Abstract
Methamphetamine (Meth) use disorder continues to be highly prevalent worldwide. Meth users have higher impulsivity and brain abnormalities that may be different between current and past Meth users. The current study assessed impulsivity and depressive symptoms in 94 participants (27 current Meth users, 32 past Meth users and 35 non-drug user controls). Additionally, brain microstructure was assessed using diffusion tensor imaging (DTI); fractional anisotropy (FA) and mean diffusivity (MD) were assessed in the striatum, and FA, MD, radial and axial diffusivity were quantified in five white matter structures using DtiStudio.
Across the three subject groups, current users had the highest self-reported impulsivity scores, while both Meth user groups had larger striatal structures than the controls. Past Meth users had the highest FA and lowest MD in the striatum, which is likely due to greater magnetic susceptibility from higher iron content and greater dendritic spine density. In white matter tracts, current Meth users had higher AD than past users, indicating greater water diffusion along the axons, and suggesting inflammation with axonal swelling. In contrast, past users had the lowest AD, indicating more restricted diffusion, which might have resulted from reactive gliosis. Although current Meth users had greater impulsivity than past users, the brain microstructural abnormalities showed differences that may reflect different stages of neuroinflammation or iron-induced neurodegeneration. Combining current and past Meth users may lead to greater variability in studies of Meth users. Longitudinal studies are needed to further evaluate the relationship between recency of Meth use and brain microstructure.
Keywords: Methamphetamine, Impulsivity, DTI, Striatum, White matter
Introduction
Methamphetamine (Meth) is a highly addictive stimulant and accounts for more than 96 % of amphetamine-type stimulants, the second most commonly used category of illicit drugs (second only to cannabis) world-wide (UNODC, 2014). Synthetic drugs, including amphetamine-type stimulants, are also the second most prevalent drug of abuse after opiates in China (INCSR 2015). Meth use disorder often leads to many psychiatric, medical and socioeconomic problems (Courtney and Ray 2014). Furthermore, the neurotoxic effects of Meth may result in neuropsychological impairments (Scott et al. 2007) and various neurochemical (Ernst et al. 2000) and structural brain abnormalities (Chang et al. 2007).
Recent studies using diffusion tensor imaging (DTI) typically demonstrate microstructural brain abnormalities in chronic Meth users, particularly in white matter and subcortical gray matter structures (Chung et al. 2007; Alicata et al. 2009; Kim et al. 2009; Salo et al. 2009; Tobias et al. 2010; Lederer et al. 2016). However, one of the DTI studies found no group difference in striatal diffusivities between Meth users and controls (Lin et al. 2015). Since brain structural, neurochemical, as well as neuropsychological abnormalities in Meth users are at least partly reversible (Volkow et al. 2001a; Wang et al. 2004), these conflicting results may be due to differences in the recency of Meth use between these cohorts.
Therefore, to clarify if abnormalities in brain microstructure are present both during active usage and after prolonged abstinence, we studied three groups of participants: active Meth users, individuals with a history of Meth use disorder but who had been abstinent for more than a month, and nondrug user control subjects. We also evaluated impulsivity in our participants since this is a common trait in individuals with substance use disorders, and Meth use disorder specifically (Lee et al. 2009; Jentsch et al. 2014). Moreover, impulsivity may contribute to Meth addiction through continuation of use, treatment non-completion, and relapses (Winhusen et al. 2013). Based on prior DTI studies of Meth users and the partial recovery of brain abnormalities discussed above, we hypothesized that the alterations in diffusion measures in the white matter regions and striatal structures may differ between current and past Meth users. Since greater impulsivity may lead to a higher likelihood of continuation or reinstatement of Meth use, we also expected that current Meth users would show greater impulsivity than past users and non-drug user controls. Lastly, we explored the possible relationships between impulsivity and diffusivity measures that showed group abnormalities in the Meth users.
Methods
Participants
A total of 94 participants were enrolled in this study, including 59 with a history of Meth use disorder (32 past users and 27 current users) and 35 non-drug user controls. Subjects were recruited by online advertisement, flyers and word-of-mouth. Participants were all verbally informed of the study and signed a written consent form that was approved by the cooperative institutional review board of the University of Hawaii and the Queen’s Medical Center (CHS 17316). All participants were evaluated with detailed medical and drug use histories and underwent physical and neuropsychiatric examinations by trained research staff and physicians to ensure they fulfilled the study criteria. Inclusion criteria were: 1) Men or women aged 18–70 years and able to provide informed consent; 2) relatively normal cognitive function with Mini-Mental status examination score ≥ 25 and Clinical Dementia Rating ≤ 0.5; 3) all Meth users had a history of moderate to severe Meth use disorder fulfilling the DSM-5 criteria, had used Meth for more than three years and described Meth as the most used substance and “drug of choice”. The current users had used Meth within the past 30 days prior to assessments, while past Meth users were abstinent for at least one month.
All participants were ineligible if they fulfilled any of the following exclusion criteria: 1) any neurological or psychiatric disorder that might confound the outcome measures; 2) chronic severe medical condition; 3) medications that might confound analyses of the study; 4) pregnancy (confirmed by urine test); 5) positive urine toxicology for substances of abuse, including amphetamines (except for current Meth users), cocaine, Δ-9-tetrahydrocannabinol, benzodiazepines, and opiates; 6) any contraindications for MRI scans; 7) history of moderate or severe substance use disorders fulfilling the DSM-5 criteria (except for tobacco smoking and caffeine use, and Meth use in the Meth users) within a 20-year period of this study.
Psychiatric, Behavioral and Neuropsychological Assessments
Depressive symptoms were assessed with the Center for Epidemiological studies – depression scale (CES-D) (Radloff 1977), a 20 item self-reported screening for depressive symptoms within the past week in which each item is rated on a scale of 0 to 4. Impulsivity was assessed with the Barratt Impulsiveness Scale (BIS-11), a 30-item self-reported measure. Each item is rated on a scale of 1 (rarely/never) to 4 (almost always/always). A total score is obtained as a measure of general impulsivity. The BIS-11 scores for the individual responses were first grouped into eleven parcels, then into two dimensions, cognitive impulsivity and behavioral impulsivity, according to the two-factor model (Reise et al. 2013).
