Abstract
Vascular dementia (VaD) is cognitive decline linked to reduced cerebral blood perfusion, yet there are few therapeutic options to protect cognitive function following cerebrovascular accidents. The purpose of this study was to profile gene expression changes unique to VaD to identify and characterize disease relevant changes that could offer clues for future therapeutic direction. Microarray-based profiling and validation studies of postmortem frontal cortex samples from VaD, Alzheimer disease, and age-matched control subjects revealed that the oxytocin receptor (OXTR) was strongly and differentially upregulated in VaD. Further characterization in fixed tissue from the same cases showed that OXTR upregulation occurs de novo around and within microinfarcts in peri-infarct reactive astrocytes as well as within vascular profiles, likely on microvascular endothelial cells. These results indicate that increased OXTR expression in peri-infarct regions may be a specific response to microvascular insults. Given the established OXTR signaling cascades that elicit antioxidant, anti-inflammatory, and pro-angiogenic responses, the present findings suggest that de novo OXTR expression in the peri-infarct space is a tissue-protective response by astroglial and vascular cells in the wake of ischemic damage that could be exploited as a therapeutic option for the preservation of cognition following cerebrovascular insults.
Keywords: Alzheimer disease, Astrocvytes, Microinfarcts, Microvascular endothelial cells, Oxytocin receptor, Vascular dementia
INTRODUCTION
Alzheimer disease (AD) and vascular dementia (VaD) represent the first and second leading causes of dementia, a syndrome of pathologically diminished cognitive functioning affecting over 50 million people worldwide (1). Recent estimates suggest a financial burden of $604 billion dollars (2), and around 40% of familial primary caregivers suffer from depression or anxiety (3). Advanced age is the greatest predictor of dementia with rates of AD doubling every 4.5 years and VaD every 5.5 years with an initial sporadic incidence at ∼65 years of age (4). As more individuals live to an advanced age, the incidence and prevalence of dementia is expected to rise (5). Therefore, the discovery of specific disease-modifying treatments or preventative strategies for these 2 most common dementias is a top priority for translational research.
AD and VaD can be difficult to differentiate clinically. This is owing in part to overlapping risk factors such as advanced age, hypertension, type 2 diabetes mellitus, and hypercholesterolemia (6). A general rule states that episodic memory deficits herald the onset of AD while executive deficits predominate initially in VaD (7). However, results from longitudinal studies like the Gothenburg mild cognitive impairment (MCI) study suggest this may only be reliable in the early stages of MCI, as all cognitive domains showed impairment once the patients progressed to AD or VaD (8). Behavioral differences are also unreliable as AD and VaD do not differ significantly in affective volatility or the presence of psychiatric symptoms (9). Currently the only definitive diagnosis of AD or VaD can be made postmortem based on pathological hallmarks (10).
The pathological hallmarks of VaD differ greatly from the amyloid plaques and neurofibrillary tangles that characterize AD (11). The cognitive impairment in VaD is typically preceded by clinical stroke or small vessel disease that reduces cerebral blood flow (CBF) without obvious stroke (12, 13). The most common injury leading to VaD is an infarction or restriction of CBF caused by either a local atherogenic thrombus or an embolism (12). This ischemic event initiates a cascade of deleterious effects including: Inflammation, neutrophil invasion and obstruction of vessels through the endothelium lining, superoxide production, nitric oxide synthase uncoupling, and deficits in oxidative phosphorylation from oxygen and glucose depletion (14). Without reperfusion or compensation by collateral branches, the surrounding tissue becomes necrotic resulting in infarcts, white matter lesions, and demyelination (15). While a single ischemic event is sufficient to produce dementia symptoms if it occurs in a particularly sensitive location like the thalamus or hippocampus, VaD may also arise from multiple, cumulative vascular accidents with increasing volume of impacted tissue predicting dementia severity (16, 17). An ischemic core of unsalvageable neural tissue forms in the immediate wake of a significant reduction in CBF. However, the surrounding penumbra of at-risk neurons represents a site where early therapeutic intervention may limit the ultimate cognitive impact of the ischemic event (18, 19). As noted above, several potential pathogenic factors contribute to the loss of brain parenchyma in the event of ischemic injury, and a successful therapy to limit neuronal death would need to exert protective effects against several of these contributors (20).
While it has been half a century since C.M. Fisher concluded that stroke alone could lead to dementia (21), the high rate of mixed pathology dementias from both AD and vascular damage has confounded efforts to combat VaD resulting from stroke or cerebrovascular disease alone (6, 22). To date, several pharmacological interventions—primarily cholinesterase inhibitors—have FDA approval for the treatment of AD, yet no drug has regulatory approval for the treatment of VaD (23, 24). Of the many clinical trials that have repurposed commonly used AD medications for the symptomatic management of VaD, there have been only small trends in cognitive improvement that skew to aiding suspected mixed pathology participants far more than VaD patients (25). The present study was undertaken to address these difficulties in finding potential therapeutic options for VaD (23).
Using microarray technology, we generated gene expression profiles of frontal cortex obtained from individuals who died with AD, VaD, or age-matched, cognitively intact controls. This analysis revealed 3495 genes that were dysregulated in AD, whereas 413 genes were selectively dysregulated in VaD. Pathway analysis and validation studies identified a VaD-specific upregulation of transcripts encoding the oxytocin receptor (Oxtr). Moreover, subsequent human tissue studies revealed focal endothelial and astroglial OXTR protein upregulation surrounding grey and white matter microinfarctions. Given the evidence for a protective role of oxytocin (OXT) signaling in peripheral ischemia (26–29), we posit that OXTR upregulation in VaD may represent a novel, druggable pathway for the therapeutic treatment of dementia resulting from cerebrovascular injury.
MATERIALS AND METHODS
Postmortem Human Tissue
Fixed and frozen frontal cortex (Brodmann Area 10) samples collected postmortem from individuals who died with AD (n = 12), VaD (primarily of the multi-infarct dementia subtype, n = 9), or without dementia (controls [CTL]; n = 10) were obtained from the University of Michigan Alzheimer’s Disease Center Brain Bank. The cases were matched for age, postmortem interval, and gender (Table 1). Cases were selected such that the AD samples had no infarcts present in the autopsied hemisphere, and the VaD cases had no evidence or very minimal evidence of any AD typical pathology. This allowed for exclusion of likely mixed dementia cases, which could obscure VaD-specific changes.
TABLE 1.
Demographic, Clinical, and Neuropathological Characteristics by Diagnosis
| Clinical Diagnosis |
Comparison by Diagnosis Group | |||
|---|---|---|---|---|
| CTL | VaD | AD | (p value) | |
| (n = 10) | (n = 9) | (n = 12) | ||
| Age at death (years) | ||||
| Mean ± SD | 78.6 ± 8.5 | 81.4 ± 10.1 | 80.9 ± 7.4 | 0.5* |
| (Range) | (60–91) | (62–96) | (63–91) | |
| No. (%) males | 5 (50%) | 5 (56%) | 4 (33%) | 0.7† |
| Postmortem interval (hours) | ||||
| Mean ± SD | 9.0 ± 3.0 | 10.0 ± 4.0 | 8.0 ± 4.0 | 0.8* |
| (Range) | (5.0–14.0) | (4.0–17.0) | (4.0–15.0) | |
| Braak scores | ||||
| 0 | 4 | 0 | 0 | |
| I–II | 2 | 2 | 1 | |
| III–IV | 0 | 0 | 11 | <0.001** |
| V–VI | 0 | 0 | 6 | |
| NR | 6 | 7 | 0 | |
| No. (%) with infarction(s)‡ | ||||
| 2 (20%) | 8 (89%) | 0 (0%) | <0.00001*** | |
Abbreviations: CTL, control; VaD, vascular dementia; AD, Alzheimer disease; NR, not recorded.
Kruskal-Wallis test with Bonferroni correction;
AD > CTL, VaD; ***VaD > CTL, AD.
Fisher exact test.
