 | 5.8 | 0.7 | 30.4 | 4.9 | 0.84 | 0.70 | 0.65 | 0.16 | 100c | 99 | 98 | ND | ||
| PrP | 1660 | 2 mg/kg QD × 5 days | 726 | 3.0 | 0.03 | 7.6 | 0.1 | 0.72 | 0 | 0.34 | 0 | 48 | 69 | ND | ND |
| 10 mg/kg × 3 doses QOD on days 2, 4, and 6 | 2179 | 8.9 | 0.1 | 28.0 | 0.3 | 0.63 | 0.02 | 0.43 | 0 | 97 | 76 | ND | ND | ||
| 10 mg/kg QD × 5 days | 3630 | 14.9 | 0.2 | 37.6 | 0.4 | 0.87 | 0.23 | 0.62 | 0 | 96 | 85 | ND | 88 | ||
| 30 mg/kg QD × 1 day | 2179 | 13.0 | 0.1 | 74.7 | 0.8 | 0.78 | 0.18 | 0.41 | 0 | 66 | 41 | ND | ND | ||
| PrP | 1649 | 2 mg/kg QD × 5 days | 514 | 2.3 | 0.1 | 5.8 | 0.3 | 0.82 | 0.58 | 0.60 | 0 | 99.8 | 85 | ND | ND |
| 6 mg/kg QD × 5 days | 1541 | 7.0 | 0.4 | 17.5 | 1.0 | 0.97 | 0.70 | 0.72 | 0 | 100c | 97 | ND | ND | ||
| 10 mg/kg QD × 5 days | 2771 | 12.6 | 0.8 | 30.1 | 1.8 | 0.95 | 0.73 | 0.74 | 0.33 | ND | ND | ND | 99 | ||
| PrP | 1812 | 20 mg/kg QD × 5 days | 452 | 3.6 | 0.5 | 22.2 | 3.3 | 0.61 | 0.41 | 0.47 | 0.28 | ND | ND | ND | 97 |
| AC | 1673 | 2 mg/kg QD × 5 days | 166 | 1.0 | 0.05 | 2.7 | 0.2 | 0.80 | 0.11 | 0.29 | 0 | 81 | 72 | ND | ND |
| 5 mg/kg QD × 5 days | 414 | 2.4 | 0.1 | 7.2 | 0.4 | 0.77 | 0.46 | 0.60 | 0 | 97 | 86 | ND | ND | ||
| 10 mg/kg QD × 5 days | 829 | 4.8 | 0.2 | 14.3 | 0.8 | 0.80 | 0.61 | 0.65 | 0 | 99.7 | 92 | ND | ND | ||
| 20 mg/kg QD × 5 days | 1658 | 9.6 | 0.5 | 28.7 | 1.5 | 0.84 | 0.67 | 0.69 | 0.01 | 100c | 97 | ND | 97 | ||
| AC | 1643b | 20 mg/kg QD × 5 days | 219 | 1.1 | 0.1 | 3.7 | 0.5 | 0.79 | 0.64 | 0.63 | 0.0 | 100c | 89 | 100c | ND |
| 50 mg/kg QD × 5 days | 546 | 2.7 | 0.3 | 9.3 | 1.1 | 0.86 | 0.71 | 0.62 | 0.07 | 100c | 88 | 100c | ND | ||
| AC | 1597 | 4 mg/kg QD × 5 days | 51 | 0.4 | 0.1 | 1.9 | 0.4 | 0.49 | 0.15 | 0.15 | 0.00 | 87 | 91 | ND | ND |