All subjects additionally were evaluated with a battery of neuropsychological tests that assessed the following domains: attention (Paced Auditory Serial Addition Task, WAIS III Digit span forward, WAIS III Letter-Number-Sequence), speed of information processing (Stroop Color, Trailmaking A) and executive function (Stroop Interference, Trailmaking B). Z-scores, adjusted for age and education, for each of these cognitive domains were generated for all participants using a normative database from 481 healthy participants who were administered the same tests in a standardized manner in the same laboratory.
Image Acquisition
All MR scans were performed on a 3 Tesla Siemens TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany). Structural MRI included 1) 3-plane localizer with TR/TE = 20/5 ms; FOV 280, 256 × 128 matrix; 2) sagittal high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence (TR/TE/TI = 2200/4.11/1000 ms, flip angle = 12 degrees, FOV 256, 256 × 256 matrix, 1 mm slices); and 3) transversal fluid-attenuated inversion recovery scan (FLAIR, TR/TE/TI = 9100/84/2500 ms, flip angle = 150, FOV 230, 256 × 256 matrix, 3 mm slices). All MR scans were reviewed by an experienced neurologist to ensure that there were no gross structural abnormalities.
Diffusion measures were acquired with diffusion tensor imaging (DTI), 12 directions, bmax = 1000s/mm2, 3700 ms repetition time, 88 ms TE, in-plane resolution 1.7 × 1.7 mm, 4 mm axial slices with 1 mm gap. Four averages with one additional b = 0 image each were acquired, for a total acquisition time of approximately 10 min.
Image Processing
The tensor field for each individual brain was calculated in DtiStudio (www.MriStudio.org) after motion correction (Jiang et al. 2006), and transformed to the JHU-MNI atlas space using dual-channel Large Deformation Diffeomorphic Metric Mapping (LDDMM) (Ceritoglu et al. 2009). The fractional anisotropy (FA), mean diffusivity (MD, mean of the three eigenvalues), axial diffusivity (AD, first eigenvalue), and the radial diffusivity (RD, mean of second and third eigenvalues) of each anatomical area defined in the JHU-MNI atlas were obtained as previously described (Oishi et al. 2009).
Based on previous findings of brain abnormalities in stimulant users (Chung et al. 2007; Alicata et al. 2009; Kim et al. 2009; Salo et al. 2009; Tobias et al. 2010; Roos et al. 2015), the following five white matter and subcortical structures were selected for analyses: anterior corona radiata, the genu of the corpus callosum, inferior fronto-occipital fasciculus, sagittal stratum and superior longitudinal fasciculus, and the striatal structures, which included the putamen, globus pallidus and caudate nucleus (caudate). Since two-way-ANCOVA of DTI measures revealed no main effects of hemisphere and no hemisphere-by-group status interactions, data from the two hemispheres were averaged for further statistical analyses.
The volumes of striatal structures (the caudate, putamen and the globus pallidus) were also obtained from the registered MP-RAGE images using MriCloud (braingps.anatomyworks.org), a fully-automated multi-atlas label-fusion method for anatomical segmentation (Tang et al. 2013; Djamanakova et al. 2014). The volumes of striatal structures were corrected for the total brain volume.
Statistical Analyses
Statistical Analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC). Analyses of variance (ANOVA), Chi-square, Fisher’s exact or Kruskal-Wallis tests were performed to assess group differences in demographic variables, CES-D score, cognitive domain Z-scores and co-morbid use of tobacco and marijuana. One-way analyses of covariance (ANCOVA) were performed to assess group differences in neuropsychological tests, self-reported impulsivity scores, DTI measures and ROI volumes. Age and sex were used as covariates for all analyses, and education was additionally included for the cognitive and BIS-11 measures. Holms-Bonferroni correction for multiple comparisons (Holm 1979) was applied for each DTI metric separately. P-values ≤0.05 that survived Holms-Bonferroni correction were considered statistically significant and p-values ≤0.05 that did not survive correction for multiple comparisons were considered trends. Relationships between group, drug use characteristics (total amount of Meth used, daily average of Meth used, age at first Meth use, duration of abstinence from Meth), and the DTI measures (FA, MD, AD and RD) or impulsivity scores were assessed, using two-way-ANCOVAs, co-varied for age, gender and education. Additionally, multivariate general linear models (mGLMs) were used to explore relationships between brain microstructure and impulsivity, with DTI metrics that showed group differences on one-way-ANCOVAs as independent variables and BIS-11 total score, cognitive impulsivity and behavioral impulsivity separately as dependent variables; education and CES-D were used as covariates, but not age and gender (which showed no significant main effects when added to the model). Additional two-way-ANCOVAs were used in a post-hoc fashion to further evaluate significant effects in the mGLM, also using education and CES-D as covariates. Relationships between continuous variables (DTI measures, volumes, impulsivity scores and clinical variables) were also assessed with Pearson correlations.
Results
Participant Characteristics (Table 1)
Table 1.