On the basis of examination of autopsied hemisphere.
mRNA Preparation and Microarray Processing
Forty to 50 mg of frozen tissue was cut on a platform in dry ice and lysed using a TissueLyser set to 20 Hz for 2 minutes in 10× volume of lysis buffer. RNA was extracted using the mirVana miRNA isolation kit (Ambion, Foster City, CA) according to the manufacturer’s instructions. Organic extraction was performed using a 1/10 volume of the miR Homogenate Additive and the acid-phenol/chloroform method. Total RNA was quantified by NanoDrop spectrophotometry (Thermo Scientific, Waltham, MA) and RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA integrity number values (mean ± SD) were as follows: CTL (6.9 ± 0.6); VaD (5.9 ± 1.2); AD (6.3 ± 1.1). Agilent’s Low Input QuickAmp Labeling Kit was used with 100 ng total RNA/sample to generate cRNA labeled with Cy3 and Cy5 dyes following the supplied protocol. The cRNA was purified using Qiagen’s RNeasy MiniElute Cleanup Kit. The yield and specific activity of each product was determined by NanoDrop analysis. The cutoff used for viable yields was above 0.825 µg and the specific activity cutoff was 6 pmol/µg. For each sample, 300 ng of labeled, linearly amplified cRNA was digested and hybridized for 17 hours at 65°C on Agilent Human 8x60k v2 microarray slides pressed to a gasket slide using the Agilent SureHyb chamber base. The slides were then sequentially washed with Gene Expression Wash Buffer 1 at room temperature and Gene Expression Wash Buffer 2, which was warmed to 37°C. After washing, the slides were scanned on an Agilent C Microarray Scanner using the G3 microarray format with the resolution set to 3 µm.
Microarray Data Processing and Analysis
Raw data files were imported into R (www.r-project.org)/Bioconductor for downstream processing and analysis (30). Data were assessed for quality via intensity distributions, principal component analysis, and mean correlation with 1 VaD sample being removed due to poor sample and data quality. All remaining data were background corrected and quantile normalized prior to differential expression. Differential expression was performed via limma using a block design to leverage technical replicates (31). Genes with a false discovery rate (FDR)-adjusted p < 0.05 were considered differentially expressed (32). Individual lists of differentially expressed genes from each comparison were imported into MetaCore version 6.23.67496 (Thomson Reuters) for Pathway Map enrichment and Shortest Paths network analysis. MetaCore Pathway Maps are rigorously curated biochemical pathways and signaling cascades. An algorithm based on the hypergeometric distribution is used to calculate enrichment p values. Shortest Paths network analysis was performed with a maximum number of steps in the path set to 1 without the use of canonical pathways. Nodes and edges were exported from MetaCore and imported into Cytoscape v3.3.0 for final visualization (33). Raw and processed microarray data are available on GEO (GSE122063).
Quantitative PCR Validation
cDNA was generated from 1 µg RNA using ThermoFisher’s RevertAid First Strand cDNA synthesis kit. TaqMan probes for Rac Family Small GTPase 1 (Rac1; probe set Hs01902432), cAMP-response element binding protein (CREB) (Crebbp; Hs00231733), 5-Oxoprolinase, ATP-Hydrolyzing (Oplah; Hs00417215), Spondin 2 (Spon2; Hs00202813), and Oxtr (Hs00168573) were multiplexed with Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh; Hs02758991; see Table 2 for gene nomenclatures). Three technical replicates were performed. Target genes used FAM as the reporter dye while the reference gene used VIC as the reporter dye. Four microliter of cDNA was used per well and 6 µL of a mixture of the 2 TaqMan probes and TaqMan Universal Master Mix II, DNA polymerase, and dNTPs were added to each well for a total volume of 10 µL per well. The plates were briefly centrifuged and analyzed on an Applied Biosystems 7500 real time PCR system over 40 cycles. Fold change between controls and dementia samples was established using the 2–ΔΔCt method with Gapdh as the reference.
TABLE 2.
Human Genome Organization (HUGO) Gene Nomenclature
| Gene | Gene Product |
|---|---|
| Aak1 | AP2 associated kinase 1 |
| Cdk5 | Cyclin dependent kinase 5 |
| Cox5a | Cytochrome c oxidase subunit 5A |
| Crebbp | cAMP-response element binding protein (CREB) binding protein |
| Cxcr4 | C-X-C chemokine receptor type 4 |
| Efnb1 | Ephrin B1 |
| Efnb3 | Ephrin B3 |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| Gfap | Glial fibrillary acidic protein |
| Grin2b | Glutamate ionotropic receptor NMDA type subunit 2B |
| Grin2d | Glutamate ionotropic receptor NMDA type subunit 2D |
| Hist1h2bm | Histone cluster 1 H2B family member M |
| Hist1h4b | Histone cluster 1 H4 family member B |
| Hist1h4d | Histone cluster 1 H4 family member D |
| Ndufa7 | NADH: ubiquinone oxidoreductase subunit A7 |
| Ndufb7 | NADH: ubiquinone oxidoreductase subunit B7 |
| Oplah | 5-Oxoprolinase, ATP-hydrolysing |
| Oxtr | Oxytocin receptor |
| Plekhg4 | Pleckstrin homology and rhoGEF domain containing G4 |
| Rab5a | rab5a, member RAS oncogene family |
| Rac1 | rac family small GTPase 1 |
| Ralbp1 | ralA binding protein 1 |
| Rcc2 | Regulator of chromosome condensation 2 |
| Satb1 | SATB homeobox 1 |
| Sh3rf1 | SH3 domain containing ring finger 1 |
| Spon2 | Spondin 2 |
| Syn1 | Synapsin 1 |
| Syn2 | Synapsin 2 |
| Trio | Trio rho guanine nucleotide exchange factor |
| Uqcr10, | Ubiquinol-cytochrome C reductase, complex III subunit X |
| Uqcrfs1 | Ubiquinol-cytochrome C reductase, Rieske iron-sulfur polypeptide 1 |
Western Blotting
Thirty to 50 mg of frozen frontal cortex tissue was dissected on dry ice and collected in a round bottom 2-mL tube. Subcellular fractionation of the samples was achieved using a protocol modified from Guillemin et al (34). 5× volume of cell lysis buffer (10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2) was added to each sample that included a volume of 1% 100 nM PMSF, 1% Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific), and 1% 0.5 M EDTA. Samples were homogenized by sonication and restored to isotonic condition with a 0.4× volume of 2.5 M sucrose. After centrifuging for 10 minutes at 6300 rpm at 4°C, the supernatant was collected and centrifuged again for 150 minutes at 14 000 rpm at 4°C. The supernatant representing the cytosolic fraction was reserved, and the pellet representing the membrane fraction was resuspended with 1x phosphate buffered saline pH 7.4 at a volume of 50 µL per pellet and the EDTA, PMSF, and inhibitor cocktail were spiked in at a 1% concentration. Protein quantification was determined with the Pierce bicinchoninic acid assay kit ([BCA], Thermo Scientific).
Proteins of the membrane fraction (30 µg/sample) were separated on 4%–20% PAGE gels (Criterion, Bio-Rad, Hercules, CA) for 25 minutes at 250 mV. They were transferred to a PVDF membrane at 400 mA for 50 minutes. After blocking with 5% dry milk in 1× Tris-buffered saline, pH 7.4, with 0.05% Tween-20 (TBST), membranes were incubated overnight at 4°C with a rabbit primary OXTR antiserum (1:500; Proteintech, Rosemont, IL) in TBST. The following day, membranes were blocked for in 10 minutes and washed twice with TBST, incubated with goat antirabbit 680 secondary antibody (1:10 000; LI-COR, Lincoln, NE) in TBST for 2 hours, followed by washes in TBST (1 × 10 minutes) and TBS (2 × 10 minutes). Blots were visualized on an Odyssey scanner (LI-COR) at a resolution of 169 µm and an intensity of 5.0 in the 700 nm channel. The blots were then stripped in Restore Western blot stripping buffer (Thermo Scientific) for 15 minutes, washed briefly with TBS and blocked for 1 hour with TBST/5% milk. Membranes were then incubated with rabbit primary calnexin antiserum (Proteintech; 1:1000) in TBST overnight at 4°C. The next day, the membranes were washed and incubated with goat antirabbit 680 secondary antibody (LI-COR; 1:10 000) and imaged on the Odyssey scanner. OXTR protein levels were normalized to calnexin levels for quantitative analysis (Odyssey Image Studio, LI-COR).