| 20 mg/kg QD × 5 days | 282 | 2.2 | 0.3 | 10.5 | 2.1 | 0.84 | 0.49 | 0.48 | 0.15 | 99.9 | 98 | ND | 99 | ||
| 60 mg/kg QD × 5 days | 764 | 6.0 | 0.7 | 28.5 | 4.6 | 0.89 | 0.76 | 0.75 | 0.29 | 100c | 97 | ND | ND | ||
| AC | 1748 | 4 mg/kg QD × 5 days | 540 | 3.9 | 0.8 | 17.0 | 3.5 | 0.90 | 0.86 | 0.84 | 0.51 | 95 | 79 | 98 | ND |
| 20 mg/kg QD × 5 days | 2700 | 19.6 | 3.9 | 86.7 | 17.4 | 0.92 | 0.87 | 0.87 | 0.80 | 100c | 86 | 100c | 99 | ||
| AC | 1757 | 4 mg/kg QD × 5 days | 382 | 2.3 | 0.3 | 6.8 | 0.7 | 0.76 | 0.73 | 0.67 | 0 | 72 | 34 | 65 | ND |
| 20 mg/kg QD × 5 days | 1908 | 11.4 | 1.4 | 33.6 | 3.7 | 0.86 | 0.77 | 0.76 | 0.55 | 98 | 79 | 99 | 99 | ||
Abbreviations: AC, 5-aminopyrazole-4-carboxamides; AUC, area under the curve; BKI, bumped kinase inhibitor; Cavg, average concentration over dosing time; Cmax, maximum concentration; EC50, 50% effective concentration; EC90, 90% effective concentration; fu, fraction unbound; ND, not determined; PP, pyrazolopyrimidines; PrP, pyrrolopyrimidines; QD, once daily; QOD, once every other day.
aBKIs and some associated data previously reported [11] as compounds 32, and 33.
bBKI and some associated data previously reported [13] as compound 35.
cValues below the assay’s lowest limit of detection are reported as 100% reduction.
Efficacy Against Acute Toxoplasmosis in Mice
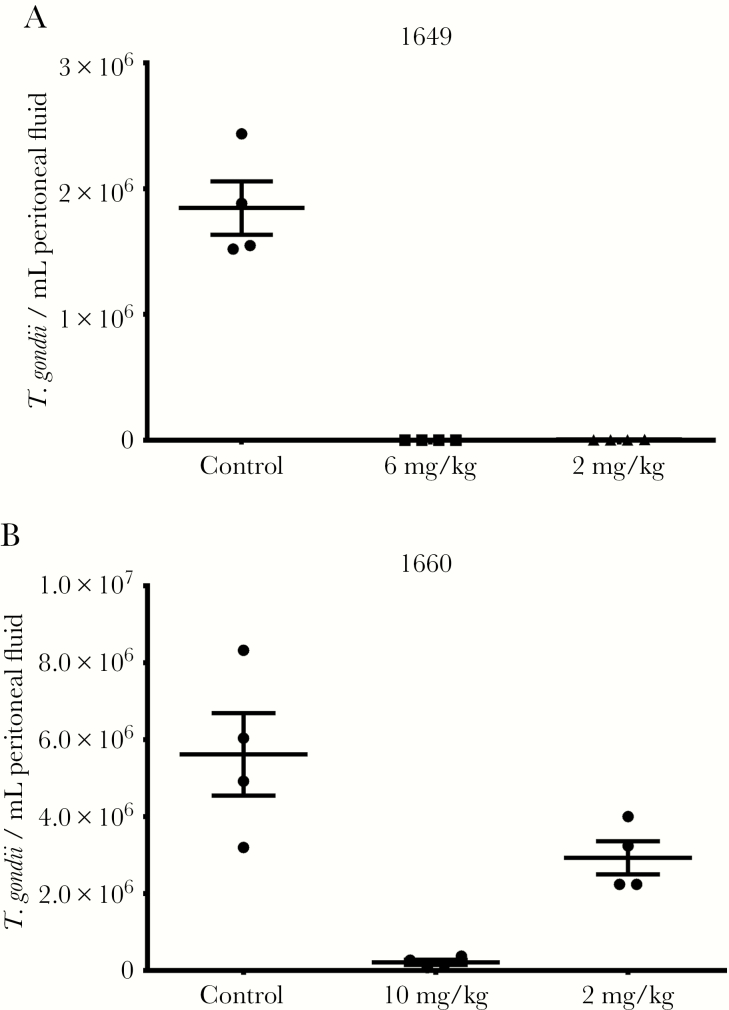
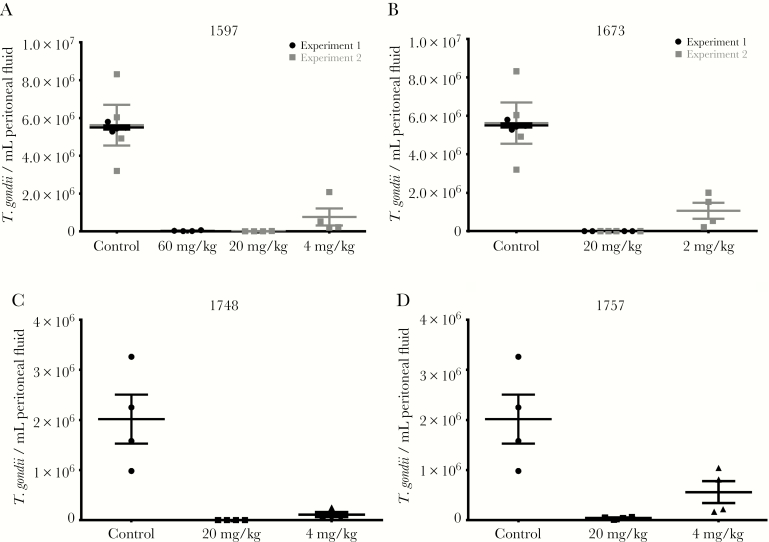
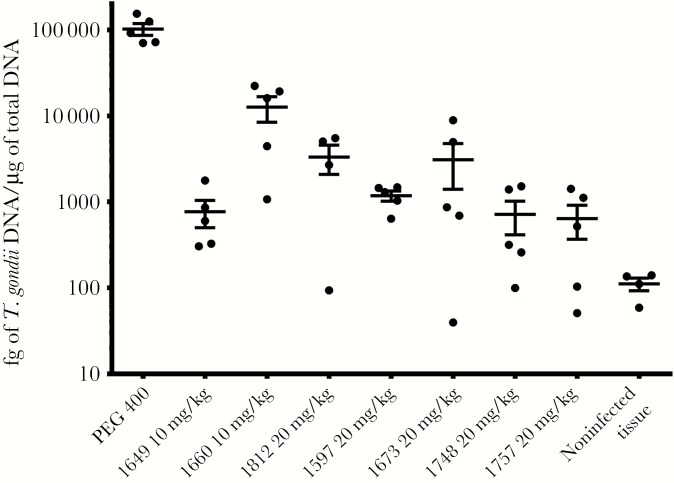
AC, PP, and PrP compounds with favorable pharmacokinetic properties and in vitro EC50s were tested in toxoplasmosis mouse models of systemic and brain infection (Table 2). In these models, CF-1 mice were infected with a high inoculum of RH strain that is fatal to mice in 8–10 days, or CBA/J mice were infected with a low inoculum of Prugniaud strain, which is not rapidly fatal and