Subject demographics and substance usage characteristics (Mean ± S.E.M)
| Controls n = 34 |
Current Meth Users n = 27 |
Past Meth Users n = 32 |
p-valuea | |
|---|---|---|---|---|
| Age (years) | 42.2 ± 2.2 | 44.1 ± 2.1 | 41.8 ± 1.9 | 0.73 |
| Education (years) | 12.9 ± 0.3 | 12.9 ± 0.3 | 12.8 ± 0.3 | 0.92 |
| Gender (% men) | 62 | 56 | 63 | 0.84 |
| Race (% White/% Asian/% Pacific Islander/%Black/% American Indian/% more than 1 race) | 47/18/9/6/3/17 | 45/25/25/0/0/5 | 16/28/34/0/0/22 | 0.03 |
| Ethnicity (% Hispanic) | 9 | 10 | 16 | 0.69 |
| CES-D (score) | 8.09 ± 1.25 | 22.83 ± 3.01 | 12.88 ± 1.63 | <0.0001 |
| Methamphetamine usage | ||||
| Cumulative lifetime used (g) | - | 3452 ± 821 | 5317 ± 970 | 0.41 |
| Median: 2278 (313–12,898) | Median: 2735 (326–18,806) | |||
| Duration of use (years) | - | 17.5 ± 1.9 | 14.6 ± 1.5 | 0.24 |
| Meth used per day (g) | - | 0.64 ± 0.12 | 0.95 ± 0.13 | 0.11 |
| Median: 0.48 (0.11–2.0) | Median: 0.73 (0.18–3.33) | |||
| Age at first use (years) | 26.9 ± 2.1 | 23.6 ± 1.6 | 0.21 | |
| Primary route of use, n (%) (Inhaled/nasal/i.v.) | - | 25(93)/1(3.5)/1(3.5) | 30(94)/2(6)/0 | 0.78 |
| Duration of abstinence (months) | - | - | 35.9 ± 12.9 | - |
| Median: 6.5 (1.0–293.7) | ||||
| Other substances used Tobacco smokers | ||||
| Current smokers, n (%) | 8 (24) | 10 (37) | 10 (31) | 0.51 |
| Lifetime smokers, n (%) | 18 (53) | 15 (56) | 24 (75) | 0.14 |
| Total Tobacco pack years (all subjects) | 10.62 ± 2.7 | 8.35 ± 2.05 | 12.51 ± 2.01 | 0.47 |
| Tobacco used per day (in current smokers, packs) | 0.66 ± 0.33 | 0.28 ± 0.13 | 0.39 ± 0.15 | 0.45 |
| Marijuana users | ||||
| Current users, n (%) | 2 (6) | 3 (11) | 4 (13) | 0.68 |
| Lifetime users, n (%) | 11 (32) | 12 (44) | 23 (72) | 0.005 |
| Cumulative lifetime marijuana used (all subjects, g) | 701 ± 313 | 4051 ± 2345 | 4373 ± 2336 | 0.28 |
| Median: 0 (0–8219) | Median: 0 (0–62,013) | Median: 429 (0–65,242) | ||
| Marijuana used per day (in current marijuana users, g) | 0.75 ± 0.00 | 1.20 ± 0.54 | 1.01 ± 0.30 | 0.78 |
p-values from 1-way-ANOVA, χ2, fisher’s exact, or Kruskal-Wallis test, as appropriate
The three subject groups had similar mean age and education, as well as similar ethnicity and gender distribution (p ≥ 0.69). However, the race distribution differed between the three groups (χ2, p = 0.03), with more Pacific Islanders in the two Meth groups than the control group, and fewer Whites among past users than the other two groups.
CES-D scores were higher in both Meth groups compared to controls (1-way-ANOVA p < 0.0001), and higher in current users than past users (post-hoc: p = 0.003). Notably, 16 current users had CES-D scores >16, indicating possible clinical depression.
More than 90 % of the Meth users smoked the drug as their primary route. The current and past Meth users reported similar cumulative amounts of Meth used during their lifetime, age of first use, duration of Meth use, and averaged daily amount of Meth used (p ≥ 0.11). The past users were abstinent from Meth for almost three years on average (mean duration of 35.9 months, median duration of 6.5 months, range 1 month to 24 years).
Co-morbid use of tobacco is common amongst Meth users (Weinberger and Sofuoglu 2009); however, the three subject groups had similar frequency and amounts of tobacco use. Concurrent marijuana (MJ) use was rare and not different amongst the three groups, although more past Meth users had used MJ than current Meth users and controls (χ2, p = 0.005).
Neuropsychological Assessments (Supplementary Table)
The three subject groups performed similarly on tests that evaluated attention and executive function. Compared to the past users and the control group, current Meth users tended to perform faster on speed of information processing tasks, including the Trail Making Test, Part A (1-way-ANCOVA p = 0.05), and on the Stroop tasks. However, the Meth user groups had non-significantly higher error rates than the controls on the Stroop Interference task.
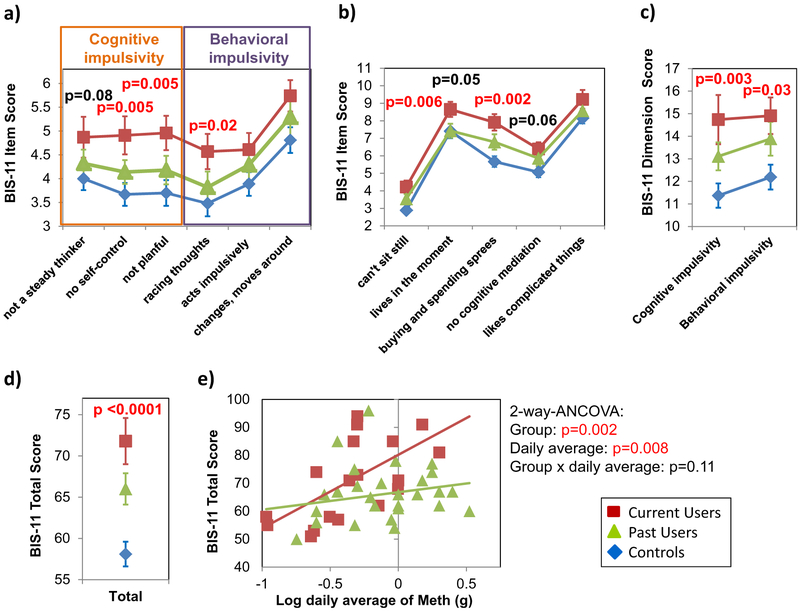
Barratt Impulsiveness Scale (Fig. 1)
Fig. 1.