Immunohistochemistry and Immunocytochemistry
For immunohistochemistry (IHC), 20-µm sections of paraffin-embedded frontal cortex tissue from the same cases used in the microarray analysis were deparaffinized, cleared in xylenes, and rehydrated using a 100%/95%/70% ethanol series. Antigen retrieval was achieved by submerging the slides in a 65°C citric acid buffer (0.1 M, pH 6.0) for 20 minutes. Slides were cooled to room temperature, washed in TBS, and incubated in a solution of 90% methanol/10% H2O2 for 10 minutes to quench endogenous peroxidase activity. The tissue was then permeabilized with TBS/0.25% Triton X-100 (Tx) for 3 × 10 minutes, blocked in TBS/0.25% Tx/1% normal goat serum (NGS) for 30 minutes, and incubated overnight at 4°C with the OXTR antiserum (1:100) in blocking buffer. Adjacent tissue sections were incubated in the absence of primary antibody. The following day, the slides were rinsed in TBS and incubated with biotinylated goat antirabbit secondary antibody (1:500; Vector Labs, Burlingame, CA) in TBS/1%NGS for 1 hour at room temperature. Afterwards, the slides were rinsed in TBS and incubated for 1 hour in Vectastain ABC solution (Vector). Once this was completed the slides were rinsed in imidazole acetate buffer (pH 7.4) and developed for 2 minutes with the ImmPact DAB peroxidase substrate kit (Vector). Once sufficient staining was observed, the slides were washed in TBS, dehydrated in an 70%/95%/100% ethanol series, cleared in xylenes, and coverslipped with Cytoseal 60 (Thermo Scientific).
For dual-labeling of both GFAP and OXTR in the same sections, GFAP antiserum (Cell Signaling; 1:200) was used following the protocol described for OXTR IHC up to the development step, when Immpact VIP peroxidase substrate kit (Vector) was used instead. After washing with TBS, the slides were blocked with avidin and biotin using Vector’s Avidin/Biotin Blocking kit. Slides were washed in TBS 3 × 10 minutes, placed in 90% methanol/10% H2O2 for 10 minutes, exchanged to TBS/0.25% Triton X-100 (Tx) for 3 × 10 minutes, blocked in TBS/0.25% Tx/1% NGS for 30 minutes, and incubated overnight at 4°C with the OXTR antiserum (1:100) in blocking buffer. Detection and development of the OXTR antiserum was performed the following day as described above.
For immunocytochemistry, HBEC-5 is human brain-derived endothelial cells (ATCC, Manassas, VA) were seeded in 8-well chamber slides at a density of 10 000 cells/well in 200 µL of DMEM/F12 media (Gibco, Waltham, MA) with l-Glutamine, 15 mM HEPES, 10% calf bovine serum, and 40 µg/mL endothelial growth supplement. The media was exchanged 3 times with 1× phosphate-buffered saline ([PBS]; 0.1 M, pH 7.4). Cultures were fixed with PBS containing 4% paraformaldehyde for fifteen minutes, permeabilized with PBS/0.2% Tx for 10 minutes and blocked with PBS/0.2% Tx/10% NGS for 1 hour (all steps at room temperature with gentle agitation). OXTR antiserum incubations were performed at 1:100 as well as no-primary antibody control overnight at 4°C with gentle agitation. The next day, wells were rinsed 5 × 5 minutes with PBS at room temperature followed by incubation with goat antirabbit 488 fluorescent secondary antibody (1:200; Life Technologies, Carlsbad, CA) in blocking buffer for 1 hour at room temperature. Afterwards, the wells were washed 5 × 5 minutes with PBS and chamber slides were processed for coverslipping using Vectashield antifade mounting media with DAPI (Vector).
RNAscope
Twenty-micrometer sections of fixed paraffin-embedded frontal cortex tissue were deparaffinized, cleared in xylenes, and submerged in 100% ethanol. All steps were performed with Advanced Cell Diagnostics (ACD, Newark, CA) reagents (RNAscope 2.5 HD Reagent Kit-BROWN Catalog No. 322300) according to the manufacturer’s instructions. Slides were incubated in H2O2 for 15 minutes at room temperature, washed twice in water, and then submerged in boiling antigen retrieval buffer for 15 minutes followed by washes with water. The slides were then incubated with ACD Protease Plus reagent for 30 minutes at 40°C, followed by 4 washes in 40°C Wash Buffer and incubation with the human GFAP probe (ACD No. 311801) for 2 hours at 40°C. The slides were removed and washed 4 times. The amplification steps proceeded 1–4 at 40°C alternating between 30 and 15 minutes with washes between each step. The final 2 amplification steps (step 5 for 30 minutes and step 6 for 15 minutes) were performed at room temperature. After the final amplification, a premixed 150 μL of premixed DAB reagents A and B were applied to the slides for 10 minutes. The DAB reaction was halted by submersion in distilled water. The slides were then dried and processed for OXTR IHC as described above.
Hematoxylin and Eosin Histology
After deparaffination and rehydration in ethanol series as described above, slides were submerged in Harris Modified Hematoxylin (Thermo Scientific) for 6 minutes, dipped in 1% acid alcohol (hydrochloric acid in 70% ethanol), differentiated in ammonia water (1/100 dilution of 28% ammonium hydroxide in water) for 30 seconds, and placed in eosin working solution (Fisher Scientific) for 1 minute before dehydration and cover slipping.
Power and Statistical Analysis
On the basis of our previous gene expression studies with human tissue samples from 3 diagnostic groups (35–39) and α = 0.05, we calculated that a sample size of 8–10 cases/group would have ∼85% power to detect an effect size of 1.25 standard deviations. Subject demographics and postmortem variables were compared by Kruskal-Wallis test or the Fisher exact test. Microarray-based differential expression was performed using limma for R/Bioconductor with technical replicates being leveraged via a block design, as described above. qPCR results were compared by one-way ANOVA with Tukey’s correction. Western results were compared via Kruskal-Wallis test to account for irregularities in variance, with Dunn’s post hoc test used for multiple comparisons. For all analyses the significance was set at p < 0.05.
RESULTS
Subject Characteristics
Demographic and neuropathological characteristics of the 31 subjects (10 CTL, 9 VaD, and 12 mild/moderate AD) are summarized in Table 1. There were no significant differences in age, gender balance, or postmortem interval. When scored for Braak stage, subjects with AD displayed significantly greater tau pathology compared with the CTL and VaD groups (p < 0.001). In contrast, there were significantly more subjects in the VaD group displaying infarctions (p < 0.00001), with 89% of the VaD cases exhibiting lacunes, large infarcts, or microinfarctions compared with 20% of CTL cases and none of the AD cases. Furthermore, 100% of the VaD cases exhibited some form of age-related cerebrovascular pathology (e.g. atherosclerosis, white matter rarefaction) compared with 67% of AD cases and 50% of CTL cases (p = 0.02).
Differential Gene Expression in VaD and AD
Frontal cortex tissue was used for microarray analysis as frontal executive function and working memory deficits are predominant in the initial stages of VaD compared with AD (40). Differential expressions analysis of AD versus controls, VaD versus controls, and AD versus VaD identified 4564, 1482, and 319 significantly differentially expressed genes, respectively (Fig. 1). Data are accessible at the NCBI GEO database (accession GSE122063).
FIGURE 1.
Venn diagram representing common and disease-specific genes in CTL, VaD, and AD subjects. Comparisons are based on significantly differentially expressed genes (FDR-adjusted p < 0.05) in frontal cortex from the 3 clinical diagnostic groups.
Pathway Analysis across the Diagnostic Groups
Pathway analysis was performed using differentially expressed genes from the following comparisons: AD versus controls, VaD versus controls, and AD versus VaD (Fig. 2). Twenty pathways were significantly enriched among differentially expressed genes in AD versus control. Among these AD-related functional pathways were synaptic processes such as vesicle fusion and recycling (e.g. Syn1, Syn2, Snap25; Table 2). Oxidative phosphorylation (Uqcr10, Uqcrfs1, Cox5a), cytoskeletal remodeling (Efnb1, Efnb3, Cxcr4), and NMDA receptor trafficking (Grin2b, Grin2d, Cdk5) were also robustly implicated in AD. By comparison, only 3 pathways were significantly enriched among differentially expressed genes in VaD compared with controls, including oxidative phosphorylation (Ndufa7, Ndufb7), cytoskeletal remodeling (Rac1, Trio), and clathrin-coated vesicle cycling (Aak1, Rab5a), whereas there were no significantly enriched pathways among genes specifically differentially expressed between VaD and AD. While these results did not provide a pathway unique to VaD, this was not unexpected since neurodegenerative processes likely involved many common functional gene families in these 2 dementia subtypes. Moreover, cerebrovascular comorbidities in the control and AD groups might also lead to the expression of overlapping pathways in the absence of frank vascular pathology (Table 1).
