Abstract
IMPORTANCE
Methamphetamine is a common illicit drug used worldwide. Methamphetamine and/or tobacco use by pregnant women remains prevalent. However, little is known about the effect of comorbid methamphetamine and tobacco use on human fetal brain development.
OBJECTIVE
To investigate whether microstructural brain abnormalities reported in children with prenatal methamphetamine and/or tobacco exposure are present at birth before childhood environmental influences.
DESIGN, SETTING, AND PARTICIPANTS
A prospective, longitudinal study was conducted between September 17, 2008, and February 28, 2015, at an ambulatory academic medical center. A total of 752 infant-mother dyads were screened and 139 of 195 qualified neonates were evaluated (36 methamphetamine/tobacco exposed, 32 tobacco exposed, and 71 unexposed controls). They were recruited consecutively from the community.
EXPOSURES
Prenatal methamphetamine and/or tobacco exposure.
MAIN OUTCOMES AND MEASURES
Quantitative neurologic examination and diffusion tensor imaging performed 1 to 3 times through age 4 months; diffusivities and fractional anisotropy (FA) assessed in 7 white matter tracts and 4 subcortical brain regions using an automated atlas-based method.
RESULTS
Of the 139 infants evaluated, 72 were female (51.8%); the mean (SE) postmenstrual age at baseline was 41.5 (0.27) weeks. Methamphetamine/tobacco-exposed infants showed delayed developmental trajectories on active muscle tone (group × age, P < .001) and total neurologic scores (group × age, P = .01) that normalized by ages 3 to 4 months. Only methamphetamine/tobacco-exposed boys had lower FA (group × age, P = .02) and higher diffusivities in superior (SCR) and posterior corona radiatae (PCR) (group × age × sex, P = .002; group × age × sex, P = .01) at baseline that normalized by age 3 months. Only methamphetamine/tobacco- and tobacco-exposed girls showed persistently lower FA in anterior corona radiata (ACR) (group, P = .04; group × age × sex, P = .01). Tobacco-exposed infants showed persistently lower axial diffusion in the thalamus and internal capsule across groups (P = .02).
CONCLUSIONS AND RELEVANCE
Prenatal methamphetamine/tobacco exposure may lead to delays in motor development, with less coherent fibers and less myelination in SCR and PCR only in male infants, but these abnormalities may normalize by ages 3 to 4 months after cessation of stimulant exposure. In contrast, persistently less coherent ACR fibers were observed in methamphetamine/tobacco- and tobacco-exposed girls, possibly from increased dendritic branching or spine density due to epigenetic influences. Persistently lower diffusivity in the thalamus and internal capsule of all tobacco-exposed infants suggests aberrant axonal development. Collectively, prenatal methamphetamine and/or tobacco exposure may lead to delayed motor development and white matter maturation in sex- and regional-specific manners.
Methamphetamine accounts for 96% of amphetamine-type stimulants, the second-most commonly abused category of illicit drugs worldwide.1 In the United States, the prevalence of pregnant women seeking treatment for methamphetamine use disorder increased from 8% to 24% between 1994 and 2006, while treatment for cocaine, alcohol, and tobacco declined.2 This trend will likely continue owing to the steady incidence of methamphetamine use between 2002 and 20133 and because twice as many women of childbearing age sought treatment for methamphetamine use disorder compared with men.4 Although 70% to 90% of methamphetamine users smoke tobacco cigarettes concurrently,5 the effect of comorbid methamphetamine and tobacco use during pregnancy on fetal brain development is rarely studied.
Methamphetamine increases synaptic dopamine,6 which may alter dopamine D1 receptors that regulate the cell cycle during corticogenesis.7 Both stimulants (methamphetamine and nicotine) also influence the developing catecholamine and cholinergic systems.8 Furthermore, rodent studies consistently demonstrate neurotoxic effects of prenatal methamphetamine or nicotine exposure, including oxidative DNA damage to fetal brain development,9 poorer motor development,10 and altered learning and memory.11 Prenatal stimulant-induced alterations in behavior and neuronal structure are often sex specific and were linked to epigenetic modifications in mouse brains, showing DNA-methylation changes with methamphetamine exposure12 and altered histone methylation with nicotine exposure.13
Human adult methamphetamine users showed larger basal ganglia,14,15 lower neuronal but higher glial metabolites,16 and higher white matter mean diffusivity than nonusers.17 Conversely, children exposed prenatally to methamphetamine showed smaller subcortical structures, including basal ganglia,18,19 and deficits on functional magnetic resonance imaging during visual attention and working memory tasks.20,21 Young children with prenatal methamphetamine and/or tobacco exposure had sex-specific alterations in brain metabolites in the frontal white matter and thalamus22,23 and lower white matter diffusivity on diffusion tensor imaging (DTI).24,25
Microstructural brain maturation can be assessed non-invasively with DTI,26 showing the greatest increases in fractional anisotropy (FA) and decreases in mean diffusivity during the first year of life.27 Mice prenatally exposed to nicotine showed increased FA and spine density on cortical neurons.13 However, whether microstructural abnormalities are present in human neonates with prenatal stimulant exposure is unknown. Therefore, we prospectively evaluated early developmental trajectories of major white matter tracts and subcortical structures in neonates with and without prenatal tobacco or methamphetamine/tobacco exposure. Studying infants minimizes potential childhood environmental influences on brain development; therefore, the findings can be attributed primarily to prenatal exposure.28 Based on prior studies,10,22-25 we expected that, compared with unexposed infants, stimulant-exposed infants would show persistently lower diffusivity in brain structures of interest and delayed quantitative motor development. In addition, since prenatal methamphetamine or nicotine exposure causes sex-specific alterations in myelination29 and myelin gene expression in animals30 and white matter metabolite abnormalities in exposed children,22,23 we expected to identify sex-specific alterations of white matter developmental trajectories in stimulant-exposed neonates.
Methods
Research Participants
A total of 752 infant-mother dyads were recruited from the local community by flyers or word of mouth and were pre-screened by telephone (between September 17, 2008, and February 28, 2015); 195 were screened for enrollment. A physician evaluated all participants to ensure they fulfilled study criteria31 (eAppendix in the Supplement). Fifty-six infants were excluded owing to either excessive maternal alcohol use (>3 drinks/mo during pregnancy [n = 17]), maternal polysubstance (n = 2) or cocaine dependency (n = 1), human immunodeficiency virus-infected mother receiving zidovudine (n = 1), prolonged (>1 week) neonatal intensive care (n = 6), incorrect magnetic resonance imaging parameters (n = 16), or incomplete (n = 6) or unusable (n = 9) DTI scans. Some infants had more than 1 reason for exclusion. Of the remaining infants, 68 had stimulant exposure (36 methamphetamine/tobacco exposed, 32 tobacco exposed), and 71 were unexposed infants serving as controls. Each mother-infant pair completed up to 3 evaluations when infants were aged approximately 1 week, 1 to 2 months, and 2 to 4 months. All infants were evaluated with a structured examination, including the Amiel-Tison Neurological Assessment at Term (ATNAT). The sections and score ranges (highest scores indicate poorer performance) for the ATNAT include neurosensory function (0-8), passive muscle tone (0-14), active muscle tone (0-19), primitive reflexes (0-8), deep tendon reflexes (0-6), cranial assessment (0-6), adaptiveness to manipulation (0-2), and sum of all neurologic examinations (0-59); score ranges varied by postmen-strual age (PMA).32 Birth records were also reviewed. The study was approved by the University of Hawaii Committee on Human Studies. The infants’ parents or legal guardians provided written and verbal informed consent and received financial compensation for their participation.