Barratt Impulsiveness Scale in Methamphetamine Users and Non-User Controls. a and b BIS-11 scores are consistently highest in current Meth users, while the scores in the controls are lowest and the past users’ scores are intermediate across all 11 parcels. c and d Consequently, current users have significantly higher scores than past users and controls (who have the lowest scores) on both the cognitive and the behavioral dimension of impulsivity, and the BIS-11 total score. e In current, but not past, Meth users, the BIS-11 total score correlated with the log-transformed average amount of daily Meth used. Data presented are means ± standard errors. P-values from 1-way ANCOVA and 2-way ANCOVA are co-varied for age, gender, and years of education
On the BIS-11, current Meth users had higher scores than the other two groups across all eleven parcels, indicating greater impulsivity (Fig. 1a and b: RM-ANCOVA p < 0.0001). The scores of past Meth users were intermediate between those of current Meth users and controls on all but one parcel (“Live in the moment”). Hence, current Meth users had the highest scores while controls had the lowest scores, on both dimensions of the BIS-11 (Fig. 1c), cognitive impulsivity (1-way-ANCOVA-p = 0.003) and behavioral impulsivity (1-way-ANCOVA- p = 0.03), as well as on the BIS-11 total score (Fig. 1d: 1-way-ANCOVA-p < 0.0001). As previously shown in Meth users (Zhang et al. 2015), impulsivity measures (BIS-11 total score, cognitive and behavioral impulsivity) correlated with depressive symptoms on CES-D (Pearson correlations: r = 0.50–0.53, p < 0.0001, for all three impulsivity measures, data not shown). Group differences on the BIS-11 total score, but not on the cognitive and behavioral dimensions of the BIS-11, remained significant when CES-D was added as a covariate. In current users, the average amount of daily Meth used correlated with the BIS-11 total score (Fig. 1e: r = 0.65, p = 0.003) and behavioral impulsivity (r = 0.75, p = 0.0006, data not shown). Two-way ANCOVA showed significant main effects of group (p = 0.002) and daily Meth used (p = 0.008) on the BIS-11 total score.
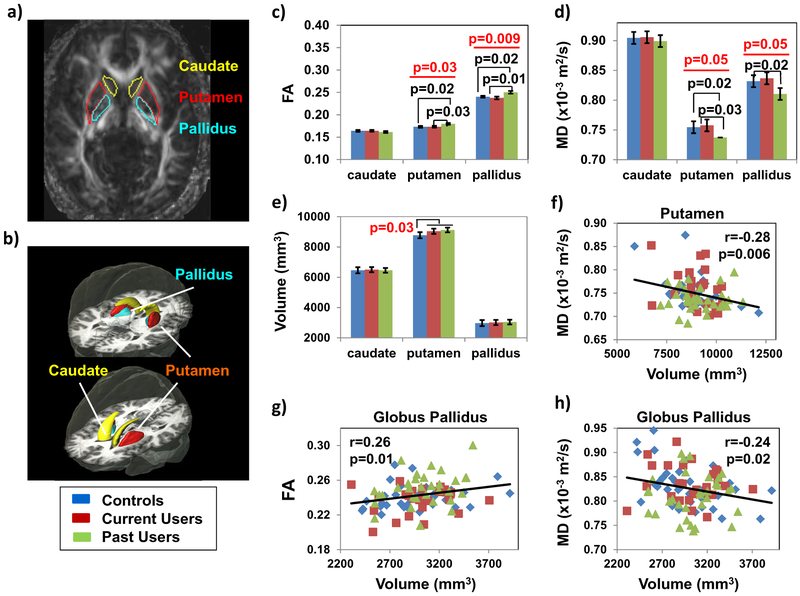
Group Differences in Striatal Regions (Fig. 2)
Fig. 2.
Fractional Anisotropy, Mean Diffusivity and Volumes in Striatal Structures a Regions of Interest are shown on an FA map of a single subject. b The Regions of Interest are shown in parcellated regions from the automated JHU-MNI atlas. c Fractional anisotropy is higher in putamen and globus pallidus of the past users compared to both current users and the controls. d Mean diffusivity is lower in putamen and pallidus in past Meth users compared to current users and controls. e Meth users as a group, combining current and past users, had larger putamen compared to controls. f) Furthermore, larger putamen was associated with lower putamen MD. g & h In the globus pallidus, larger volume was associated with higher FA and lower MD across all groups. Data presented are means ± standard errors; p-values in the bar graphs from 1-way ANCOVA and post-hoc analyses are co-varied for age and gender
Past Meth users had higher FA than current users and controls in the putamen (Fig. 2c: 1-way ANCOVA-p = 0.03) and globus pallidus (Fig. 2c: 1-way ANCOVA-p = 0.009), but not in the caudate. However, only the group effect of FA in globus pallidus remained significant after correction for multiple comparisons. Furthermore, in the same regions (putamen and globus pallidus), past Meth users tended to have lower MD than current users or controls (Fig. 2d: 1-way-ANCOVA-p = 0.05 for both), whereas MD values were similar between current users and controls. Furthermore, DTI metrics in these regions did not correlate with Meth use parameters evaluated.
The volumes of caudate nucleus, putamen and globus pallidus were similar across the three groups (Fig. 2e); however, all Meth users combined showed larger putamen than controls (Fig. 2e: 1-way-ANCOVA-p = 0.03). Across all subjects, the putamen volume correlated inversely with MD (Fig. 2f: r = −0.28, p = 0.006). Similarly, the globus pallidus volume correlated positively with FA (Fig. 2g: r = 0.26, p = 0.01) and inversely with MD (Fig. 2h: r = −0.24, p = 0.02).