allows for better infection of brain tissue. Compounds and vehicle control were administered orally for 5 days, starting on day 2 postinfection for RH strain or day 9 postinfection for Prugniaud strain. BKIs 1597, 1649, 1660, 1673, 1748, and 1757, along with previously reported BKIs 1553, 1561 [11], and 1643 [13], all reduced the number of RH strain T. gondii in the peritoneal fluid by more than 95% at doses of 20 mg/kg or less (Figures 3 and 4). BKIs 1643, 1673, and 1748 at 20 mg/kg and 1649 at 6 mg/kg all reduced the burden of infection in the peritoneal fluid to below limits of detection (Table 2). BKIs 1597, 1649, 1660, 1673, 1748, 1757, and 1812 were dosed in Prugniaud strain infected mice at or above levels that showed strong reductions against the RH strain (Table 2). All significantly reduced the brain infections (P < .0001), with PrP BKIs reducing brain infections by 88%–99% and the AC BKIs by 97%–99% over controls (Table 2 and Figure 5). This outstanding efficacy demonstrates that the cyclopropyloxynaphthyl and cyclopropyloxyquinoline R1 groups were associated with efficacy for all 3 central scaffolds. BKIs 1597 and 1812, with a 2-ethoxyquinolin-6-yl R1 substituent, also showed high reductions of infection across 2 of the 3 scaffolds, with BKI 1597 showing reduction in the peritoneum, with the highest dose of 60 mg/kg reducing infection to below the limits of detection. Efficacy was also maintained with changes to AC compounds at the R2 group for BKI 1748 and the R1 group for 1757. BKI 1547 was not tested in either in vivo efficacy model due to its substantially lower systemic exposure (Table 1) and the BKIs 1553 and 1561 were not tested in the Prugniaud strain model because the PP scaffold has been previously shown to reduce brain infections in mice [11].