FIGURE 2.
Pathway analysis of dysregulated genes in VaD compared with AD frontal cortex. Horizontal bar graph shows that gene expression alterations in AD were heavily enriched for synaptic function, cytoskeletal remodeling, and glutamatergic signaling compared with controls. In contrast, VaD displayed an enrichment of genes dysregulated in oxidative phosphorylation and clathrin-coated transport compared with controls. Pathways most strongly differentiating AD and VaD were associated with cell adhesion and RhoA-mediated G protein-coupled signaling. Red hashed line: p < 0.05 (FDR-adjusted).
VaD-Specific Network Analysis Reveals Genes of Interest
A network analysis was performed using all genes that were found to be up or downregulated in VaD cases but not in AD (n = 413; Fig. 3). Two hub genes and a highly upregulated receptor were noted. Rac1, a Rho-type small GTPase implicated in both reducing apoptosis-mediated cell death and, by contrast, contributing to higher incidence of myocardial infarction (41), was downregulated in VaD (logFC = –0.40, p = 0.01; Table 2). Interestingly, several RAC1 protein activators such as Plekhg4 (logFC = –0.78, p = 0.02) and Sh3rf1 (logFC = –0.33, p = 0.04) were also downregulated, while RAC1 inhibitors such as Rcc2 (logFC = 0.41, p = 0.04) and Ralbp1 (logFC = 0.40, p = 0.02 rfrf) were upregulated. On the other hand, Crebbp mRNA was upregulated in VaD (logFC = 0.37, p = 0.02), as were several core histones targeted by CREBBP acetyltransferase activity (42) such as Hist1h4b (logFC = 0.59, p = 0.01), Hist1h4d (logFC = 0.53, p = 0.02), and Hist1h2bm (logFC = 0.60, p = 0.04). Finally, Oxtr, which encodes the OXTR G-protein coupled receptor linked to phospholipase C activation, was significantly upregulated in VaD (logFC = 0.86, p = 0.03; Fig. 3).
FIGURE 3.
Network analysis of VaD-specific gene dysregulation. Two major interaction hubs were identified centering on Rac1 and Crebbp (Table 2). Rac1 was significantly downregulated in VaD compared with AD and controls (log fold change = –0.4, FDR adjusted p < 0.01). Likewise, several genes encoding RAC1 inhibitors (e.g. Ralbp1) were upregulated whereas genes encoding RAC1 activators (e.g. Plekhg4) were downregulated in VaD. In contrast, Crebbp gene expression was significantly upregulated in VaD samples (log fold change = 0.4, FDR-adjusted p < 0.02). Crebbp is a modulatory hub for several upregulated genes encoding core histones (e.g. Hist1h2bm, Hist1h4b) and regulators of chromatin remodeling (e.g. Satb1). Notably, Oxtr was among the most strongly upregulated genes within the VaD network (log fold change = 1.86, FDR-adjusted p < 0.04). Light to dark blue = increased downregulation in VaD relative to controls and AD; light to dark red = increased upregulation in VaD relative to controls and AD.
Validation of VaD-Specific Gene Changes
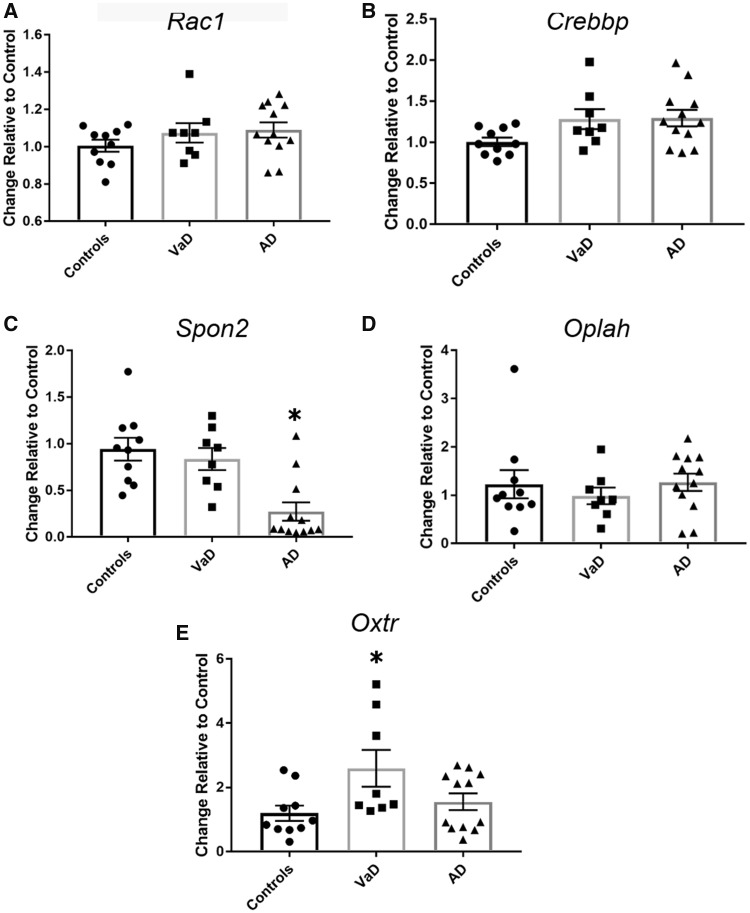
qPCR analysis of frontal cortex samples was used to validate microarray-based observations in VaD-related gene expression. In addition to Rac1, Crebbp, and Oxtr, we selected 2 additional genes for analysis based upon their differential expression in VaD compared with AD and controls, Oplah and Spon2. The OPLAH gene product is an enzyme that aids in the conversion of 5-oxoproline to glutamate and in cases of genetic insufficiency has been associated with oxidative stress and delayed motor skills (43). SPON2 is a cell adhesion protein important for neurogenesis (44, 45). As shown in Figure 4, levels of Rac1 (Fig. 4A), Crebbp (Fig. 4B), and Oplah (Fig. 4D) did not differ across the diagnostic groups, whereas Spon2 was significantly downregulated in AD compared with controls (Fig. 4C). In contrast, Oxtr was significantly upregulated in VaD compared with AD and controls (Fig. 4E), suggesting a VaD-specific alteration in this receptor. Notably, no sex differences were found when analysis was performed independent of diagnostic group (p = 0.79 via Student’s t test) or within the diagnostic groups (p = 0.31 via Kruskal-Wallis testing). Western blot analysis of frontal cortex membrane fractions revealed that OXTR protein levels were significantly upregulated in VaD compared with controls, with intermediate levels detected in AD cases (Fig. 5).
FIGURE 4.
qPCR validation of selected genes dysregulated via microarray analysis. (A) qPCR analysis of Crebbp mRNA in the same tissue blocks of frontal cortex examined by microarrays revealed a nonsignificant trend of upregulation in VaD and AD samples compared with control levels (one-way ANOVA [F = 2.99, p = 0.06]). (B)Rac1 levels did not differ between the 3 diagnostic groups (F = 1.22, p = 0.31). (C)Spon2 was significantly downregulated in AD compared with control samples (F = 11.18, p = 0.0003). (D)Oplah mRNA levels were unchanged among the 3 diagnostic groups (F = 0.4850, p = 0.6209). (E)Oxtr mRNA levels were significantly upregulated in the VaD samples (F = 3.886, p = 0.03). *p < 0.05.
FIGURE 5.
Western blot confirmation of OXTR protein upregulation in VaD frontal cortex. Membrane fractions of the same tissue blocks of frontal cortex were used to detect protein levels of OXTR. (A) Representative western blot showing OXTR and calnexin immunoreactivity in CTL, VaD, and AD cases. (B) Quantitative analysis revealed that protein levels were significantly different among the 3 diagnostic groups (Kruskal-Wallis test [H = 7.426, p = 0.03]). OXTR levels in VaD were significantly different than CTL (Dunn's multiple comparison test [mean rank = −10.63, multiplicity-adjusted p = 0.033]), with intermediate but nonsignificant levels detected in AD. *p < 0.05. Samples were run as technical replicates with means used for statistical analysis.