Assessments of Maternal Characteristics
Mothers completed the (1) Substance Abuse Subtle Screening Inventory (SASSI) to estimate the probability of having a substance dependency disorder (categories and score ranges: face value alcohol total, 0-36 [least to most used], face value other drugs total, 0-42 [least to most used], defensiveness, 0-11 [least to most defensive], and random answering pattern, 0-6 [>2 suggest data are invalid])33; (2) Beck Depression Inventory II (BDI-II) (score range, 0-63; ≤13 indicates minimal depression)34; (3) Edinburgh Postnatal Depression Scale, which identified mothers at risk for perinatal or postnatal depression (score range, 0-30, ≥10 indicates possible depression)35; (4) Symptom Checklist-90-Revised, which assessed 9 domains of psychopathology (T scores of 30 [2 SD] indicate below-average and 80 [3 SD] indicate above-average levels of psychopathology)36; and (5) Hollingshead Two-Factor Index of Social Position (ISP) (score range, 11-77, indicating highest to lowest socioeconomic status),37 which quantified the primary caregivers’ socioeconomic status. A study physician (L.C. or D.A.) performed a structured interview of each mother for a detailed list and amounts of potential substances or medications used during the pregnancy for each trimester.
Imaging Acquisition and Processing
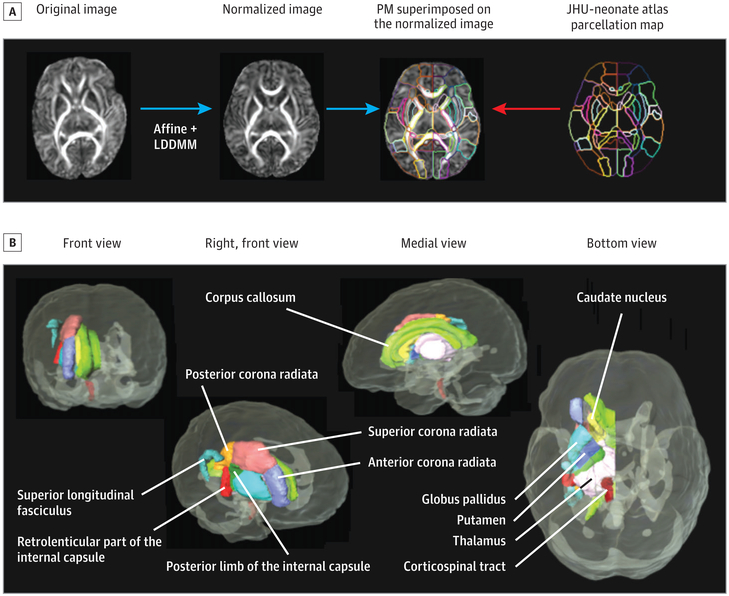
All infants were sleeping naturally without sedation and were visually monitored during scans. Magnetic resonance imaging was performed with a 12-channel head coil (Trio TIM 3.0T; Siemens). The protocol included a sagittal, 3-dimensional, magnetization-prepared, rapid-acquisition gradient-echo sequence (repetition time [TR]/inversion time, 3200/1400 milliseconds; echo time [TE], 4.47 milliseconds, version B15; TE, 4.15 milliseconds, version B 17), a T2-weighted scan (to exclude lesions), and DTI (single-shot, spin-echo, echo-planar; 12 noncollinear diffusion directions; b = 1000 s/mm2; 44 axial sections; 2.5-mm thickness; 2 averages; 2-mm in-plane resolution; TR/TE, 9500/90 milliseconds). All images were visually reviewed for structural abnormalities, excess movement, or other artifacts immediately following image reconstruction by experienced research staff. Scans with excess motion were repeated, provided the infants remained asleep or when they returned for another scanning session. Diffusion-weighted images were coregistered to one of the minimally diffusion-weighted images using a linear transformation of automated image registration. From these images, 6 elements of the diffusion tensor were calculated for each pixel with multivariate linear fitting using DtiStudio, version 2.03.38-40 The automated outlier rejection function41 of DtiStudio eliminated sections with relative fitting errors of >3%. An experienced neurologist (K.O.) performed secondary quality control by visually inspecting color-coded orientation maps calculated from the tensor field. The quality control-passed tensor fields were transformed to the Johns Hopkins University (JHU)-neonate DTI atlas42,43 using dual-channel, large deformation diffeomorphic metric mapping.44,45 Mean diffusivity (average of tensor eigenvalues), axial diffusivity (first eigenvalue), radial diffusivity (second and third eigenvalues averaged), and FA were calculated from each tensor field and transformed into atlas space (Figure 1A). Mean FA and diffusivity values (left and right averaged) for 7 major fiber tracts and 4 subcortical regions were extracted using the anatomical parcellation map of the JHU-neonate atlas (Figure 1B). These regions were selected based on their rapid growth during infancy.
Figure 1. Automated Atlas-Based Analyses for Diffusion Tensor Imaging (DTI) From Infants.
A, The steps involved in matching the template to the final atlas are illustrated. After the affine transformation, the large deformation diffeomorphic metric mapping (LDDMM)44,45 was performed to match the new DTI to the neonatal atlas developed for infants,42,43 which is available at http://www.mristudio.org. The atlas parcellation map (PM) automatically segmented 122 brain regions, yielding diffusivity (mean, radial, and axial) and fractional anisotropy in each region. (Modified with permission from Deshpande et al.43) B, Seven major fiber tracts and 4 subcortical regions were selected for the current analyses; the superior longitudinal fasciculus was not included owing to its slow development at this age. JHU indicates Johns Hopkins University.
Of 325 DTI scans attempted from 139 infants, 102 scans (31.4%) were excluded because of infants’ inability to remain asleep, excessive head motion, or different scanning parameters during an optimization phase. Forty-eight infants required 62 repeat sessions. A total of 223 DTI scans from 109 infants (32 methamphetamine/tobacco exposed, 30 tobacco exposed, and 47 unexposed controls) passed final quality assurance; 109 infants completed 1 time point, 72 completed 2 time points, and 44 completed 3 time points.