Figure 3.
Efficacy of pyrrolopyrimidines in Toxoplasma gondii type I RH strain infected CF-1 mice: (A) BKI 1649, (B) BKI 1660. The T. gondii burden of infection was measured 7 days postinfection by counting parasites recovered from peritoneal lavage (n = 4). Mean (central bar) and standard error of the mean (error bars) are shown.
Figure 4.
Efficacy of 5-aminopyrazole-4-carboxamides in Toxoplasma gondii type I RH strain infected CF-1 mice: (A) BKI 1597, (B) BKI 1673, (C) BKI 1748, (D) BKI 1757. The T. gondii burden of infection was measured 7 days postinfection by counting parasites recovered from peritoneal lavage (n = 4). Mean (central bar) and standard error of the mean (error bars) are shown.
Figure 5.
Efficacy of pyrrolopyrimidines and 5-aminopyrazole-4-carboxamides in Toxoplasma gondii type II Prugniaud strain infected CBA/J mice. The T. gondii burden of infection was measured 7 days after the initiation of treatment, or day 16 postinfection, by polymerase chain reaction analysis of brain tissue (n = 5). Mean (central bar) and standard error of the mean (error bars) are shown.
Simulated Plasma Concentrations in Efficacy Experiments
The single-dose PK studies were used to simulate the total and unbound plasma concentrations for the various efficacy experiment dosing regimens (Table 2 and Supplementary Figure 2). Simulated Cmax, fu Cmax, average concentration (Cavg), and fu Cavg levels were calculated for each BKI dose. The simulated AUC and the fraction of time (ft) both total and unbound concentrations remained above the EC50 and EC90 were also calculated (Table 2). Neither a specific central scaffold nor an R1 substituent alone could be directly associated with superior efficacy, with different structural combinations all showing strong efficacy, depending upon the dose concentration and duration used.
DISCUSSION
Though clearly defined pharmacodynamic (PD) properties do not emerge from this data set, several observations can be made in regards to the PK. The simulated unbound concentrations for most of the compounds are not predicted to reach the EC90 at many of the different treatment regimens, but still show significant clearance of peritoneal infection (Table 2). For the cyclopropyloxynaphthyl PP compound 1553, the simulated unbound concentration for the lowest dose of 2 mg/kg once daily for 5 days also did not reach the EC50 but reduced peritoneal infection by 48% (Table 2). It is possible that the assumption that BKIs have linear PK is invalid and simulated plasma concentrations may not accurately predict the actual plasma concentrations for efficacy studies. However, these data suggest that the total plasma concentration of compound is more important in determining efficacy than the unbound concentrations.
