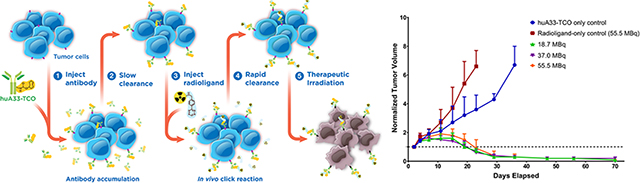
Abstract
Pretargeted radioimmunotherapy (PRIT) based on the inverse electron demand Diels-Alder (IEDDA) reaction between tetrazine (Tz) and trans-cyclooctene (TCO) represents a promising strategy for leveraging the affinity and specificity of antibodies without their pharmacokinetic drawbacks. Herein, we present an investigation of the in vivo efficacy and dosimetry of a PRIT strategy for colorectal carcinoma based on the ligation between a 177Lu-labeled Tz radioligand (177Lu-DOTA-PEG7-Tz) and a TCO-bearing immunoconjugate of the huA33 antibody (huA33-TCO). Biodistribution studies in tumor-bearing mice using intervals of 24, 48, and 72 h between the administration of huA33-TCO and 177Lu-DOTA-PEG7-Tz revealed that a 24 h lag time produced the most promising in vivo results: high activity concentrations in the tumor (21.2 %ID/g ± 2.9 at 24 h post-injection), low uptake in non-target tissues, and favorable dosimetry (an effective dose of 0.054 mSv/MBq). A subsequent longitudinal therapy study revealed striking differences between both the survival and tumor growth of the treatment and control cohorts, clearly underscoring the promise of this approach for the radiotherapy of colorectal carcinoma.
Keywords: radioimmunotherapy, click chemistry, pretargeting, pretargeted radioimmunotherapy, Lu-177, colorectal cancer, inverse electron demand Diels-Alder reaction, tetrazine, trans-cyclooctene
Graphical Abstract
The promise of radioimmunotherapy (RIT) for the treatment of cancer rests in large part on the exquisite specificity and selectivity of monoclonal antibodies for cancer biomarkers.1 A critical limitation of this approach, however, stems from the long serum half-lives of antibodies. Indeed, immunoglobulins can circulate in the blood for days or weeks before reaching their optimal biodistribution. As a result, radioimmunoconjugates for RIT must be labeled with long-lived isotopes such as 177Lu (t1/2 = 6.8 d) or 131I (t1/2 = 8.0 d) to ensure that significant amounts of radioactivity remain once the antibody has reached the tumor tissue. Unfortunately, the use of these long-lived radionuclides presents a significant drawback: high radiation doses to healthy tissues during the circulation of the radioimmunoconjugate.2
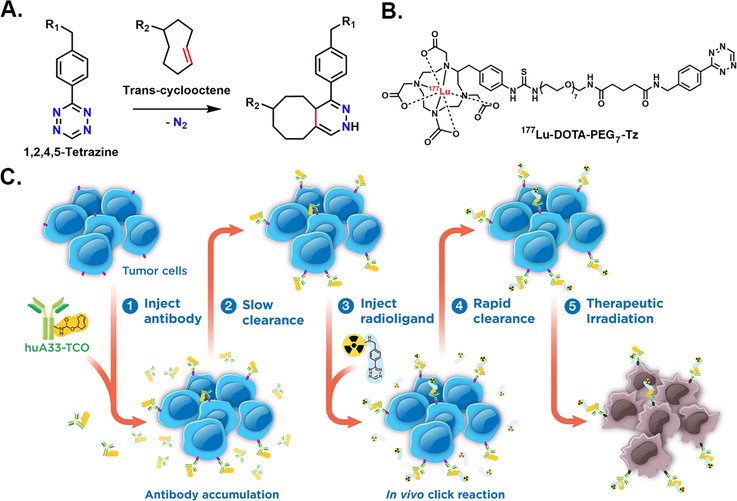
Pretargeted radioimmunotherapy (PRIT) is an alternative approach to RIT that combines the specificity and affinity of antibodies with the pharmacokinetics of small molecules. In vivo pretargeting achieves this by decoupling the targeting vector and the radioactivity, administering the two components separately, and allowing them to combine within the body. A number of different mechanisms for pretargeting have been explored, most notably the use of bispecific antibodies, which have had preclinical success and are currently being investigated in clinical trials.3, 4 However, due to the complexity and expense of such systems, the creation of more modular approaches to pretargeting is crucial for its widespread application. A particularly promising pretargeting strategy that has emerged over the last few years is based on the rapid and bioorthogonal inverse electron demand Diels-Alder (IEDDA) reaction between a Tz-bearing radioligand and a TCO-modified antibody (mAb-TCO; Figure 1A). In vivo pretargeting based on the IEDDA reaction typically has four steps: (1) the administration of an mAb-TCO immunoconjugate; (2) the accumulation of the immunoconjugate at the site of antigen expression and its concurrent clearance from the blood and non-target organs; (3) the injection of a Tz-bearing radioligand; and (4) the in vivo click ligation between the two components followed by the rapid excretion of the radioligand from the bloodstream (Figure 1C). Pretargeted PET and SPECT imaging using this approach have proven very effective, producing high quality images at time points as early as 1 h following the administration of the radioligand.5, 6
Figure 1.