Statistical Analysis
Demographic variables were compared across groups using 1-way analysis of variance or a χ2 test. Group effects and group × age interactions on longitudinal FA and diffusivity measures were performed using mixed models with a random intercept and unstructured covariance matrix. Because plots of FA and diffusivity against PMA were curvilinear, a quadratic model was applied with mean centering of PMA (without age-squared interactions). Demographic variables showing group differences (sex, ISP, BDI, maternal weight gain, and maternal alcohol and marijuana use during pregnancy) were included as covariates in the mixed models but were retained for final models only if they showed significant effects. Missing covariates (1 each for BDI and weight gain) were imputed for covariate analyses. Sex differences on ATNAT scores and DTI metrics were examined using 3-way analysis of covariance (ANCOVA) (group × age × sex interactions). For each DTI metric, a Bonferroni correction for multiple (n = 10) regions was applied; ie, P < .05/10 (double-sided) were considered statistically significant, whereas P < .05 were considered trends for significance. Statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc).
Results
Neonatal Characteristics
The 3 infant groups had similar gestational age, racial and ethnic background, delivery method, Apgar score, birth head circumference, birth weight, and body mass index (Table 1). The mean (SE) PMA at baseline was 41.5 (0.27) weeks. However, tobacco-exposed infants had shorter mean (SE) birth length than did methamphetamine/tobacco-exposed infants (47.99 [1.07] vs 51.23 [0.42]; P = .003). The unexposed group had more girls than boys (44 [62%] vs 27 [38%]), whereas the tobacco-exposed group had more boys than girls (21 [65.6%] vs 11 [34.4%]) (P = .03; χ2 test), but the proportions were similar in the final groups (unexposed: 29 girls, 18 boys; methamphetamine/tobacco exposed: 14 girls, 18 boys; tobacco exposed: 11 girls, 19 boys; P = .07; χ2 test).
Table 1.
Clinical Characteristics of the Infants
| Characteristic | Unexposed (n = 71) |
Tobacco Exposed (n = 32) |
Methamphetamine/ Tobacco Exposed (n = 36) |
P Valuea |
|---|---|---|---|---|
| Characteristics of Infants at Birth | ||||
| Sex, No. (%) | ||||
| Female | 44 (62.0) | 11 (34.4) | 17 (47.2) | .03 |
| Male | 27 (38.0) | 21 (65.6) | 19 (52.8) | |
| Race, No. (%) | ||||
| Asian | 12 (16.9) | 3 (9.4) | 3 (8.3) | .18 |
| Native Hawaiian/other Pacific Islander | 43 (60.6) | 21 (65.6) | 27 (75) | |
| White | 15 (21.1) | 6 (18.8) | 3 (8.3) | |
| Black or African American | 1 (1.4) | 1 (3.1) | 3 (8.3) | |
| American Indian or Alaska Native | 0 | 1 (3.1) | 0 | |
| Ethnicity, No. (%) | ||||
| Hispanic | 15 (21.1) | 11 (34.4) | 15 (41.7) | .07 |
| Non-Hispanic (%) | 56 (78.9) | 21 (65.6) | 21 (58.3) | |
| Delivery method, No. (%) | ||||
| Cesarean | 17 (23.9) | 13 (40.6) | 6 (16.7) | .07 |
| Vaginal | 45 (63.4) | 12 (37.5) | 22 (61.1) | |
| Unknown | 9 (12.7) | 7 (21.9) | 8 (22.2) | |
| Gestational age, mean (SE), wk | 38.39 (0.36) | 37.47 (0.69) | 39.04 (0.28) | .10 |
| Weight, mean (SE), kg | 3.19 (0.08) | 2.92 (0.17) | 3.22 (0.07) | .15 |
| Length, mean (SE), cm | 49.78 (0.52) | 47.99 (1.07) | 51.23 (0.42) | .01 |
| BMI, mean (SE) | 12.61 (0.21) | 12.15 (0.41) | 12.23 (0.21) | .38 |
| Head circumference, mean (SE), cm | 33.90 (0.36) | 32.85 (0.74) | 34.07 (0.22) | .19 |
| Apgar score, mean (SE)b | ||||
| 1 min | 7.67 (0.16) | 7.04 (0.36) | 7.88 (0.19) | .06 |
| 5 min | 8.81 (0.08) | 8.74 (0.11) | 8.91 (0.05) | .48 |
| Characteristics of Infants at Baseline Imaging | ||||
| Postmenstrual age, mean (SE), wk | 40.56 (0.19) | 41.38 (0.56) | 43.44 (0.77) | <.001 |
| Weight, mean (SE), kg | 3.50 (0.06) | 3.74 (0.18) | 4.10 (0.19) | .003 |
| Length, mean (SE), cm | 51.54 (0.30) | 51.69 (0.88) | 53.48 (0.70) | .03 |
| Head circumference, mean (SE), cm | 35.37 (0.18) | 35.67 (0.37) | 36.51 (0.47) | .03 |
| Amiel-Tison Neurological Assessment at Term Agec | ||||
| Baseline | (n = 55) | (n = 30) | (n = 34) | |
| Postmenstrual age, mean (SE), wk | 41.23 (0.33) | 41.40 (0.60) | 43.74 (0.80) | .002 |
| Neurosensory function, mean (SE) | 0.35 (0.08) | 0.14 (0.11) | 0.14 (0.11) | .17 |
| Passive muscle tone, mean (SE) | 0.11 (0.04) | 0.01 (0.05) | 0.06 (0.05) | .33 |
| Active muscle tone, mean (SE) | 3.10 (0.23) | 3.03 (0.31) | 4.34 (0.30) | .002 |
| Primitive reflexes, mean (SE) | 0.09 (0.04) | 0.03 (0.06) | 0.09 (0.05) | .68 |
| Deep tendon reflexes, mean (SE) | 0 (0) | 0 (0) | 0 (0) | NA |
| Cranial assessment, mean (SE) | 0 (0) | 0 (0) | 0 (0) | NA |
| Adaptiveness to manipulation, mean (SE) | 0 (0.02) | 0.06 (0.03) | 0.08 (0.03) | .07 |
| Sum of all neurologic examinations, mean (SE) | 3.64 (0.25) | 3.28 (0.34) | 4.70 (0.33) | .009 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Determined using χ2, 1-way analysis of covariance, or analysis of variance, with P values and means (covaried for postmenstrual age) determined from baseline scores; higher scores indicate poorer performance (Figure 2A provides longitudinal data).
Activity, pulse, grimace, appearance, and respiration evaluated.