OXTR Expression in Neocortex and Human Cerebrovascular Endothelial Cell Culture
OXTR IHC revealed immunopositive profiles in frontal cortex consistent with a predominant vascular localization (Fig. 6A, B), supporting previous work suggesting that OXTR is abundantly expressed by cerebrovascular endothelial cells (46). Accordingly, fluorescence immunocytochemistry using an immortalized line of human brain-derived endothelial cells revealed robust OXTR positive staining that was not observed in control primary-delete experiments (Fig. 6C). Hence, OXTR expression may be enriched in the brain cerebrovasculature.
FIGURE 6.
OXTR expression in cerebrovascular endothelial cells. (A, B) OXTR immunoreactivity is enriched in vascular profiles in human frontal cortex tissue (left). Deletion of primary OXTR antibody supports antigen specificity (right). Images shown at 20× (A) and at 40× (B). (C) Fluorescence immunocytochemical detection of OXTR with DAPI counterstain in cultures of human brain derived endothelial cells, shown with (left) and without (right) primary antibody.
De Novo OXTR Expression Surrounding Grey and White Matter Microinfarctions
Although we identified specific and significant regional increases in Oxtr gene and OXTR protein expression in frozen frontal cortex tissue samples from individuals who died with VaD, these experiments did not test the extent to which OXTR upregulation was associated with microvascular lesions. We hypothesized that a specific OXTR response to ischemic injury would be expected to peak in the penumbra region of an infarction and dissipate with distance. To test this possibility, we processed adjacent paraffin-embedded frontal cortex tissue sections from the same cases using hematoxylin and eosin staining to identify microinfarcts (Fig. 7A, B) and IHC to label OXTR-expressing profiles (Fig 7C, D). A strong, de novo expression of the receptor was noted around sites of both white matter (Fig. 7A, C) and grey matter (Fig. 7B, D) infarcts. Notably, OXTR antibody labeling was observed in vessels, but also in cells resembling polarized astrocytes (Fig. 7C, D). Subsequently, dual labeling for OXTR and the reactive astrocyte marker glial fibrillary acidic protein (GFAP) was performed on additional series of tissue. Around established microinfarcts (Fig. 8A, B), we found prominent overlap of OXTR and GFAP labeling on both astrocytic and vascular profiles (Fig. 8C, D), the latter likely reflecting astrocytic end-feet. Upon further examination, colocalization of OXTR and GFAP was at its highest in fields close to the infarction and minimal to nonexistent in fields remote from the infarction (Fig. 9).
FIGURE 7.
De novo peri-infarct expression of OXTR. (A, B) H&E staining identifies both white (A) and grey (B) matter infarcts in paraffin embedded frontal cortex tissue from VaD cases. Shown at 4× magnification. (C, D) OXTR IHC in adjacent sections revealed prominent expressions surrounding the white (C) and grey (D) matter microinfarcts. Shown at 4× (top) and 10× (bottom) magnification. The OXTR-immunoreactive profiles showed an astroglial typical morphology.
FIGURE 8.
Peri-infarct astroglial and vascular expression of OXTR. (A, B) Photomicrographs show white matter microinfarction via H&E staining at 4× (A) and 10× (B, panel A inset). (C, D) Photomicrographs show dual-label IHC of OXTR (brown reaction product) and GFAP (black reaction product) at 4× (C) and 10× (D, panel C inset) in an adjacent section.
FIGURE 9.
Gradient of OXTR expression in astrocytes and blood vessels relative to microinfarction site. (A) Photomicrograph shows dual-label IHC of OXTR and GFAP from Figure 8 with numbered insets as shown in (B). (B) OXTR expression is enriched in GFAP-immunopositive profiles in a field close to the microinfarction [3] and in the left part of fields [1] and [4] proximal to the lesion. In contrast, OXTR is minimal in GFAP-immunopositive profiles in a field farther from the microinfarction [2] and in the right part of fields [1] and [4] distal to the lesion. All fields shown at 20×. (C) OXTR-expressing astrocytes are indicated with black arrows, whereas vessels labeled with OXTR are indicated with black arrowheads and OXTR-negative astrocytes are labeled with asterisks. All fields shown at 40×. (D) Another field from the same section further demonstrates dual-labeled GFAP positive astrocytes and vessels expressing OXTR (40×).
OXTR Expressing Cells with Astrocytic Profiles Also Contain GFAP mRNA
In order to further validate the presence of OXTR positive astrocytes within the penumbra of microinfarctions, we performed RNAscope combining GFAP mRNA probe amplification with OXTR IHC. OXTR was detected in both vascular profiles and strongly arborous cells, which also exhibited the presence of GFAP mRNA (Fig. 10A). While the GFAP mRNA signal remained with omission of the OXTR primary, the vessels and arborous limbs of the cells did not, supporting the concept that de novo expression of OXTR occurs within parenchymal microvessels and astroglia at the sight of microinfarction (Fig. 10B).
FIGURE 10.
RNAscope detection of GFAP mRNA in profiles expressing OXTR. (A) Photomicrograph shows OXTR IHC (blue-black reaction product) combined with GFAP mRNA amplification via RNAscope (brown reaction product) in a peri-infarct field (20×). Insets 1 and 2 are shown at 40× magnification. (B) GFAP mRNA labeling in the absence of OXTR primary antibody validates specificity of co-expression at 20× (left) and 40× (right, insert).
DISCUSSION
Currently, there is no FDA approved therapy for VaD and only rapid thrombolysis is indicated for acute ischemic stroke (47). Failures to find interventions of significant benefit in experimental and clinical studies of VaD have been partially attributed to a lack of emphasis on probable VaD (48–50). This study was designed to identify gene expression changes reflecting putative pathologic or protective mechanisms unique to VaD that might be amenable to therapy. Using microarray analysis to quantify differences in gene expression patterns and highly connected networks among individuals who died with VaD, AD or no dementia, we identified and prioritized several dysregulated genes of interest for further validation including Rac1, Crebbp, Oxtr, Opah, and Spon2. To our knowledge, this is the first microarray study comparing cortical gene expression profiles among VaD, AD and CTL subjects. The main finding of this study is the novel observation via independent, complementary methods that Oxtr gene expression is differentially upregulated in VaD and appears to manifest as de novo, peri-infarct OXTR expression localized to cerebrovascular endothelial cells and reactive astroglia surrounding microinfarctions. Given the multiple lines of evidence supporting a protective role for OXTR signaling in peripheral infarction (26–28), as well as studies suggesting that OXT may protect against cerebral damage in stroke in both rats (51) and postpartum women (52), we posit that this phenomenon is a compensatory response to rescue the ischemic penumbra and that manipulations of OXTR signaling may provide a therapeutic benefit in VaD and perhaps mixed dementia, as well.
The OXTR is a G-protein coupled receptor linked to Gαq/11-mediated activation of phospholipase C (PLC), which in turn activates a variety of calcium-dependent intracellular signaling pathways through the hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2) (53). Its endogenous ligand OXT is a neuropeptide synthesized primarily in the paraventricular nucleus and supraoptic nucleus of the hypothalamus (54). Fiber projections to the posterior pituitary allows for the centrally produced OXT to be stored and released into the general circulation (54). The activation of OXT signaling has been routinely studied for its role in social affiliation and reproduction (55–58), as well as uterine contractions (59, 60) during labor and milk ejection during nursing (61, 62). In fact, the OXTR antagonist Atosiban is effective in halting premature labor (59), and OXTR agonists such as Carbetocin can be used to induce labor and minimize the incidence of postpartum hemorrhage (63). Intranasal OXT has been found to increase the likelihood of adapting to the opinions of one’s in-group when presented with diverging opinions of an in-group and out-group member and thus has been closely tied to social conformity (64). In contrast, far less is known about the vascular effects of OXTR signaling, but the reported effects of OXT signaling during experimental peripheral infarction (26, 65) suggest a striking propensity to counter many of the deleterious mechanisms of the ischemic cascade in stroke.
In this regard, while the current study does not address the functional consequences of increased OXTR expression surrounding infarction, an examination of the available literature supports a protective role for OXTR upregulation in VaD. The ischemic cascade comprises an array of negative consequences following oxygen and glucose deprivation that leads to apoptotic tissue death. These consequences include NADPH oxidase production of superoxide free radicals (66), induction of pro-inflammatory cytokines leading to immune cell activation and adhesion (67), endothelial disruption weakening the blood brain barrier (68), and disturbances in nitric oxide synthase (NOS) isoforms resulting in toxic uncoupling by inducible NOS (69) and impairment of pro-angiogenic endothelial NOS (eNOS) (70).