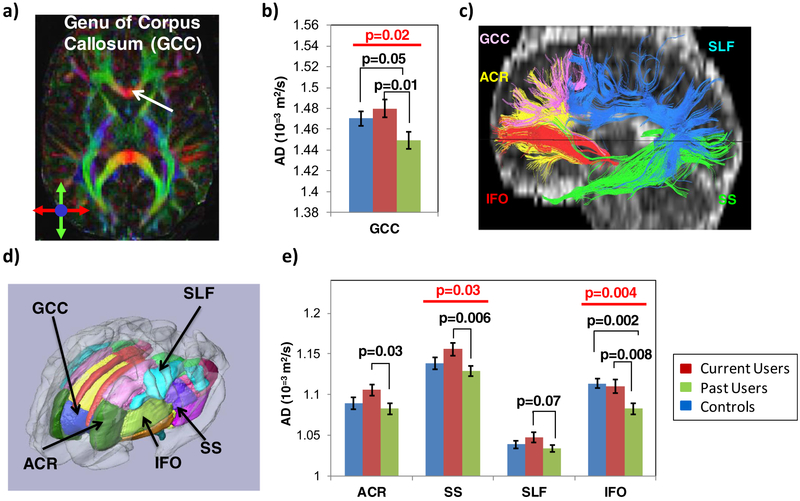
Diffusivities and Group Differences in White Matter Structures (Fig. 3)
Fig. 3.
Axial Diffusivity in White Matter Structures of Interest a Color map of the fractional anisotropy from a single subject showing the location of the genu of the corpus callosum. b In the genu of the corpus callosum, axial diffusivity (AD) is lower in past Meth users than in current users and controls. c In a single subject, fiber-tracts of the five white matter structures of interest are illustrated to demonstrate the anatomical relationships of these structures. d Regions of Interest (ROIs) from various white matter structures were parcellated using the JHU-MNI atlas, and the ROIs from the five tracts of interest that were evaluated are labeled. e In addition to the abnormal AD in the genu of the corpus callosum (GCC), the AD is also higher in current users but normalized or lower in past Meth users compared to controls, in the anterior corona radiata (ACR), the sagittal stratum (SS) and the superior longitudinal fasciculus (SLF). However, in the inferior fronto-occipital fasciculus (IFO), past Meth users had lower AD than both current Meth users and controls. Data presented are means ± standard errors; p-values from 1-way ANCOVA and post-hoc analyses are co-varied for age and gender
All of these structures showed the typical age-dependent decreases in FA (Pearson correlation: p < 0.0001–0.05, data not shown) and sex differences, with higher FA in men than women in the inferior fronto-occipital fasciculus (1-way-ANCOVA p = 0.001, data not shown) and the anterior corona radiata (1-way-ANOVA-p = 0.06, data not shown).
Group differences or trends for group differences were found on AD and MD, but not on FA and RD, in the five white matter structures of interest. Specifically, AD was significantly different between the three groups in the inferior fronto-occipital fasciculus (1-way ANCOVA p = 0.004) and tended to be different in the genu of the corpus callosum (1-way-ANCOVA p = 0.02) and the sagittal stratum (1-way ANCOVA p = 0.03), after correction for multiple comparisons.
In the genu of the corpus callosum, current Meth users had higher AD than past users (Fig. 3b, p-0.01), who in turn had lower AD than the controls (Fig. 3b, p=0.05). Current users also had higher AD than past users in the other four white matter tracts (Fig. 33: post-hoc p = 0.006–0.07). A similar pattern with the highest AD in current Meth users, lowest AD in the past users and intermediate AD in controls was observed in four of the five white matter structures, the genu of the corpus callosum, anterior corona radiata, sagittal stratum, and superior longitudinal fasciculus (Fig. 3b and e).
Although the MDs were not significantly different in the white matter across groups, post-hoc analyses showed a similar pattern with higher MD in current users than in past users in the anterior corona radiata, the genu of the corpus callosum and the inferior fronto-occipital fasciculus (1-way-ANCOVA p = 0.03–0.04, data not shown).
DTI metrics in these regions did not correlate with any of the drug use parameters.
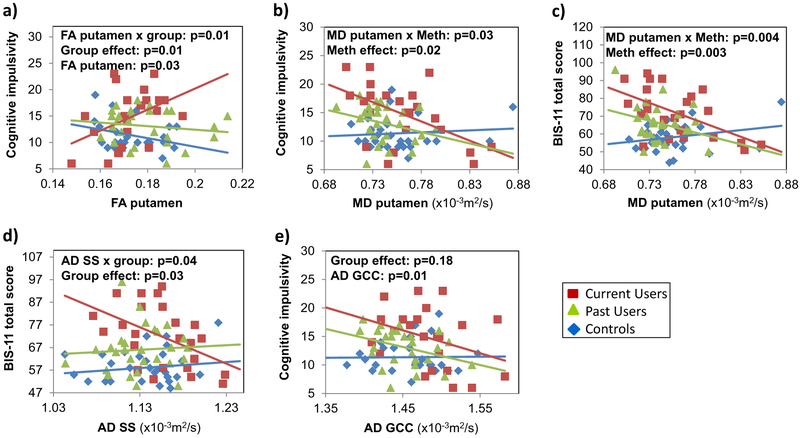
Relationships between Impulsivity and DTI Measures (Fig. 4)
Fig. 4.
Relationships between DTI measures and impulsivity. a Only current Meth users with higher FA in the putamen had higher cognitive impulsivity scores. b and c Both current and past Meth users with lower MD had higher cognitive impulsivity and BIS-11 scores. d Only current Meth users with lower AD in the sagittal stratum (SS) had greater BIS-11 scores. e Meth users, regardless of recency of use, with lower AD in the genu of the corpus callosum (GCC) tended to have higher cognitive impulsivity scores. P-values are from two-way-ANCOVAs, co-varied for education and CES-D, for the DTI metrics that had significant effects or interactions in the exploratory multivariate general linear models on the impulsivity measures and were still significant on the post-hoc two-way-ANCOVA
On the multivariate general linear models (mGLMs), across the groups, FA in the putamen tended to be different (p = 0.07) and showed different relationships with cognitive impulsivity (p = 0.06). Similarly, across subject groups, MD in the putamen was related to cognitive impulsivity (p = 0.04), but the groups tended to show different relationships with the BIS-11 total score (p = 0.07). Lastly, AD in the genu of the corpus callosum of all subjects showed a main effect on cognitive impulsivity (p = 0.005) while the groups had different relationships between AD in the sagittal stratum and the BIS-11 total score (p = 0.03) or the behavioral impulsivity (p = 0.02).