(A) The inverse electron demand Diels-Alder (IEDDA) reaction between trans-cyclooctene and tetrazine; (B) the structure of the 177Lu-DOTA-PEG7-Tz radioligand; (C) cartoon schematic of pretargeted radioimmunotherapy based on the IEDDA reaction.
Until recently, the application of the IEDDA reaction to PRIT had not been investigated beyond preliminary biodistribution data.6 In 2017, however, Houghton et al. performed the first longitudinal therapy study of 177Lu-based PRIT in a murine model of pancreatic ductal adenocarcinoma (PDAC) and produced extremely promising results.7 Herein, we present an investigation into the efficacy and dosimetry of click-mediated PRIT using a TCO-bearing immunoconjugate of the huA33 antibody, a 177Lu-labeled Tz radioligand, and a murine model of A33 antigen-expressing human colorectal carcinoma. We have previously demonstrated the efficacy of pretargeted PET imaging utilizing the huA33 antibody/antigen system in the same murine model of colorectal carcinoma.8, 9
The huA33 antibody targets the A33 antigen, a transmembrane glycoprotein present on the surface of >95% of all colorectal carcinomas.10 The A33 antigen is an extraordinarily promising target for in vivo pretargeting for two reasons. First, upon binding the A33 antigen, the huA33 antibody persists on the surface of the cell for weeks, meaning that the huA33-TCO immunoconjugate will remain readily available for click ligations with any incoming Tz-based radioligands.10 Second, while the A33 antigen is most highly expressed in colorectal cancer cells, it is also expressed at lower levels by the epithelium of healthy small and large intestines.10, 11 Not surprisingly, this presents a major challenge for traditional RIT. However, an interesting wrinkle makes this an enticing opportunity for PRIT. It has been shown that the A33 antigen is shed from healthy bowel tissue in a few days, while the same antigen is retained on tumor tissue for weeks.12 One can imagine a PRIT approach in which the huA33-TCO immunoconjugate is injected and provided an accumulation interval time long enough to allow it to bind its antigen and then shed from healthy tissue while remaining on malignant tissue. By the time the Tz-based radioligand is injected, the huA33-TCO will only be present at the tumor. In this way, this strategy could simultaneously increase the specificity of this therapeutic approach for tumor tissue and limit the radiation dose to the healthy bowel tissue compared to traditional RIT.
Due to its effectiveness in previous studies, 177Lu-DOTA-PEG7-Tz was selected as the radioligand for this investigation (Figure 1B).7 The synthesis of this chelator-bearing precursor is a facile, modular, three-step procedure. First, a Boc-protected polyethylene glycol linker is conjugated to the tetrazine-NHS ester via a simple coupling reaction. Next, the intermediate is deprotected using trifluoroacetic acid in dichloromethane. And finally, the DOTA chelator is attached through chemistry similar to that used in the first step, and the final product is isolated and purified via semi-preparative, reversed-phase C18 HPLC in ~74% yield. The radiosynthesis to produce the final product — 177Lu-DOTA-PEG7-Tz — is also straightforward: DOTA-PEG7-Tz is incubated with 177LuCl3 in a solution of 0.25 mM NH4OAc (pH 5.5) for 10 minutes at room temperature. A radiochemical yield of >98% was determined using instant thin layer chromatography (iTLC), and no further purification was necessary prior to administration. The huA33-TCO immunoconjugate was likewise synthesized according previously reported protocols (~2 TCO/mAb; see Supporting Information).8
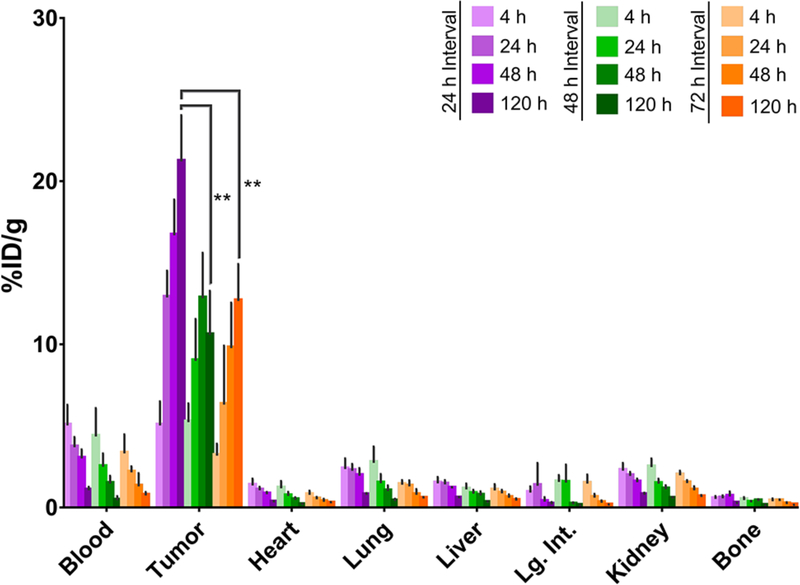
In vivo biodistribution experiments were carried out to determine the optimal time interval between the injection of the huA33-TCO and the administration of the 177Lu-labeled radioligand. To this end, athymic nude mice bearing A33 antigen-expressing subcutaneous SW1222 human colorectal carcinoma xenografts were administered huA33-TCO (100 μg, 0.6 nmol) either 24, 48, or 72 hours prior to the injection of 177Lu-DOTA-PEG7-Tz (11.1 MBq, 0.7 nmol). At prescribed time points following the administration of the radioligand, select organs were harvested, weighed, and their activity quantified via gamma counting (Figure 2).