Significantly, several studies have suggested an ability of OXTR signaling to modify several of these aspects of the ischemic cascade that might ultimately prove beneficial in preventing cognitive impairment due to strokes. For instance, OXTR stimulation by OXT was shown to decrease NADPH oxidase-mediated superoxide production in endothelial cells by 24%–48% at baseline and by ∼40% upon tumor necrosis factor α (TNFα) stimulation (71). Because of its ability to attenuate oxidative stress in the vasculature, OXT has been linked to the noted protective effect of social affiliation in atherosclerosis-prone animal models as a probable physiological intermediator (72). Moreover, Jankowski et al found that OXT treatments after surgical induction of myocardial infarction in rats led to a significant decrease in pro-inflammatory cytokines (e.g. IL-6 and TNFα), and an increase in anti-inflammatory cytokines (e.g. IL-10) (26). This ability to regulate the inflammatory environment may explain promising results that led to previous interest in OXT as a prosurvival factor in tissue grafts (73). OXTR activation is also known to contribute to eNOS phosphorylation via the activation of the phosphatidylinositol-3-kinase (PI3-K)/AKT pathway, which stimulates cell migration and angiogenesis to promote reperfusion (74). In contrast, the noted ability of OXTR activation to stimulate tube formation in endothelial cell cultures at a similar rate to that experienced with vascular endothelial growth factor has led to suggestions that its repression may be a viable method to control aberrant angiogenesis such as occurs in endometriosis (75). A final putative, protective consequence of OXTR activation in the ischemic penumbra is its ability to induce proteolytic shedding of full-length receptor for advanced glycation end-products (RAGE) up to 5-fold over basal turnover through PLC and PI3-K controlled mechanisms (76). This induction of RAGE shedding has attracted interest as a means to prevent diabetic complications by limiting the number of receptors for AGEs to bind to and provoke inflammation and oxidative stress particularly in an early metabolic syndrome stage (77).
We identified astrocytes and the cerebrovascular endothelium as the likely cellular sources of de novo OXTR upregulation surrounding ischemic injury. This is intriguing as both cell types have attracted attention as mediators of acute and chronic responses to ischemia. The endothelium, or the innermost monolayer of endothelial cells comprising the first line of the blood-brain barrier in cerebral blood vessels, has noted vasoactive and immunity mediating responses to ischemic damage (78, 79). The vasodilation of blood vessels due to enhanced eNOS activity promotes tissue preservation in ischemic injury and has been suggested to be a secondary means through which statins protect against cerebral ischemia (78, 79). In the acute phases of hypoxia, the endothelium produces cytokines and reactive oxygen species similar to innate immune cells (80–83). Basal levels of cytokines are often minimal in endothelium, but in response to hypoxic signals they can adopt either a pro-inflammatory (IL-1α, IL-1β, IL-6, TNFα) or anti-inflammatory (IL-10, transforming growth factor β [TGFβ]) phenotype (83). The environmental conditions, particularly transmitters and cytokines secreted by other cells, have a vital role in driving endothelial cell phenotype (82). Reactive oxygen species, particularly the superoxide anion O2–, are produced by both the NAPDH oxidase enzyme and uncoupled NOS in dysfunctional endothelial cells and further damages the blood-brain barrier in cerebral vascular disorders (80, 81). In later stages of posthypoxic injury, the endothelium can take an important reparative role through angiogenesis and the secretion of neurotrophic factors, which re-establishes blood perfusion and supports neurogenesis to restore damaged tissues (84–86). Hence, OXTR upregulation and signaling in the cerebrovasculature may provide a mechanism for rescuing these deleterious pathways following stroke.
Astrocytes also play a role in the acute and chronic stages of postischemia. Although there is less evidence for potential OXTR signaling consequences in astrocytes versus endothelial cells, astrocytes have been shown to express the OXTR (87, 88), and it is exciting to speculate on OXTR function given canonical astrocyte-mediated pathways in hypoxic conditions. The most commonly known aspect of astrocytic intervention in cerebral injury is the formation of a glial scar through the interaction of astrocytic processes produced by intermediate filaments with the extracellular matrix to isolate the lesion from salvageable tissue and sometimes provide paths for neuroblasts to restore vital circuits (84, 89–91). Astrocytes also act as inflammatory mediators in hypoxia, producing cytokines and ROS through both a p38 mitogen-activated protein kinase pathway and nuclear factor-kappa β (NF-kβ) (91). Importantly, astrocytes can adopt either pro-inflammatory (NF-kβ) or anti-inflammatory (gp130) responses (89). Given the anti-inflammatory signaling profile for OXTR described above, we posit that de novo OXTR expression in peri-infarct reactive astrocytes supports neuroprotection. Moreover, in a more acute protective role, astrocytes can limit excitotoxicity through increased glutamate uptake and metabolism via TGFβ activation (91, 92), which leads to an increase in glutamate transporter 1 (92) and the glutamate-aspartate transporter (91).
In contrast, we cannot rule out the possibility that de novo expression of the OXTR in astrocytes and the endothelium in the wake of ischemia is deleterious. For instance, it has been suggested that OXT enhances proliferation of endothelial cells (74), yet this also includes an increase in matrix metalloproteinases to degrade extracellular matrix proteins (65). While necessary for angiogenesis, the role of these enzymes in infarction has also raised concerns about hemorrhagic transformation due to blood-brain barrier degradation (93–95) and destructive immune responses (96, 97). Hence, OXTR signaling in endothelial cells in the proximity of the ischemic lesion could lead to expansion of the lesion rather than reduction, especially after the therapeutic administration of tissue plasminogen activator (93). Moreover, while reactive astrocytes can have a neuroprotective role in cerebral ischemia, there are also opposing mechanisms by which reactive astrocytes can lead to expansion of the lesion (98, 99) or hinder plasticity during recovery (100, 101). Reactive astrocytes can interdigitate and express inhibitory molecules like chondroitin sulfate proteoglycans that restrict axonal regeneration (101). They can also contribute to oxidative stress and inflammation through inducible nitric oxide synthase (98) and the transcription factor NF-kβ (102). Like many ischemic responses, controversy exists as to whether signaling of the endothelium and activated astrocytes ultimately fall under neuroprotective or neurotoxic (103, 104). Ultimately, timing, degree of injury, and the presence of multiple modulators in the tissue likely contribute to the sum effect (91, 105). Further research on the pathways affected by the OXTR on endothelium and astrocytes under hypoxic and metabolic stress is necessary to support or refute our hypothesis that peri-infarct expression of OXTR represents a compensatory response.
In summary, we have identified OXTR upregulation as a novel, potentially protective phenomenon associated with microinfarction lesions, which may modify many of the negative outcomes associated pathways of the ischemic cascade though increased OXT signaling. Moreover, the persistence of peri-infarct OXTR expression in vessels and astrocytes at autopsy suggests that this phenomenon is a protracted rather than acute response to injury with a wide therapeutic window for target engagement. This is particularly compelling given the safety profile of its centrally acting properties in clinical settings and the availability of FDA-approved OXTR ligands (106, 107). Significantly, our results may have therapeutic implications for stroke recovery irrespective of putative cognitive outcomes, as well as for mixed AD dementia. A reduction in ROS production by NADPH oxidase could reduce the oxidation of DNA in impacted regions and enhance the percentage of newborn neurons surviving in the environmentally harsh penumbra (108). Increased eNOS production of nitric oxide by OXTR signaling could contribute to angiogenic vessel remodeling after a stroke to restore sufficient blood flow to the penumbra region (109). A reduction in pro-inflammatory cytokines in favor of anti-inflammatory cytokines, and initiation of full-length RAGE shedding could limit the activation of innate immune cells and their extravasation through the vessel wall into the brain parenchyma where they can produce cytotoxic and self-reactive damage (110). Future studies will dissect the precise intracellular pathways mediated by astroglial and endothelial OXTR that might represent a novel mechanism unique to VaD with therapeutic potential.
ACKNOWLEDGMENTS
The authors thank Matthew Perkins, University of Michigan Brain Bank Coordinator, for his expert assistance in tissue procurement and selection.
This study was supported by NIH grants AG014449, AG053581, AG053760, as well as the Saint Mary's Foundation and Miles for Memories of Battle Creek, MI.