Highest scores indicate poorer performance: neurosensory function (0-8), passive muscletone (0-14), active muscle tone (0-19), primitive reflexes (0-8), deep tendon reflexes (0-6), cranial assessment (0-6), adaptiveness to manipulation (0-2), and sum of all neurologic examinations (0-59).32
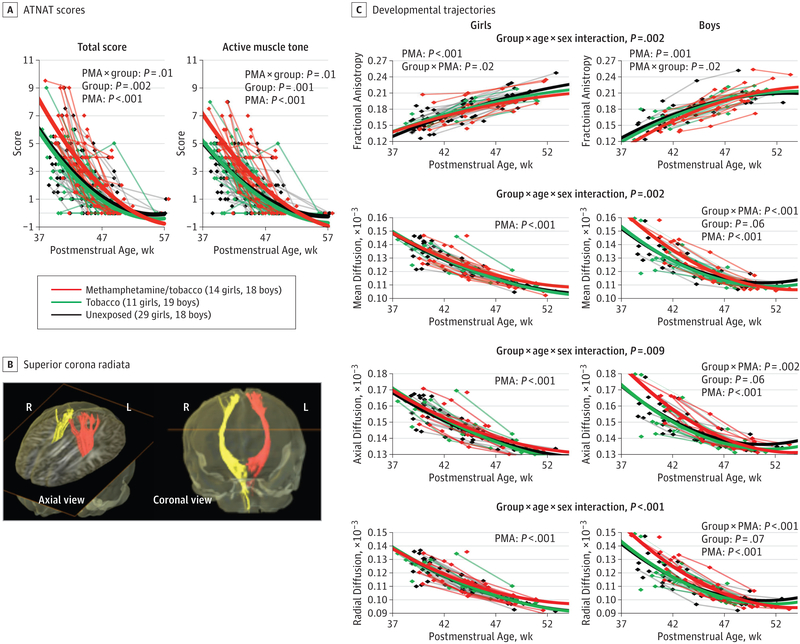
At baseline imaging, methamphetamine/tobacco-exposed infants were older (mean [SE], 43.44 [0.77] weeks PMA) compared with the tobacco-exposed (41.38 [0.56]) and unexposed (40.56 [0.19]) groups (P < .001) and hence had greater weight, length, and head circumference but showed poorer active muscle tone and total scores on the ATNAT that normalized by 3 to 4 months PMA (Figure 2A).
Figure 2. Developmental Trajectories of the Amiel-Tison Neurological Assessment at Term (ATNAT) and Diffusion Tensor Imaging Metrics in Superior Corona Radiata.
A, ATNAT showing delayed active muscle tone and total scores in methamphetamine/tobacco-exposed infants compared with the other groups. B, Fiber tracts in bilateral superior corona radiata from a 1-month-old infant are shown in the axial and coronal views. C, Fractional anisotropy (FA) increases with age while diffusivities decrease with age in all groups. However, the developmental trajectory in the FA of methamphetamine/tobacco-exposed boys showed a slightly steeper trajectory than the other 2 groups at an earlier age but normalized at later postmenstrual age (PMA), with no group differences in the age-dependent changes among the female infants. Similarly, diffusivities in both the axial and radial directions, and hence mean diffusivity values, were also higher at baseline in the methamphetamine/tobacco-exposed boys compared with the other 2 groups of boys, but no group differences in diffusivities were observed in either direction in the girls. L indicates left; R, right.
Maternal Characteristics
Mothers in the 3 groups had similar age at the infants’ birth and similar head circumferences and self-reported body mass index at baseline (Table 2). However, methamphetamine/tobacco group mothers gained more weight during pregnancy than women in the other 2 groups (mean [SE], 21.46 [1.84] vs 11.53 [1.74] and 13.74 [0.79] kg in the tobacco-exposed and unexposed groups, respectively; P < .001). Among groups, methamphetamine/tobacco group mothers had the lowest educational level and socioeconomic status. All infants lived with at least 1 biological parent.
Table 2.
Clinical Characteristics of the Biological Parents
| Mean (SE) |
||||
|---|---|---|---|---|
| Characteristic | Unexposed (n = 71) |
Tobacco Exposed (n = 32) |
Methamphetamine/ Tobacco Exposed (n = 36) |
P Value |
| Mother’s age at delivery, y | 28.89 (0.68) | 27.50 (0.91) | 28.36 (1.12) | .55 |
| Mother’s pregnancy weight gain, kg | 13.74 (0.79) | 11.53 (1.74) | 21.46 (1.84) | <.001 |
| Mother’s head circumference, cm | 56.57 (0.34) | 57.12 (0.44) | 56.97 (0.35) | .56 |
| Mother’s BMI by self-report | 30.51 (0.77) | 31.89 (1.40) | 29.22 (0.87) | .26 |
| Father’s BMI by self-report | 30.34 (0.72) | 29.08 (1.07) | 29.21 (1.10) | .53 |
| Mother’s educational level, y | 14.28 (0.30) | 12.50 (0.30) | 11.71 (0.32) | <.001 |
| Socioeconomic statusa | 40.89 (1.74) | 53.28 (2.43) | 64.39 (0.83) | <.001 |
| Maternal substance use during pregnancyb | ||||
| Mothers with any methamphetamine use, No. (%) | 0 | 0 | 36 (100) | <.0001 |
| Trimesters with methamphetamine use | 0 | 0 | 1.81 (0.15) | <.001 |
| Total methamphetamine used during pregnancy, g | 0 | 0 | 96.56 (18.95) | <.001 |
| Mothers with any tobacco use, No. (%) | 0 | 32 (100) | 35 (97.2) | <.001 |
| No. of trimesters with tobacco exposure | 0 | 1.78 (0.16) | 2.28 (0.15) | <.001 |
| Total No. of cigarettes smoked during pregnancy | 0 | 1364 (341) | 2717 (407) | <.001 |
| Mothers with any alcohol use, No. (%) | 13 (18.3) | 10 (31.3) | 15 (41.7) | .03 |
| No. of trimesters with alcohol exposure | 0.27 (0.07) | 0.34 (0.09) | 0.56 (0.13) | .11 |
| Total No. of drinks during pregnancy | 0.83 (0.38) | 2.78 (0.96) | 7.92 (2.94) | .002 |
| Mothers with any marijuana use, No. (%) | 1 (1.4) | 7 (21.9) | 15 (41.7) | <.001 |
| No. of trimesters with marijuana exposure | 0.01 (0.01) | 0.31 (0.11) | 0.78 (0.18) | <.001 |
| Total No.of marijuana cigarettes smoked during pregnancy | 0.03 (0.03) | 32.41 (18.84) | 36.71 (31.97) | .19 |
| Mothers’ depressive symptoms at the infants’ baseline imaging | ||||
| Edinburgh Postnatal Depression Scalec | 5.44 (0.68) | 7.40 (0.93) | 7.85 (1.07) | .09 |
| Beck Depression Inventory-IId | 7.48 (0.91) | 9.91 (1.40) | 11.94 (1.71) | .04 |
| Symptom Checklist-90-Revisede | ||||
| Depression T score | 52.20 (1.28) | 52.77 (1.69) | 53.94 (2.01) | .73 |
| Globalseverity index T score | 50.08 (1.44) | 51.81 (2.04) | 54.26 (2.42) | .27 |
| Positive symptom distress index T score | 52.42 (1.36) | 51.39 (1.78) | 55.97 (1.92) | .18 |
| Positive symptom total T score | 49.39 (1.46) | 50.74 (2.21) | 52.57 (2.24) | .47 |
| Substance Abuse Subtle Screening Inventoryf | ||||
| Probability of moderate to severe substance use disorder, No. (%) | ||||
| High | 15 (21.4) | 8 (25.8) | 35 (97.2) | <.001 |
| Low | 55 (78.6) | 23 (74.2) | 1 (2.8) | |
| Face value alcohol total | 4.67 (0.64) | 5.81 (0.81) | 11.37 (1.72) | <.001 |
| Face value other drugs total | 3.91 (1.03) | 7.48 (1.47) | 29.20 (1.56) | <.001 |
| Defensiveness score | 6.63 (0.26) | 4.94 (0.34) | 4.03 (0.41) | <.001 |
| Random answering pattern score | 0.29 (0.06) | 0.39 (0.17) | 0.28 (0.09) | .76 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Based on self-report from the Hollingshead Two-Factor Index of Social Position; score range, 11 to 77, indicating highest to lowest socioeconomic status.37
Six mothers also had prescription drug use during pregnancy. Unexposed mothers: sertraline in the first trimester (n = 1), clonazepam and methadone (n = 1), and lamotrigine and duloxetine (n = 1); tobacco group mothers: acetaminophen/hydrocodone combination (n = 1), paroxetine in the first 2 to 3 weeks of pregnancy (n = 1), and risperidone and gabapentin in the first 2 months (n = 1).