To further assess these findings from the mGLMs, we conducted two-way-ANCOVAs as post-hoc analyses. In the putamen, higher FA was associated with greater cognitive impulsivity only in the current users (Fig. 4a: FA-by-group-interaction-p = 0.01), while lower MD was associated with greater cognitive impulsivity (Fig. 4b: MD-by-Meth-interaction-p = 0.03) and BIS-11 total scores (Fig.4c: MD-by-Meth-interaction p = 0.004) in both Meth user groups, but not in controls. In the sagittal stratum, lower AD was associated with greater BIS-11 scores in current users only (Fig. 4d: AD-by-group-interaction p = 0.04). In the genu of the corpus callosum, lower AD was associated with higher cognitive impulsivity across all three groups (Fig. 4e: p = 0.01).
Discussion
This study demonstrated the following: 1) We validated prior findings (Mahoney et al. 2015) of higher impulsivity scores in Meth users compared to controls, and further showed that current Meth users had greater impulsivity than past users. 2) The Meth user groups combined had enlarged putamen, as shown previously (Chang et al. 2005; Jan et al. 2012). However, compared to current users, the elevated FA in striatal structures of the past users is likely due to greater magnetic susceptibility from higher iron content, while the lower MD may be due to greater dendritic spine density. 3) In contrast, the higher axial diffusivity in the white matter tracts of current users compared to the past users may reflect different stages of Meth-induced neuroinflammation. 4) The correlations between impulsivity scores and diffusion measures in several brain regions (putamen, the genu of the corpus callosum and the sagittal stratum) of the current Meth users further suggest that these microstructural alterations might modulate the impulsivity symptoms.
Higher Impulsivity in Meth Users
Consistent with prior reports (Lee et al. 2009; Mahoney et al. 2015), the Meth users in both groups had greater impulsivity than the controls, but the current users had the highest scores for impulsivity. In PET studies, higher impulsivity during early abstinence from Meth use was associated with lower D2 receptor availability (Lee et al. 2009). D2 receptors may be down regulated due to excess dopamine release from chronic Meth use (Volkow et al. 2001b); however, the decreased D2 receptor availability may normalize with prolonged abstinence (Volkow et al. 2001a). The normalized D2 receptors might in turn explain the lower impulsivity in the past Meth users compared to current or recently abstinent users. Similarly, rats exposed chronically to high doses of Meth showed greater impulsive behaviors (Richards et al. 1999), while cocaine users showed decreased BIS-11 scores one year after reducing cocaine intake (Hulka et al. 2015). However, since greater impulsivity is a core feature of addictions (Jentsch et al. 2014), it may also be a premorbid risk-factor for Meth use disorder, as shown in non-drug using siblings of stimulant users (Ersche et al. 2010).
Furthermore, Meth users with greater impulsivity had more depressive symptoms, similar to prior reports (Semple et al. 2005; Zhang et al. 2015). However, the past Meth users had lower CES-D scores than the current users, which suggests that cessation of Meth use may reduce depressive symptoms, as shown in Meth users after three weeks of abstinence (Bagheri et al. 2015). Meth users with greater depressive symptoms also showed greater craving for Meth (Nakama et al. 2008), which together with greater impulsivity may increase the likelihood for relapses in stimulant users (Winhusen et al. 2013). Therefore, treatment interventions should focus on treating both the depressive symptoms and reducing impulsivity.
Microstructural Abnormalities in the Striatum
The Meth users had larger putamen than controls, which is consistent with prior reports of enlarged striatal structures in chronic Meth users (Chang et al. 2005; Jan et al. 2012), and in monkeys treated with Meth for 30 days (Groman et al. 2013). However, compared to current users and controls, the putamen and globus pallidus of past Meth users had higher FA and lower MD, which likely resulted from greater magnetic susceptibility that was associated with higher iron content (Xu et al. 2015). Similarly, the basal ganglia of Meth-exposed monkeys also showed levels of reactive iron that were elevated to those observed in aged monkeys (Melega et al. 2007). Accumulation of ferritin-bound iron, especially in the basal ganglia, may occur both in aging due to changes in iron homeostasis (Killilea et al. 2004) and in neurodegenerative disorders, such as Parkinson’s or Alzheimer’s disease (Ward et al. 2014). The excess iron may induce neurodegeneration by oxidative stress, ferroptosis, an iron-dependent form of cell death, and oxidation of catecholamines (e.g. dopamine) to toxic quinones via reduction of ferric iron, as well as reactive neuroinflammation (Ward et al. 2014). Therefore, increased iron content in the brains of Meth users may be a cause for the greater than age-related brain atrophy (Nakama et al. 2011) and the increased risk for Parkinson’s disease (Callaghan et al. 2012; Curtin et al. 2015).
Additionally, lower MD in the basal ganglia of past Meth users compared to current users and controls indicates restricted water diffusion, which may be due to increased dendritic spine density, as shown in the dorsolateral caudate-putamen of rats after repeated exposure to amphetamines (Li et al. 2003; Jedynak et al. 2007). However, in the current Meth users, ongoing neuroinflammation with greater water content may counterbalance this restricted diffusion, leading to the apparently normal MD in these striatal structures, as reported in another study (Lin et al. 2015).
Microstructural Abnormalities in White Matter Tracts
In the white matter tracts, current Meth users consistently had higher AD than past Meth users with prolonged abstinence, which is consistent with the higher than normal MD in the genu of the corpus callosum of recently abstinent Meth users (Lederer et al. 2016). Higher AD in white matter tracts indicates greater water diffusion along the axons, which is typically observed during acute inflammation, with increased cytokines (Singh et al. 2016) and fluid leaking into myelin space causing axonal swelling (Kimura-Ohba et al. 2016), or from loss of axonal fibers (Hikishima et al. 2015). Neuroinflammation, including microglial and astroglial activation (Thomas et al. 2004; Borgmann and Ghorpade 2015), was shown in Meth-induced brain injury (Yu et al. 2015). Since prolonged abstinence from Meth use may lead to partial recovery of D2 receptors and brain metabolism (Volkow et al. 2001a; Wang et al. 2004), the lower AD in the past Meth users compared to current users may reflect resolution of the axonal swelling. However, ongoing reactive gliosis, with activated microglia or hypertrophic astrocytes, as seen during chronic repair of brain injury (Singh et al. 2016), may further restrict water diffusion (lower AD) in the axons of past Meth users, especially in the genu of the corpus callosum and the inferior fronto-occipital fasciculus. Consistent with this interpretation, past Meth users had persistent microglial activation on PK-11, 195 PET even after two years of abstinence from Meth use (Sekine et al. 2008).