Footnotes
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. Towards a Dementia Plan: A WHO Guide [Internet]. Dementia: A Public Health Priority – World Health Organization; 2017 [Google Scholar]
- 2. Wimo A, Jönsson L, Bond J, et al. The worldwide economic impact of dementia 2010. Alzheimer’s Dement 2013;9:1–11 [DOI] [PubMed] [Google Scholar]
- 3. Livingston G, Barber J, Rapaport P.. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: Pragmatic randomised controlled trial. BMJ 2013;347:f6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jorm AF, Jolley D.. The incidence of dementia: A meta-analysis. Neurology 1998;51:728–33 [DOI] [PubMed] [Google Scholar]
- 5. Corrada MM, Brookmeyer R, Paganini-Hill A, et al. Dementia incidence continues to increase with age in the oldest old the 90+ study. Ann Neurol 2010;67:114–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attems J, Jellinger KA.. The overlap between vascular disease and Alzheimer’s disease – Lessons from pathology. BMC Med 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brookes RL, Hannesdottir K, Lawrence R, et al. Brief memory and executive test: Evaluation of a new screening test for cognitive impairment due to small vessel disease. Age Ageing 2012;41:212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallin A, Nordlund A, Jonsson M, et al. Alzheimer’s disease-subcortical vascular disease spectrum in a hospital-based setting: Overview of results from the Gothenburg MCI and dementia studies. J Cereb Blood Flow Metab 2016;36:95–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bathgate D, Snowden JS, Varma A, et al. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 2001;103:367–78 [DOI] [PubMed] [Google Scholar]
- 10. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–60 [DOI] [PubMed] [Google Scholar]
- 11. Zheng L, Vinters HV, Mack WJ, et al. Differential effects of ischemic vascular disease and Alzheimer disease on brain atrophy and cognition. J Cereb Blood Flow Metab 2015;16570:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thal DR, Grinberg LT, Attems J.. Vascular dementia: Different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 2012;47:816–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorelick PB, Counts SE, Nyenhuis D.. Vascular cognitive impairment and dementia. Biochim Biophys Acta Mol Basis Dis 2016;1862:860–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dirnagl U, Iadecola C, Moskowitz M.. Pathobiology of ischaemic stroke: An integrated view. 4441. Trends Neurosci 1999;22:391–7 [DOI] [PubMed] [Google Scholar]
- 15. Iadecola C. The pathobiology of vascular dementia. Neuron 2013;80:844–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment – A critical update. Front Aging Neurosci 2013;5:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomlinson BE, Blessed G, Roth M.. Observations on the brains of demented old people. J Neurol Sci 1970;11:205–42 [DOI] [PubMed] [Google Scholar]
- 18. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol 1994;36:557–65 [DOI] [PubMed] [Google Scholar]
- 19. Liu Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurol Res 2012;6412:331–7 [DOI] [PubMed] [Google Scholar]
- 20. Shichinohe H, Tan C, Abumiya T, et al. Neuroprotective effects of cilostazol are mediated by multiple mechanisms in a mouse model of permanent focal ischemia. Brain Res 2015;1602:53–61 [DOI] [PubMed] [Google Scholar]
- 21. Fisher C, Dementia in cerebral vascular disease Cereb Vasc Dis Sixth Princet Conf New York. NY: Greene Strat, 1968:232–6 [Google Scholar]
- 22. O'Brien JT, Thomas A.. Vascular dementia. Lancet 2015;386:1698–706 [DOI] [PubMed] [Google Scholar]
- 23. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kavirajan H, Schneider LS.. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: A meta-analysis of randomised controlled trials. Lancet Neurol 2007;6:782–92 [DOI] [PubMed] [Google Scholar]
- 25. Baskys A, Cheng JX.. Pharmacological prevention and treatment of vascular dementia: Approaches and perspectives. Exp Gerontol 2012;47:887–91 [DOI] [PubMed] [Google Scholar]
- 26. Jankowski M, Bissonauth V, Gao L, et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol 2010;105:205–18 [DOI] [PubMed] [Google Scholar]
- 27. Düşünceli F, Işeri SÖ, Ercan F, et al. Oxytocin alleviates hepatic ischemia-reperfusion injury in rats. Peptides 2008;29:1216–22 [DOI] [PubMed] [Google Scholar]
- 28. Tuǧtepe H, Şener G, Biyikli NK, et al. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept 2007;140:101–8 [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez-Reyes A, Menaouar A, Yip D, et al. Molecular mechanisms underlying oxytocin-induced cardiomyocyte protection from simulated ischemia-reperfusion. Mol Cell Endocrinol 2015;412:170–81 [DOI] [PubMed] [Google Scholar]
- 30. Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods 2015;12:115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaujoux R, Seoighe C.. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics 2010;11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merico D, Isserlin R, Stueker O, et al. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS One 2010;5(11):e13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guillemin I, Becker M, Ociepka K, et al. A subcellular prefractionation protocol for minute amounts of mammalian cell cultures and tissue. Proteomics 2005;5:35–45 [DOI] [PubMed] [Google Scholar]
- 35. Tiernan CT, Ginsberg SD, He B, et al. Pretangle pathology within cholinergic nucleus basalis neurons coincides with neurotrophic and neurotransmitter receptor gene dysregulation during the progression of Alzheimer’s disease. Neurobiol Dis 2018;117:125–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tiernan CT, Ginsberg SD, Guillozet-Bongaarts AL, et al. Protein homeostasis gene dysregulation in pretangle-bearing nucleus basalis neurons during the progression of Alzheimer’s disease. Neurobiol Aging 2016;42:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Counts SE, Alldred MJ, Che S, et al. Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 2014;79:172–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Counts SE, Mufson EJ, Ginsberg SD, et al. α7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol 2007;64:1771. [DOI] [PubMed] [Google Scholar]
- 39. Kelly SC, He B, Perez SE, et al. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stuss DT, Levine B.. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annu Rev Psychol 2002;53:401–33 [DOI] [PubMed] [Google Scholar]
- 41. Marei H, Malliri A.. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases 2016;1248:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogryzko VV, Schiltz RL, Russanova V, et al. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 1996;87:953–9 [DOI] [PubMed] [Google Scholar]
- 43. Almaghlouth IA, Mohamed JY, Al-Amoudi M, et al. 5-Oxoprolinase deficiency: Report of the first human OPLAH mutation. Clin Genet 2012;82:193–6 [DOI] [PubMed] [Google Scholar]
- 44. He Y-W, Li H, Zhang J, et al. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat Immunol 2004;5:88–97 [DOI] [PubMed] [Google Scholar]
- 45. Feinstein Y, Borrell V, Garcia C, et al. F-spondin and mindin: Two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development 1999;126:3637–48 [DOI] [PubMed] [Google Scholar]
- 46. Thibonnier M, Conarty DM, Preston J, et al. Human vascular endothelial cells express oxytocin receptors. Endocrinology 1999;140:1301–9 [DOI] [PubMed] [Google Scholar]
- 47. Tsivgoulis G, Zand R, Katsanos AH, et al. Safety of intravenous thrombolysis in stroke mimics. Stroke 2015;46:1281–7 [DOI] [PubMed] [Google Scholar]
- 48. Herrmann N, Lanctôt KL, Hogan DB.. Pharmacological recommendations for the symptomatic treatment of dementia: The Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimers Res Ther 2013;5:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perry E, Ziabreva I, Perry R, et al. Absence of cholinergic deficits in “pure” vascular dementia. Neurology 2005;64:132–3 [DOI] [PubMed] [Google Scholar]
- 50. Knopman DS, Parisi JE, Boeve BF, et al. Vascular dementia in a population-based autopsy study. Arch Neurol 2003;60:569–75 [DOI] [PubMed] [Google Scholar]
- 51. Karelina K, Stuller KA, Jarrett B, et al. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke 2011;42:3606–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seo HG, Lee HH, Oh BM.. The possible effect of oxytocin in postpartum recovery from a stroke: A case report. PM R 2018;10:1422–5 [DOI] [PubMed] [Google Scholar]
- 53. Zingg HH, Laporte SA.. The oxytocin receptor. Trends Endocrinol Metab 2003;14:222–7 [DOI] [PubMed] [Google Scholar]
- 54. Gimpl G, Fahrenholz F, Gene C.. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev 2001;81:629–83 [DOI] [PubMed] [Google Scholar]
- 55. Insel TR, Shapiro LE.. Oxytocin receptors and maternal behavior. Ann N Y Acad Sci 1992;652:122–41 [DOI] [PubMed] [Google Scholar]