Figure 2.
Biodistribution data for in vivo pretargeting with huA33-TCO and 177Lu-DOTA-PEG7-Tz in athymic nude mice (n = 4 per cohort) bearing subcutaneous SW1222 human colorectal cancer xenografts using pretargeting intervals of 24 (purple), 48 (green), or 72 (orange) hours. Data is reported with standard errors, and a statistical analysis was performed using an unpaired Student’s t-test, **P < 0.01.
The biodistribution data plainly illustrate that excellent tumoral activity concentrations can be achieved using each of the three pretargeting time intervals, with the highest tumoral activity concentration (21.2 %ID/g ± 2.9, n = 4) observed using an interval of 24 h. The 24 h interval also affords the highest tumor-to-background organ activity concentration ratios (see Supporting Information). Indeed, with this injection interval, the tumor-to-blood, tumor-to-liver, and tumor-to-muscle activity concentration ratios at 120 h after the administration of the radioligand are 20 ± 5, 37 ± 7, and 184 ± 30, respectively. Taken together, these two factors led us to adopt it as the interval of choice for subsequent longitudinal therapy studies. We also observed that the uptake of radioactivity in the tumor increases from 4 to 120 h post-injection, even when the antibody is given 72 h to accumulate at the tumor and clear from the bloodstream prior to the administration of the radioligand. The most likely explanation for this phenomenon is the occurrence of click reactions between the radioligand and the TCO-bearing immunoconjugate in the blood, followed by the localization of the 177Lu-labeled antibody to the tumor tissue. Given that we have observed varying degrees of this behavior with different tetrazine-based radioligands, the binding of the radioligand to plasma proteins — and thus the extension of its serum half-life — may also play a role. Investigations into this phenomenon are ongoing in our laboratory.
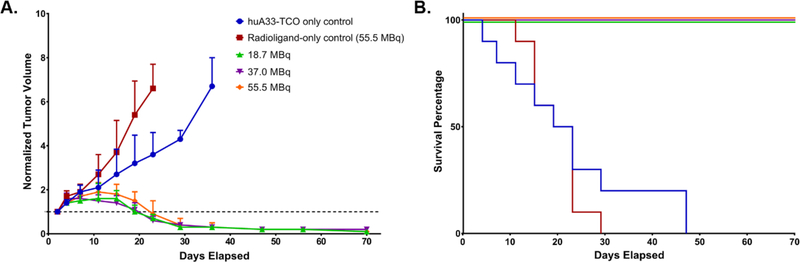
The next step in the investigations was the in vivo validation of the therapeutic efficacy of the system. To this end, athymic nude mice bearing subcutaneous SW1222 xenografts were randomly assigned into one of five cohorts (n = 10 each): two control groups and three treatment groups. The control groups consisted of one cohort in which the mice received huA33-TCO but not the radioligand and one cohort in which the mice received 177Lu-DOTA-PEG7-Tz (55.5 MBq, 0.78nmol) but not the immunoconjugate. The three treatment groups each received the same amount of huA33-TCO (100 μg, 0.6 nmol) and then ⎯ following a 24 h interval ⎯ three different doses of 177Lu-DOTA-PEG7-Tz: 18.5 (0.76 nmol), 37.0 (0.79 nmol), or 55.5 (0.78 nmol) MBq. Tumor volumes were calculated from caliper measurements taken every 3–4 d for the first three weeks of the study followed by weekly measurements taken until the conclusion of the study at 70 d (10 half-lives of 177Lu). As can be seen in Figure 3A, the treated cohorts exhibited a brief period of tumor growth followed by drastic and sustained reductions in tumor volume. These data very clearly validate the merits of this pretargeted approach to radioimmunotherapy. In addition, the efficacy of the therapy is plainly illustrated in the Kaplan-Meier plot of the study. To wit, no mice from the control cohorts survived past 47 d, while the mice from the experimental groups boasted a perfect survival record over the course of the study. Critically, a similar dose-dependent therapeutic response was also observed in a parallel huA33-TCO/177Lu-DOTA-PEG7-Tz PRIT study using a 72 h pretargeting interval and lower activities of the 177Lu-labeled radioligand (Supporting Information Figure S2).