Score range, 0 to 30; 10 or higher indicates possible depression.35
Score range, 0 to 63; 13 or lower indicates minimal depression.34
T scores less than 30 (2 SDs) indicate below-average and greater than 80 (3 SDs) indicate above-average levels of psychopathology.36
Face value alcohol total, 0 to 36 (least to most used); face value other drugs total, 0 to 42 (least to most used); defensiveness, 0 to 11 (least to most defensive); and random answering pattern, 0 to 6 (>2 suggest data are invalid).33
Methamphetamine/tobacco group mothers used methamphetamine variably (mean [SE] total, 96.6 [18.9] g; median: 47 g; range, 0.15-388 g) during pregnancy. Although none of the methamphetamine/tobacco group mothers met DSM-5 criteria for moderate or severe use of substances other than methamphetamine or tobacco, they drank more alcohol and tended to smoke more marijuana during pregnancy than women in the other 2 groups. Compared with tobacco group mothers, methamphetamine/tobacco group mothers smoked twice the number of cigarettes and more continued tobacco use (19 of 36 [52.8%] vs 10 of 32 [31.2%]; P = .07, χ2 test], but only 7 of 36 (19.4%) continued methamphetamine use through more than two-thirds of the third trimester.
Although methamphetamine/tobacco group mothers had higher BDI-II scores than the unexposed and tobacco groups (11.94 [1.71] vs 7.48 [0.91] and 9.91 [1.40]; P = .04), their scores indicated minimal depressive symptoms. Similarly, the 3 groups showed no significant psychopathological symptoms on Edinburgh Postnatal Depression Scale and Symptom Checklist-90-Revised. On the SASSI across groups, methamphetamine/tobacco group mothers more commonly had a high probability of moderate to severe substance use disorder as well as higher self-reported alcohol total and other drugs total, but both stimulant groups had lower defensiveness scores (Table 2).
DTI Findings
In the superior corona radiata (SCR), FA increased with age and diffusivities decreased with age in all groups (Figure 2B and C). The trajectories of FA were lower in the methamphetamine/tobacco-exposed boys than in the other 2 groups at earlier PMA but normalized at later PMA; conversely, girls showed no group differences in SCR FA trajectories (age-dependent changes: group × age × sex interaction; P = .002) (Figure 2C, top graphs). Similarly, diffusivity measures were higher in the methamphetamine/tobacco-exposed boys at baseline but had steeper declines compared with the other 2 male infant groups; however, the girls showed no group difference in diffusivities in either direction (mean diffusivity: group × age × sex interaction, P = .002; axial diffusivity: group × age × sex interaction, P = .009; radial diffusivity: group × age × sex interaction, P < .001) (Figure 2C, bottom graphs).
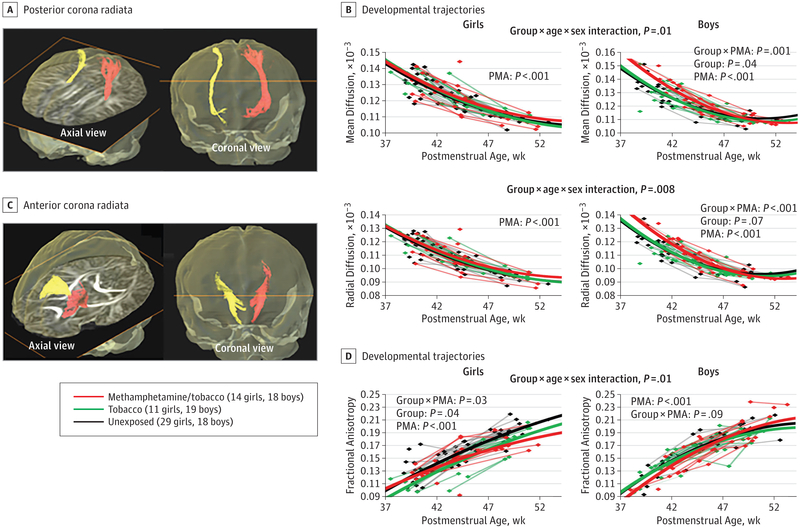
Across groups, FA in the anterior corona radiata (ACR) and posterior corona radiata (PCR) increased (P < .001) and diffusivities decreased with age (P < .001). In the PCR (Figure 3A and B), the trajectories of mean and radial diffusivity in methamphetamine/tobacco-exposed boys started higher and declined steeper compared to the other 2 groups, but these trajectories were not different across female groups (mean diffusivity: group × age × sex interaction, P = .01; radial diffusivity: group × age × sex interaction, P = .008). Methamphetamine/tobacco-exposed boys also had higher mean PCR diffusivity than the unexposed group (post hoc ANCOVA, P = .05), mostly due to higher radial diffusivity (post hoc ANCOVA, P = .05).
Figure 3. Developmental Trajectories of Diffusivities and Fractional Anisotropy (FA) in Anterior Corona Radiata (ACR) and Posterior Corona Radiata (PCR) Across Groups.