- 56. Kosfeld M, Heinrichs M, Zak PJ, et al. Oxytocin increases trust in humans. Nature 2005;435:673–6 [DOI] [PubMed] [Google Scholar]
- 57. Bale TL, Davis AM, Auger AP, et al. CNS region-specific oxytocin receptor expression: Importance in regulation of anxiety and sex behavior . J Neurosci 2001;21:2546–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Argiolas A, Melis MR, Gessa GL.. Oxytocin: An extremely potent inducer of penile erection and yawning in male rats. Eur J Pharmacol 1986;130:265–72 [DOI] [PubMed] [Google Scholar]
- 59. Akerlund M, Stromberg PER, Hauksson A, et al. Inhibition of uterine contractions of premature labour with an oxytocin analogue. Results from a pilot study. BJOG 1987;94:1040–4 [DOI] [PubMed] [Google Scholar]
- 60. Caldeyro-Barcia R, Poseiro JJ.. Oxytocin and contractility of the pregnant human uterus. Ann N Y Acad Sci 1959;75:813–30 [DOI] [PubMed] [Google Scholar]
- 61. Nishimori K, Young LJ, Guo Q, et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A 1996;93:11699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Newton M, Newton NR.. The let-down reflex in human lactation. J Pediatr 1948;33:698–704 [DOI] [PubMed] [Google Scholar]
- 63. Phaneuf S, Rodríguez Liñares B, TambyRaja RL, et al. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil 2000;120:91–7 [DOI] [PubMed] [Google Scholar]
- 64. Stallen M, De Dreu CKW, Shalvi S, et al. The herding hormone: Oxytocin stimulates in-group conformity. Psychol Sci 2012;23:1288–92 [DOI] [PubMed] [Google Scholar]
- 65. Kobayashi H, Yasuda S, Bao N, et al. Postinfarct treatment with oxytocin improves cardiac function and remodeling via activating cell-survival signals and angiogenesis. J Cardiovasc Pharmacol 2009;54:510–9 [DOI] [PubMed] [Google Scholar]
- 66. Manzanero S, Santro T, Arumugam TV.. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem Int 2013;62:712–8 [DOI] [PubMed] [Google Scholar]
- 67. Siniscalchi A, Gallelli L, Malferrari G, et al. Cerebral stroke injury: The role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol 2014;25:131–7 [DOI] [PubMed] [Google Scholar]
- 68. Khatri R, McKinney M, Swenson B, et al. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012;79:S52–7 [DOI] [PubMed] [Google Scholar]
- 69. Rodrigo R, Fernandez-Gajardo R, Gutierrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets 2013;12:698–714 [DOI] [PubMed] [Google Scholar]
- 70. Poittevin M, Bonnin P, Pimpie C, et al. Diabetic microangiopathy: Impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes 2015;64:999–1010 [DOI] [PubMed] [Google Scholar]
- 71. Szeto A, Nation D, Mendez AJ, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab 2008;295:E1495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Szeto A, Rossetti MA, Mendez AJ, et al. Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits. Psychoneuroendocrinology 2013;38:685–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Petersson M, Lundeberg T, Sohlström A, et al. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch Pharmacol 1998;357:701–4 [DOI] [PubMed] [Google Scholar]
- 74. Cattaneo MG, Chini B, Vicentini LM.. Oxytocin stimulates migration and invasion in human endothelial cells. Br J Pharmacol 2008;153:728–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cattaneo MG, Lucci G, Vicentini LM.. Oxytocin stimulates in vitro angiogenesis via a Pyk-2/Src-dependent mechanism. Exp Cell Res 2009;315:3210–9 [DOI] [PubMed] [Google Scholar]
- 76. Metz VV, Kojro E, Rat D, et al. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS ONE 2012;7(7):e41823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koyama H, Shoji T, Yokoyama H, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 2005;25:2587–93 [DOI] [PubMed] [Google Scholar]
- 78. Hiroi Y, Noma K, Kim H-H, et al. Neuroprotection mediated by upregulation of endothelial nitric oxide synthase in rho-associated, coiled-coil-containing kinase 2 deficient mice. Circ J 2018;82:1195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laufs U, Gertz K, Dirnagl U, et al. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res 2002;942:23–30 [DOI] [PubMed] [Google Scholar]
- 80. Taniyama Y, Griendling KK.. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension 2003;42:1075–81 [DOI] [PubMed] [Google Scholar]
- 81. Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 2007;38:3000–6 [DOI] [PubMed] [Google Scholar]
- 82. O’Carroll SJ, Kho DT, Wiltshire R, et al. Pro-inflammatory TNFaα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation 2015;12:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mai J, Virtue A, Shen J, et al. An evolving new paradigm: Endothelial cells – Conditional innate immune cells. J Hematol Oncol 2013;6:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruan L, Wang B, Zhuge Q, et al. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 2015;1623:166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994;25:1794–8 [DOI] [PubMed] [Google Scholar]
- 86. Qin W, Li Z, Luo S, et al. Exogenous fractalkine enhances proliferation of endothelial cells, promotes migration of endothelial progenitor cells and improves neurological deficits in a rat model of ischemic stroke. Neurosci Lett 2014;569:80–4 [DOI] [PubMed] [Google Scholar]
- 87. Di Scala-Guenot D, Strosser MT.. Oxytocin receptors on cultured astroglial cells. Regulation by a guanine-nucleotide-binding protein and effect of Mg2+. Biochem J 1992;284:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mittaud P, Labourdette G, Zingg H, et al. Neurons modulate oxytocin receptor expression in rat cultured astrocytes: Involvement of TGF-β and membrane components. Glia 2002;37:169–77 [DOI] [PubMed] [Google Scholar]
- 89. Cekanaviciute E, Buckwalter MS.. Astrocytes: Integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics 2016;13:685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pekny M, Pekna M, Messing A, et al. Astrocytes: A central element in neurological diseases. Acta Neuropathol 2016;131:323–45 [DOI] [PubMed] [Google Scholar]
- 91. Roy Choudhury G, Ding S.. Neurobiology of disease reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis 2016;85:234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 2008;28:468–81 [DOI] [PubMed] [Google Scholar]
- 93. Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107:598–603 [DOI] [PubMed] [Google Scholar]
- 94. Rosell A, Lo EH.. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 2008;8:82–9 [DOI] [PubMed] [Google Scholar]
- 95. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol 2011;10:471–80 [DOI] [PubMed] [Google Scholar]
- 96. Worthmann H, Tryc AB, Deb M, et al. Linking infection and inflammation in acute ischemic stroke. Ann N Y Acad Sci 2010;1207:116–22 [DOI] [PubMed] [Google Scholar]
- 97. Amantea D, Micieli G, Tassorelli C, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci 2015;9: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Matsui T, Mori T, Tateishi N, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: Enhanced astrocytic synthesis of S-100β in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab 2002;22:711–22 [DOI] [PubMed] [Google Scholar]
- 99. Tateishi N, Mori T, Kagamiishi Y, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: Suppression of astrocytic activation by a novel agent (R)-(−)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early. J Cereb Blood Flow Metab 2002;22:723–34 [DOI] [PubMed] [Google Scholar]
- 100. Yiu G, He Z.. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006;7:617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Galtrey CM, Fawcett JW.. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 2007;54:1–18 [DOI] [PubMed] [Google Scholar]
- 102. Zhang P, Liu X, Zhu Y, et al. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci Lett 2013;534:123–7 [DOI] [PubMed] [Google Scholar]
- 103. De Pablo Y, Nilsson M, Pekna M, et al. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem Cell Biol 2013;140:81–91 [DOI] [PubMed] [Google Scholar]
- 104. Caleo M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neuroscience 2015;311:180–94 [DOI] [PubMed] [Google Scholar]
- 105. Pekny M, Wilhelmsson U, Pekna M.. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 2014;565:30–8 [DOI] [PubMed] [Google Scholar]
- 106. Tachibana M, Kagitani-Shimono K, Mohri I, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol 2013;23:123–7 [DOI] [PubMed] [Google Scholar]
- 107. Finger EC, MacKinley J, Blair M, et al. Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology 2015;84:174–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Choi DH, Kim JH, Lee KH, et al. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS One 2015;10:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bir SC, Xiong Y, Kevil CG, et al. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res 2012;95:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chamorro Á, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol 2012;8:401–10 [DOI] [PubMed] [Google Scholar]