In contrast to several other studies that found lower than normal FA in several white matter regions of Meth users (Chung et al. 2007; Alicata et al. 2009; Kim et al. 2009; Tobias et al. 2010), the current study and another recent study (Lederer et al. 2016) did not find group differences in FA in white matter structures. These discrepancies may be related to the different methodologies used or differences in the cohorts evaluated. Specifically, while prior studies evaluated smaller regions of interest, we assessed parcellations of five major white matter tracts, which may be less sensitive in detecting smaller or regional decreases in FA but more sensitive in detecting axial diffusivities. Furthermore, unlike the current study, the majority of the prior studies did not match the groups for education or socioeconomic status, which are often lower in Meth user groups than in controls, and may account for the lower FA (Johnson et al. 2013; Noble et al. 2013).
Relationship between Impulsivity and Microstructural Alterations
The correlation between higher FA in the putamen of current Meth users, or lower MD in putamen of all Meth users, and greater cognitive impulsivity suggest that microstructural alterations in the putamen might have contributed to the greater impulsivity. In a prior study, abstinent Meth users with higher striatal gray matter density had greater impulsivity (Schwartz et al. 2010). In the current study, higher FA and lower MD in the Meth users correlated with larger striatal volumes, as well as greater impulsivity. Thus, both studies support the hypothesis that alterations in striatal structures might contribute to greater impulsivity in Meth users. Furthermore, activation of striatal D2 receptors may play a role in impulsivity since current and recently abstinent Meth users with greater impulsivity had lower D2 receptors availability (Lee et al. 2009; Ballard et al. 2015). Future studies are needed to evaluate whether D2 receptor availability are influenced by Meth-induced striatal structural alterations.
The axial diffusivity in sagittal stratum and the genu of the corpus callosum also show possible relationships with impulsivity. These fiber tracts both project to the orbitofrontal and prefrontal cortices, which are involved in inhibition (McClure et al. 2004). Furthermore, reduced FA or white matter integrity in the sagittal stratum were reported in other conditions with high impulsivity, such as obesity (Shott et al. 2015) and ADHD (Cortese et al. 2013), while lower FA in the anterior corpus callosum was found in cocaine users with greater impulsivity (Moeller et al. 2005).
Limitations
The interpretation of our findings may have several limitations. First, the cross-sectional study design cannot evaluate causal relationships between Meth usage and behavioral or brain structural alterations. However, our findings were generally consistent with preclinical studies and prior studies in human Meth users. Second, the group differences on brain diffusivity between current and past Meth users may be due to different cohort characteristics, or the acute effects of Meth (i.e., within minutes to hours), rather than the different stages of Meth use. Longitudinal studies are needed to further evaluate microstructural changes from current to past Meth use. Third, despite the moderate sample size and the similar sex proportion in the current study, a larger sample size is needed to further evaluate sex-specific differences in brain abnormalities and impulsivity, which are often seen in Meth users (Kogachi et al. 2016).
In summary, although current Meth users had higher impulsivity than past users, the brain microstructural abnormalities showed differences that may reflect different stages of neuroinflammation or iron-induced neurodegeneration. Combining current and past Meth users may lead to greater variability in studies of Meth users. Longitudinal studies are needed to further evaluate the relationship between recency of Meth use and brain microstructure.
Supplementary Material
Acknowledgments
NIH Grant support: R24-DA27318; K24-DA16170; U54-NS56883; G12MD-007601; P41EB015909. We thank our study participants, and the many clinical and technical staff from the UH Neuroscience and MR Research Center who were involved in the data collection. We also thank Caroline Jiang and Vanessa Douet for their advice on statistical analyses.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11481-016-9675-8) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Alicata D, Chang L, Cloak C, Abe K, Ernst T (2009) Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Res 174:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri M, Mokri A, Khosravi A, Kabir K (2015) Effect of abstinence on depression, anxiety, and quality of life in chronic methamphetamine users in a therapeutic community. Int J High Risk Behav Addict 4: e23903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, Dean AC, London ED (2015) Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum 18:pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann K, Ghorpade A (2015) HIV-1, methamphetamine and astrocytes at neuroinflammatory Crossroads. Front Microbiol 6:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ (2012) Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120:35–40 [DOI] [PubMed] [Google Scholar]
- Ceritoglu C, Oishi K, Li X, Chou MC, Younes L, Albert M, Lyketsos C, van Zijl PC, Miller MI, Mori S (2009) Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. NeuroImage 47:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T (2005) Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57:967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102(Suppl 1):16–32 [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF (2007) Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol 10:765–775 [DOI] [PubMed] [Google Scholar]
- Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, Ramos-Olazagasti MA, Milham MP, Kelly C, Castellanos FX (2013) White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry 74:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA (2014) Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR (2015) Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend 146:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamanakova A, Tang X, Li X, Faria AV, Ceritoglu C, Oishi K, Hillis AE, Albert M, Lyketsos C, Miller MI, Mori S (2014) Tools for multiple granularity analysis of brain MRI data for individualized image analysis. NeuroImage 101:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O (2000) Evidence for longterm neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 54:1344–1349 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW (2010) Drug addiction endophenotypes: impulsive versus sensationseeking personality traits. Biol Psychiatry 68:770–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD (2013) Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology 229:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikishima K, Ando K, Yano R, Kawai K, Komaki Y, Inoue T, Itoh T, Yamada M, Momoshima S, Okano HJ, Okano H (2015) Parkinson disease: diffusion MR imaging to detect Nigrostriatal pathway loss in a marmoset model treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Radiology 275:430–437 [DOI] [PubMed] [Google Scholar]