Diffusivities decreased with age and FA increased with age across all groups in both fiber tracts. A, Fiber tracts in the PCR from a 1-month-old infant are shown in the axial and coronal views. B, In the PCR, the developmental trajectories of both the mean diffusivity and radial diffusivity in the methamphetamine/tobacco-exposed boys declined slower than those in the tobacco-exposed and unexposed boys, but the age-dependent changes in diffusivities are not significantly different across the groups in the girls. The male methamphetamine/tobacco-exposed infants also had higher mean and radial diffusivities than did male unexposed infants in the PCR (post hoc analysis of covariance, P = .05 for both measures). C, Fiber tracts in the ACR from a 1-month-old infant are shown in the axial and coronal views. D, In the ACR, the developmental trajectories of the FA in the 2 female stimulant-exposed groups remained lower than the FA in the unexposed girls across the age span, but the age-dependent changes in FA were not significantly different across the groups in the boys. PMA indicates postmenstrual age.
In the ACR (Figure 3C and D), FA in female stimulant-exposed groups was lower compared with FA in unexposed girls (post hoc ANCOVA, covarying for PMA: methamphetamine/tobacco vs unexposed, P = .06; tobacco vs unexposed, P = .03), whereas age-dependent FA changes were not different across male groups (group × age × sex interaction, P = .01). In addition, stimulant-exposed girls, but not boys, tended to have slower development than did the unexposed infants regarding radial ACR diffusivity (group × age × sex interaction, P = .07).
Independent of sex, the developmental trajectories of the thalamus and internal capsule (posterior limb of the internal capsule and retrolenticular part of the internal capsule) differed across groups (eFigure 1A-F in the Supplement). In the posterior limb internal capsule, tobacco-exposed infants tended to show lower mean diffusivity (group, P = .06), mostly due to lower axial diffusivity (P = .02), compared with the other groups (eFigure 1B and C in the Supplement). In the retrolenticular internal capsule, the 2 stimulant-exposed groups showed altered age-dependent decreases in axial diffusivity compared with the unexposed group (eFigure 1E in the Supplement). Furthermore, tobacco-exposed infants had lower axial diffusivity in the thalamus compared with the other groups (post hoc: tobacco vs unexposed group, P = .009) (eFigure 1F in the Supplement).
Discussion
To our knowledge, this is the first study to demonstrate altered developmental trajectories of brain microstructure and abnormal active muscle tone in infants with prenatal tobacco or methamphetamine/tobacco exposure. Methamphetamine/tobacco-exposed boys showed lower FA and higher diffusivities in the SCR and PCR at baseline, but these measures normalized at later time points. In contrast, stimulant-exposed girls showed lower FA in ACR, and all tobacco-exposed infants showed lower axial diffusion in the thalamus and posterior limb internal capsule across time points. These brain abnormalities were likely due to prenatal stimulant exposure, possibly via epigenetic effects,12,13,46 genetic predisposition,47 or other prenatal factors not evaluated. Normalization of the motor examination, as well as SCR and PCR white matter trajectories, in methamphetamine/tobacco-exposed infants over the first 3 to 4 months suggests improved myelination after cessation of stimulant exposure.
Neonatal Physical and Neurologic Development
Unlike studies48,49 showing that prenatal methamphetamine exposure is associated with lower birth weight and higher incidence of being small for gestational age, we found no group differences in birth weight, which is consistent with a large study50 of pregnancy outcomes in methamphetamine-using women. These discrepancies may be attributable to differences in racial or ethnic distributions or participant criteria across studies. The present study enrolled primarily healthy, term-born infants and mothers without significant comorbid disorders. The shorter lengths despite similar birth weights in our tobacco-exposed infants compared with the unexposed infants may be a result of the higher proportion of boys than girls, which likely masked the well-documented fetal growth restriction due to tobacco exposure51,52 since boys usually weigh more than girls at birth.53
The normal physical examination and normal-appearing brain magnetic resonance imaging are consistent with results from the multicenter Infant Development, Environment, and Lifestyle (IDEAL) study, which found no increased incidence of congenital abnormalities54 or abnormal head sonograms55 in infants with prenatal methamphetamine exposure. However, on neurologic evaluation, our methamphetamine/tobacco group had delayed development on active muscle tone and total ATNAT scores, although these scores appeared to normalize at 3 to 4 months of age. These findings contrast with the poorer fine motor (grasping) scores at ages 1 and 3 years56 and poorer inhibitory control at school age57 in IDEAL children with high meconium methamphetamine metabolite concentrations. Another cohort of young methamphetamine-exposed children (aged 3-4 years) also showed poorer visual-motor integration, which correlated with lower glial metabolite myoinositol levels in the thalamus.22,23 Similarly, rat pups prenatally exposed to methamphetamine showed impaired development of postural motor movements on the rotarod test during the first 3 postnatal weeks.10 The normalization of motor scores at later time points in methamphetamine/tobacco-exposed infants may result from improved myelination (eg, in SCR and PCR) when infants are no longer exposed to stimulants. Follow-up evaluations are needed to evaluate their fine motor development.
Maternal Behaviors
While the 3 groups of mothers had similar weights at their baseline evaluations, methamphetamine/tobacco group mothers had greater weight gain during pregnancy than the other 2 groups. This finding suggests lower prepregnancy weights and possibly poorer nutrition during early pregnancy stages due to the stimulants’ powerful appetite-suppressant effects and subsequent excess weight gain during abstinence58 in the last trimester. Compared with tobacco group mothers, methamphetamine/tobacco group mothers smoked more tobacco cigarettes for more trimesters, which is consistent with greater addictive behaviors during active substance use5 and higher rates of tobacco use among Native Hawaiian women.59 The higher levels of nicotine exposure might have contributed to greater abnormalities on DTI in methamphetamine/tobacco-exposed compared with tobacco-exposed neonates. Furthermore, maternal factors that might contribute to these abnormal findings include higher probabilities of having moderate to severe substance use disorder and alcohol use on SASSI, higher BDI-II scores,60 and lower socioeconomic status61 or educational levels,62 which are all typical of methamphetamine users63 compared with non-drug users. However, none of these variables, except for greater stimulant use, contributed to the DTI abnormalities.
DTI Findings
Our infants had typical and rapid FA increases and diffusivity decreases during the first months of life due to ongoing myelination and brain growth.42,64 The lower baseline and faster increases of FA in the SCR of methamphetamine/tobacco-exposed boys, but not methamphetamine/tobacco-exposed girls, indicate less coherent fibers at birth, with delayed white matter maturation during the first 3 months of age. Higher mean diffusivities in SCR and PCR in methamphetamine/tobacco-exposed boys during the early weeks of life suggest lesser and delayed myelination in these tracts. This interpretation is consistent with the reduced myelin content in the optic nerves of rats with prenatal methamphetamine exposure,29,65 and smaller optic nerve diameters and areas in male–but not female–methamphetamine-exposed rats as early as postnatal day 7.65 Similarly, only male juvenile (postnatal day 21) rats with gestational nicotine exposure showed lesser myelin gene expression in the striatum, compared with saline-exposed controls.66
However, lower FA in the ACR of girls exposed to stimulants, particularly tobacco, suggests that axons are less coherent in this tract, perhaps attributable to greater dendritic branching and spine densities67 as well as delayed myelination and deformed axons, as observed in rodents with prenatal tobacco or methamphetamine exposure.29,65 Altered glial or neuronal metabolites were also observed primarily in young girls with prenatal tobacco23 or methamphetamine exposure.22 Similarly, reduced expression of myelin genes was found in periadolescent (postnatal days 35-36) female rats with gestational nicotine exposure.30 Together, these findings suggest that prenatal stimulant exposure might lead to epigenetic effects, with reduced myelin gene expression and less mature white matter development in the ACR of girls exposed to stimulants (especially tobacco). Unlike prior studies68,69 that suggest nicotine’s neuroprotective effects on methamphetamine-induced neurotoxic effects in adults, our methamphetamine/tobacco-exposed infants had greater white matter abnormalities.