For all AC compounds, BKIs 1597, 1643, 1673, 1748, and 1757, efficacy and all simulated PK parameters increased as dose concentration was increased (Table 2). Comparing previous dose fractionation studies with single daily dosing of the AC compound, BKI 1517, suggests that efficacy is primarily related to Cmax. AC BKI 1517, previously reported as compound 1 [11, 13] and mostly identical in structure to BKI 1597, but with 7-ethoxyquinolin-3-yl in place of the 2-ethoxyquinolin-6-yl R1 group, had improved efficacy against the RH strain at 60 mg/kg once daily over a fractionated doses of 20 mg/kg in the morning and 40 mg/kg in the evening over the same period of time (Supplementary Figure 3) [13]. The 60 mg/kg once daily doses had a higher Cmax, but substantially lower ft/EC50 than the 20 mg/kg morning 40 mg/kg evening doses. A lower concentration dose of 5 mg/kg in the morning and 10 mg/kg in the afternoon also displayed a larger ft/EC50 than the 60 mg/kg once daily dose, but showed further reduced efficacy with an even lower Cmax (Supplementary Figure 3) [13]. These data suggest that the AC compounds act predominantly in a concentration-dependent rather than time-dependent manner. Additional dose fractionation studies on the other AC compounds will help to determine if this pharmacodynamic property is scaffold specific [22].
For the PP and PrP compounds, the association between PK and PD is not clear from these studies. For cyclopropyloxynaphthyl PP BKI 1553, dosing at 2 mg/kg once daily for 5 days was compared to a single dose of 10 mg/kg (Table 2). The simulated Cmax for these doses is inversely related to the peritoneal efficacy while the ft/EC90 correlated with efficacy, suggesting the compound may be time dependent. However, the same doses of cyclopropyloxyquinoline PP BKI 1561 showed an increase in peritoneal efficacy as Cmax increased and identical ft/EC90 for the 2 dosing regimens (Table 2), suggesting concentration-dependent action. For cyclopropyloxynaphthyl PrP BKI 1660, a treatment of 10 mg/kg every other day was compared to a single dose of 30 mg/kg. Here, the Cmax and ft/EC50 are both inversely related to peritoneal efficacy (Table 2). Although the minor differences between these molecules do affect their PK properties, it is unlikely that such minor structural changes would drastically alter the pharmacodynamics within a set sharing the same central scaffold. It is also possible that these results reflect other pharmacokinetic properties, such as tissue distribution, that would lead to greater efficacy than expected based on plasma concentrations alone. Results from future studies on several BKIs at multiple concentrations where total drug administered is divided into once or twice daily dosing over the same period of time would provide a clearer indication of PD for the PP and PrP scaffolds.
Despite questions remaining regarding PK/PD properties of these molecules, all BKIs tested here have properties that allow for daily oral dosing and show substantial reductions in systemic and brain infections and a lack of toxicity in cellular and in vivo assays. Previous studies have shown that inhibiting TgCDPK1 effectively decreases the burden of T. gondii brain tissue cysts [11, 13, 23]. Future studies will examine the efficacy of these new BKIs against latent brain tissue cysts. A drug that is well tolerated and that eradicates Toxoplasma brain tissue cysts would represent a major advance over treatments currently available.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Geno De Hostos and Robert Choy (PATH Drug Solutions), for their consultation in the drug development pathway, and Gail M. Freiberg and Dale J. Kempf (AbbVie) for establishing hERG inhibition of the BKIs.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Department of Agriculture National Institute of Food and Agriculture.
Financial support. This work was supported by the US Department of Veterans Affairs Biomedical Laboratory Research and Development Career Development Award (grant number BX002440); National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers R21AI123690, R01AI089441, R01AI111341, R01A1112427, and R01HD080670), and the US Department of Agriculture, National Institute of Food and Agriculture (grant number 2014-06183).
Potential conflicts of interest. W. C. V. is the President of ParaTheraTech Inc., a company that is developing BKIs for animal health. W. C. V. did not perform the experiments, nor interpret the results of the experiments, but did edit this paper and helped plan the experiments. W. C. V., E. F., D. J. M., W. H., and K. K. O. are named on University of Washington patents relevant to the compounds used in this paper. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 14th Biennial Conference of the Toxoplasma gondii Research Community, Tomar, Portugal, 31 May–4 June 2017.