- Holm S (1979) A simple sequentially rejective multiplpe test procedure. Scand J Stat 6 [Google Scholar]
- Hulka LM, Vonmoos M, Preller KH, Baumgartner MR, Seifritz E, Gamma A, Quednow BB (2015) Changes in cocaine consumption are associated with fluctuations in self-reported impulsivity and gambling decision-making. Psychol Med 45:3097–3110 [DOI] [PubMed] [Google Scholar]
- INCSR (2015) International Narcotis control strategy report. United States Department of State - Bureau for International Narcotics and Law Enforcement Affairs. [Google Scholar]
- Jan RK, Lin JC, Miles SW, Kydd RR, Russell BR (2012) Striatal volume increases in active methamphetamine-dependent individuals and correlation with cognitive performance. Brain Sci 2:553–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE (2007) Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci 25:847–853 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT (2014) Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci 1327:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Prog Biomed 81:106–116 [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Gold BT (2013) Socioeconomic status is positively correlated with frontal white matter integrity in aging. Age 35:2045–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Wong SL, Cahaya HS, Atamna H, Ames BN (2004) Iron accumulation during cellular senescence. Ann N Y Acad Sci 1019: 365–367 [DOI] [PubMed] [Google Scholar]
- Kim IS, Kim YT, Song HJ, Lee JJ, Kwon DH, Lee HJ, Kim MN, Yoo DS, Chang Y (2009) Reduced corpus callosum white matter microstructural integrity revealed by diffusion tensor eigenvalues in abstinent methamphetamine addicts. Neurotoxicology 30:209–213 [DOI] [PubMed] [Google Scholar]
- Kimura-Ohba S, Yang Y, Thompson J, Kimura T, Salayandia VM, Cosse M, Yang Y, Sillerud LO, Rosenberg GA (2016) Transient increase of fractional anisotropy in reversible vasogenic edema. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogachi S, Chang L, Alicata D, Cunningham E, Ernst T (2016) Sex differences in impulsivity and brain Morphometry in methamphetamine users. Brain Struct Funct. doi: 10.1007/s00429-016-1212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer K, Fouche JP, Wilson D, Stein DJ, Uhlmann A (2016) Frontal white matter changes and aggression in methamphetamine dependence. Metab Brain Dis 31:53–62 [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE (2003) The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 28:1082–1085 [DOI] [PubMed] [Google Scholar]
- Lin JC, Jan RK, Kydd RR, Russell BR (2015) Investigating the microstructural and neurochemical environment within the basal ganglia of current methamphetamine abusers. Drug Alcohol Depend 149: 122–127 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ 3rd, Thompson-Lake DG, Cooper K, Verrico CD, Newton TF, De La Garza R 2nd (2015) A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. J Psychopharmacol 29:50–56. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004) Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507 [DOI] [PubMed] [Google Scholar]
- Melega WP, Lacan G, Harvey DC, Way BM (2007) Methamphetamine increases basal ganglia iron to levels observed in aging. Neuroreport 18:1741–1745 [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA (2005) Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 30:610–617 [DOI] [PubMed] [Google Scholar]
- Nakama H, Chang L, Cloak C, Jiang C, Alicata D, Haning W (2008) Association between psychiatric symptoms and craving in methamphetamine users. Am J Addict 17:441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T (2011) Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 106:1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM (2013) Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci 16:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S (2009) Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. NeuroImage 46:486–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED (2013) The Barratt impulsiveness scale-11: reassessment of its structure in a community sample. Psychol Assess 25:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146:432–439 [DOI] [PubMed] [Google Scholar]
- Roos A, Kwiatkowski MA, Fouche JP, Narr KL, Thomas KG, Stein DJ, Donald KA (2015) White matter integrity and cognitive performance in children with prenatal methamphetamine exposure. Behav Brain Res 279:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Galloway GP, Leamon MH (2009) Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry 65:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF (2010) Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage 50:1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297 [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL (2008) Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL (2005) Impulsivity and methamphetamine use. J Subst Abus Treat 29:85–93 [DOI] [PubMed] [Google Scholar]
- Shott ME, Cornier MA, Mittal VA, Pryor TL, Orr JM, Brown MS, Frank GK (2015) Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes 39:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Trivedi R, Devi MM, Tripathi RP, Khushu S (2016) Longitudinal changes in the DTI measures, anti-GFAP expression and levels of serum inflammatory cytokines following mild traumatic brain injury. Exp Neurol 275(Pt 3):427–435 [DOI] [PubMed] [Google Scholar]
- Tang X, Oishi K, Faria AV, Hillis AE, Albert MS, Mori S, Miller MI (2013) Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PLoS One 8:e65591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7 [DOI] [PubMed] [Google Scholar]
- Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED (2010) White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology 209:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) (2014) World Drug Report. United Nations Publication. [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001a) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N (2001b) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021 [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS (2004) Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 161:242–248 [DOI] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M (2009) The impact of cigarette smoking on stimulant addiction. AmJ Drug Alcohol Abuse 35:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, Seamans CL, Hodgkins CC, Dicenzo JC, Botero CL, Jones DR, Somoza E (2013) Impulsivity is associated with treatment noncompletion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J Subst Abus Treat 44:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang Q, Zhong J, Zhang M (2015) Iron deposition influences the measurement of water diffusion tensor in the human brain: a combined analysis of diffusion and iron-induced phase changes. Neuroradiology 57:1169–1178 [DOI] [PubMed] [Google Scholar]
- Yu S, Zhu L, Shen Q, Bai X, Di X (2015) Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol 2015:103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Su H, Tao J, Xie Y, Sun Y, Li L, Zhang XY, Hu Z, He J (2015) Relationship of impulsivity and depression during early methamphetamine withdrawal in Han Chinese population. Addict Behav 43:7–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.