Furthermore, persistently lower axial diffusivity in the thalamus and posterior limb internal capsule of tobacco-exposed infants might have resulted from reduced myelination between compacted axons or from increased dendritic branching and spine densities, as observed in young adult mice with these long-lasting alterations, along with epigenetic changes (upregulation of histone methylation complexes) after prenatal nicotine exposure.13,67 In contrast, in the retrolenticular internal capsule, altered age-dependent changes in axial diffusivity, which are higher at baseline but lower at 4 months PMA in the stimulant-exposed groups compared with the unexposed group, suggest less mature development, possibly with less myelination initially followed by aberrant dendritic branching later.67
Limitations
This study has several limitations. First, because not all of the infants completed their follow-up scans, the developmental trajectories may be skewed at later time points; future studies with larger sample sizes and complete follow-up visits are needed to validate these findings. Second, despite strict inclusion criteria, mothers who used methamphetamine/tobacco also likely had more unstable social circumstances70 and stress, which may contribute to epigenetic reprogramming of fetal brain development.71 Third, the potential neurotoxic effects of stimulants might influence only subregions of white matter tracts and subcortical structures and lead to smaller effect sizes. Fourth, because we excluded mothers with clinical depression, which would be common among methamphetamine users during abstinence, our findings cannot be generalized to infants whose mothers additionally had depression during pregnancy or post partum.
Conclusions
The altered white matter developmental trajectories, which are often sex specific, in several major white matter tracts of infants with prenatal stimulant exposure may be due to epigenetic influences that lead to sex-specific delayed or arrested myelination, or aberrant neuronal growth, as observed in preclinical studies. However, in some fiber tracts, these effects on myelination may normalize when stimulant exposure ceases postnatally, as seen in methamphetamine/tobacco-exposed boys.
Supplementary Material
Key Points.
Question Do infants with prenatal methamphetamine and/or tobacco exposure show brain abnormalities?
Findings In this case-control study of 139 neonates, methamphetamine- and tobacco-exposed infants showed delayed developmental trajectories on active muscle tone, and the exposed boys also had significantly delayed trajectories in superior and posterior corona radiatae that normalized by ages 3 to 4 months. However, persistently lower fractional anisotropy was found in anterior corona radiata of methamphetamine/tobacco- and tobacco-exposed girls as well as lower diffusion in the thalamus and internal capsule of all tobacco-exposed infants.
Meaning Prenatal methamphetamine/tobacco or tobacco exposure may lead to delayed motor development and white matter maturation in sex- and regional-specific manners.
Acknowledgments
Funding/Support: This work was supported in part by grant U54-NS56883 from the National Institute of Neurological Disorders and Stroke and National Institute on Drug Abuse; grants K24-DA16170-LC, K02-DA16991-TE, and K01-DA021203-CC from the National Institute on Drug Abuse; core resources with grant G12 MD003061-MB from the National Institute on Minority Health and Health Disparities; and grant DABK39-03-C-0060-LC&TE from the Office of National Drug Control Policy.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
Linda Chang, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Kenichi Oishi, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Jon Skranes, Department of Pediatrics, Sørlandet Hospital, Arendal, Norway, Department of Laboratory Medicine, Children’s and Women’s Health, Norwegian University of Science and Technology, Trondheim, Norway.
Steven Buchthal, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Eric Cunningham, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Robyn Yamakawa, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Sara Hayama, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Caroline S. Jiang, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Daniel Alicata, Department of Psychiatry, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Antonette Hernandez, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Christine Cloak, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Tricia Wright, Department of Obstetrics, Gynecology and Women’s Health, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
Thomas Ernst, Department of Medicine, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu.
REFERENCES
- 1.United Nations Office on Drugsand Crime. World Drug Report. Vienna, Austria: United National Office on Drugs and Crime; 2014. United Nations publication, sales No. E.14.XI.7. [Google Scholar]
- 2.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113(6):1285–1291. [DOI] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results From the 2014 National Survey on Drug Use and Health. Rockville, MD: Office of Applied Studies; September 2015. [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality. The TEDS Report: Gender Differences in Primary Substance of Abuse Across Age Groups. Rockville, MD: Center for Behavioral Health Statistics and Quality; April 3, 2014. [Google Scholar]
- 5.Weinberger AH, Sofuoglu M The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3(4):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Lidow MS. D1 dopamine receptor regulation of cell cycle in FGF- and EGF-supported primary cultures of embryonic cerebral cortical precursor cells. Int J Dev Neurosci. 2002;20(8):593–606. [DOI] [PubMed] [Google Scholar]
- 8.Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190(suppl 1):S8–S21. [DOI] [PubMed] [Google Scholar]
- 9.Jeng W, Wong AW, Ting-A-Kee R, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005;39(3):317–326. [DOI] [PubMed] [Google Scholar]
- 10.Slamberova R, Pometlova M, Charousová P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30 (1):82–88. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Bo L, Zhang P, et al. Exposure to nicotine during pregnancy and altered learning and memory in the rat offspring. Nicotine Tob Res. 2015;17(6): 661–666. [DOI] [PubMed] [Google Scholar]
- 12.Itzhak Y, Ergui I, Young JI. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol Psychiatry. 2015;20(2):232–239. [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, Hsieh LS, Lee AM, et al. An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nat Neurosci. 2016;19(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57 (9):967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162(8):1461–1472. [DOI] [PubMed] [Google Scholar]
- 16.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54(6):1344–1349. [DOI] [PubMed] [Google Scholar]
- 17.Alicata D, Chang L, Cloak C, Abe K, Ernst T. Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Res. 2009;174(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang L, Smith LM, LoPresti C, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132(2):95–106. [DOI] [PubMed] [Google Scholar]
- 19.Sowell ER, Leow AD, Bookheimer SY, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci. 2010;30(11):3876–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32(12):2453–2464. [DOI] [PubMed] [Google Scholar]