References
- 1. Julliac B, Théophile H, Begorre M, Richez B, Haramburu F. Side effects of spiramycin masquerading as local anesthetic toxicity during labor epidural analgesia. Int J Obstet Anesth 2010; 19:331–2. [DOI] [PubMed] [Google Scholar]
- 2. Meneceur P, Bouldouyre MA, Aubert D, et al. . In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother 2008; 52:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008; 47:554–66. [DOI] [PubMed] [Google Scholar]
- 4. Van Voorhis WC, Doggett JS, Parsons M, et al. . Extended-spectrum antiprotozoal bumped kinase inhibitors: a review. Exp Parasitol 2017; 180:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy RC, Ojo KK, Larson ET, et al. . Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett 2010; 1:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 2010; 465:359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moine E, Dimier-Poisson I, Enguehard-Gueiffier C, et al. . Development of new highly potent imidazo[1,2-b]pyridazines targeting Toxoplasma gondii calcium-dependent protein kinase 1. Eur J Med Chem 2015; 105:80–105. [DOI] [PubMed] [Google Scholar]
- 8. Moine E, Denevault-Sabourin C, Debierre-Grockiego F, et al. . A small-molecule cell-based screen led to the identification of biphenylimidazoazines with highly potent and broad-spectrum anti-apicomplexan activity. Eur J Med Chem 2015; 89:386–400. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Ojo KK, Johnson SM, et al. . Benzoylbenzimidazole-based selective inhibitors targeting Cryptosporidium parvum and Toxoplasma gondii calcium-dependent protein kinase-1. Bioorg Med Chem Lett 2012; 22:5264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother 2014; 58:3547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vidadala RS, Rivas KL, Ojo KK, et al. . Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitor with minimal human ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 2016; 59:6531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vidadala RSR, Golkowski M, Hulverson MA, et al. . 7 H-Pyrrolo[2,3- d]pyrimidin-4-amine-based inhibitors of calcium-dependent protein kinase 1 have distinct inhibitory and oral pharmacokinetic characteristics compared with 1 H-Pyrazolo[3,4- d]pyrimidin-4-amine-based inhibitors. ACS Infect Dis 2018; 4:516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W, Ojo KK, Zhang Z, et al. . SAR studies of 5-aminopyrazole-4-carboxamide analogues as potent and selective inhibitors of Toxoplasma gondii CDPK1. ACS Med Chem Lett 2015; 6:1184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Ojo KK, Vidadala R, et al. . Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 2014; 5:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ojo KK, Eastman RT, Vidadala R, et al. . A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J Infect Dis 2014; 209:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hulverson MA, Vinayak S, Choi R, et al. . Bumped-kinase inhibitors for cryptosporidiosis therapy. J Infect Dis 2017; 215:1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang W, Choi R, Hulverson MA, et al. . 5-Aminopyrazole-4-carboxamide-based compounds prevent the growth of Cryptosporidium parvum. Antimicrob Agents Chemother 2017; 61:pii: e00020-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McFadden DC, Seeber F, Boothroyd JC. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother 1997; 41:1849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tatipaka HB, Gillespie JR, Chatterjee AK, et al. . Substituted 2-phenylimidazopyridines: a new class of drug leads for human African trypanosomiasis. J Med Chem 2014; 57:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danker T, Möller C. Early identification of hERG liability in drug discovery programs by automated patch clamp. Front Pharmacol 2014; 5:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McConnell EV, Bruzual I, Pou S, et al. . Targeted structure-activity analysis of endochin-like quinolones reveals potent qi and qo site inhibitors of Toxoplasma gondii and Plasmodium falciparum cytochrome bc1 and identifies ELQ-400 as a remarkably effective compound against acute experimental toxoplasmosis. ACS Infect Dis 2018; 4:1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielsen EI, Cars O, Friberg LE. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 2011; 55:4619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rutaganira FU, Barks J, Dhason MS, et al. . Inhibition of calcium dependent protein kinase 1 (CDPK1) by pyrazolopyrimidine analogs decreases establishment and reoccurrence of central nervous system disease by Toxoplasma gondii. J Med Chem 2017; 60:9976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.