- 21.Roussotte FF, Bramen JE, Nunez SC, et al. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54(4): 3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Cloak C, Jiang CS, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48(2):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L, Cloak CC, Jiang CS, Hoo A, Hernandez AB, Ernst TM. Lower glial metabolite levels in brains of young children with prenatal nicotine exposure. J Neuroimmune Pharmacol. 2012;7(1):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72(24):2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos A, Kwiatkowski MA, Fouche JP, et al. White matter integrity and cognitive performance in children with prenatal methamphetamine exposure. Behav Brain Res. 2015;279:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17(12):2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng X, Gouttard S, Sharma A, et al. Quantitative tract-based white matter development from birth to age 2 years. Neuroimage. 2012;61(3):542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon U, Fahim C, Perusse D, Evans AC. Lateralized genetic and environmental influences on human brain morphology of 8-year-old twins. Neuroimage. 2010;53(3):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo P, Pinazo-Durán MD, Salgado-Borges J, Tavares MA. Correlation of axon size and myelin occupancy in rats prenatally exposed to methamphetamine. Brain Res. 2008;1222:61–68. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Wang J, Dwyer JB, et al. Gestational nicotine exposure modifies myelin gene expression in the brains of adolescent rats with sex differences. Transl Psychiatry. 2013;3:e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland D, Chang L, Ernst TM, et al. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 2014;71(10):1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosselin J, Gahagan S, Amiel-Tison C. The Amiel-Tison Neurological Assessment at Term: conceptual and methodological continuity in the course of follow-up. Ment Retard Dev Disabil Res Rev. 2005;11(1):34–51. [DOI] [PubMed] [Google Scholar]
- 33.Miller GA. The Substance Abuse Subtle Screening Inventory (SASSI) Second Edition Manual. Springfield, IN: SASSI Institute; 1999. [Google Scholar]
- 34.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996; 67(3):588–597. [DOI] [PubMed] [Google Scholar]
- 35.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 36.Derogatis LR. SCL-90-R: Administration, Scoring, and Procedures Manual II. Baltimore, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- 37.Adams J, Weakliem D, August B. Hollingshead’s “Four Factor Index of Social Status”: from unpublished paper to citation classic. Yale J Sociol. 2011;8:11–20. [Google Scholar]
- 38.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6, pt 1):1174–1185. [DOI] [PubMed] [Google Scholar]
- 39.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–116. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Shea SM, Lorenz CH, Jiang H, Chou MC, Mori S. Image corruption detection in diffusion tensor imaging for post-processing and real-time monitoring. PLoS One. 2013;8(10):e49764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 2011;56(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshpande R, Chang L, Oishi K. Construction and application of human neonatal DTI atlases. Front Neuroanat. 2015;9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ceritoglu C, Oishi K, Li X, et al. Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. Neuroimage. 2009;47 (2):618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oishi K, Faria A, Jiang H, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage. 2009;46(2): 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godino A, Jayanthi S, Cadet JL. Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics. 2015;10(7):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall FS, Drgonova J, Jain S, Uhl GR. Implications of genome wide association studies for addiction: are our a priori assumptions all wrong? Pharmacol Ther. 2013;140(3):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr 2003;24(1):17–23. [DOI] [PubMed] [Google Scholar]
- 49.Smith LM, LaGasse LL, Derauf C, et al. The Infant Development, Environment, and Lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149–1156. [DOI] [PubMed] [Google Scholar]
- 50.Wright TE, Schuetter R, Tellei J, Sauvage L. Methamphetamines and pregnancy outcomes. J Addict Med. 2015;9(2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cnattingius S The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl 2):S125–S140. [DOI] [PubMed] [Google Scholar]
- 52.Ko TJ, Tsai LY, Chu LC, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55(1):20–27. [DOI] [PubMed] [Google Scholar]
- 53.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah R, Diaz SD, Arria A, et al. Prenatal methamphetamine exposure and short-term maternal and infant medical outcomes. Am J Perinatol. 2012;29(5):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith LM, Diaz S, LaGasse LL, et al. Developmental and behavioral consequences of prenatal methamphetamine exposure: a review of the Infant Development, Environment, and Lifestyle (IDEAL) study. Neurotoxicol Teratol. 2015; 51:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith LM, LaGasse LL, Derauf C, et al. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 2011;33(1):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derauf C, Lagasse LL, Smith LM, et al. Prenatal methamphetamine exposure and inhibitory control among young school-age children. J Pediatr. 2012; 161(3):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. [DOI] [PubMed] [Google Scholar]
- 59.Wright TE, Tam E. Disparate rates of persistent smoking and drug use during pregnancy of women of Hawaiian ancestry. Ethn Dis. 2010;20(1)(suppl 1):S1–S215, 8. [PubMed] [Google Scholar]
- 60.Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry. 2015;(12):15. [DOI] [PubMed] [Google Scholar]
- 61.Johnson NF, Kim C, Gold BT. Socioeconomic status is positively correlated with frontal white matter integrity in aging. Age (Dordr). 2013;35(6): 2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noble KG, Korgaonkar MS, Grieve SM, Brickman AM.Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci. 2013;16(5):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wouldes TA, LaGasse LL, Derauf C, et al. Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug Alcohol Depend. 2013;127(1-3): 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu A, Fortier MV, Bai J, et al. Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. Neuroimage. 2013;65:315–323. [DOI] [PubMed] [Google Scholar]
- 65.Melo P, Moreno VZ, Vázquez SP, Pinazo-Durán MD, Tavares MA. Myelination changes in the rat optic nerve after prenatal exposure to methamphetamine. Brain Res. 2006;1106(1):21–29. [DOI] [PubMed] [Google Scholar]
- 66.Cao J, Dwyer JB, Gautier NM, Leslie FM, Li MD. Central myelin gene expression during postnatal development in rats exposed to nicotine gestationally. Neurosci Lett. 2013;553:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mychasiuk R, Muhammad A, Gibb R, Kolb B. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res. 2013;1499:53–60. [DOI] [PubMed] [Google Scholar]
- 68.Sung YH, Yurgelun-Todd DA, Kondo DG, et al. Gender differences in the effect of tobacco use on brain phosphocreatine levels in methamphetamine-dependent subjects. Am J Drug Alcohol Abuse. 2015;41(4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baladi MG, Nielsen SM, McIntosh JM, Hanson GR, Fleckenstein AE. Prior nicotine self-administration attenuates subsequent dopaminergic deficits of methamphetamine in rats: role of nicotinic acetylcholine receptors. Behav Pharmacol. 2016;27(5):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rommel N, Rohleder NH, Wagenpfeil S, Haertel-Petri R, Kesting MR Evaluation of methamphetamine-associated socioeconomic status and addictive behaviors, and their impact on oral health. Addict Behav. 2015;50:182–187. [DOI] [PubMed] [Google Scholar]
- 71.Bock J, Poeschel J, Schindler J, et al. Transgenerational sex-specific impact of preconception stress on the development of dendritic spines and dendritic length in the medial prefrontal cortex. Brain Struct Funct. 2016;221(2): 855–863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.