Figure 3.
A longitudinal therapy study of 5 groups of mice (n = 10 each) bearing subcutaneous SW1222 tumors depicted in (A) a graph of normalized tumor volume as a function of time and (B) the corresponding Kaplan-Meier survival curve. The control groups received either the immunoconjugate without the radioligand (blue) or the radioligand without the immunoconjugate (red). The three treatment groups received huA33-TCO (100 μg, 0.6 nmol) followed 24 h later by either 18.5 (green), 37.0 (purple), or 55.5 (orange) MBq (~0.8 nmol in each case) of 177Lu-DOTA-PEG7-Tz. By log-rank (Mantel–Cox) test, survival was significant (P < 0.0001) for all treatment groups.
During the therapy study, pretargeted PET imaging was performed on representative mice from each cohort in order to visualize the amount of A33 antigen remaining on the tumor surface and to probe if additional cycles of huA33-TCO/177Lu-DOTA-PEG7-Tz PRIT could be feasible (Supporting Information Figure S3). These imaging protocols were performed on day 27 of the imaging study in order to ensure that representative mice from every cohort were available for the study. To this end, the mice in question were injected with huA33-TCO (100 μg, 0.6 nmol), and, after a 24 h interval period, a 64Cu-labeled tetrazine radioligand (64Cu-SarAr-Tz, 18.5 MBq, 0.95 nmol). PET images collected 24 h after the administration of the radioligand clearly show uptake of the 64Cu-SarAr-Tz at the tumor site, suggesting that the initial round of PRIT did not saturate the A33 antigen at the tumor site and that an iterative therapeutic approach is possible. An exploration of this fractionated approach is ongoing in the laboratory.
In order to properly compare the dosimetry of this PRIT approach to conventional RIT, it was necessary to collect in vivo data with a directly-labeled antibody. To this end, an immunoconjugate of huA33 bearing the CHX-A″-DTPA chelator — CHX-A″-DTPA-huA33 — was synthesized and radiolabeled with 177Lu in high radiochemical purity and specific activity (see Supporting Information). Subsequently, biodistribution studies were performed in athymic nude mice bearing SW1222 human colorectal cancer xenografts. Time activity curves were generated for both the PRIT and RIT modalities, and the integrals under the curves were determined to calculate the residence times of 177Lu in each organ. These residence times were next entered into the OLINDA program to estimate the radiation dose to selected organs as well as the total effective dose (Table 1).13 The effective dose of the PRIT system was found to be 0.054 mSv/MBq, slightly lower than the 0.068 mSv/MBq effective dose produced by the RIT approach. This reduction is admittedly not as dramatic as we had hoped and had observed previously in our pretargeted PET imaging studies.8 In the latter, however, the adoption of a pretargeted approach facilitates the use of short-lived radionuclides that would normally be incompatible with antibody-based vectors, such as 64Cu (t1/2 = 12.7 h), 18F (t1/2 = 107 min), and 68Ga (t1/2 = 68 min). In this case, however, we do not have that option, as effective therapeutic radionuclides with short half-lives are few and far between. It is important to note, however, that the red marrow is typically the dose limiting organ for RIT, with a maximum tolerated dose of 150 cGy.14 As can be seen in our dosimetry estimates, the dose received by the red marrow during PRIT is nearly half of that produced by RIT: 0.059 mGy/MBq for the former compared to 0.105 mGy/MBq for the latter. Consequently, the dose reductions observed in PRIT could enable the administration of larger initial doses to the patient without marrow-related toxicity.
Table 1.
Radiation dose estimates for a 70-kg adult male based on the biodistribution data for the huA33-TCO/177Lu-DOTA-PEG7-Tz PRIT system with a 24 h interval (left) and the directly-labeled 177Lu-CHX-A˝-DTPA-huA33 radioimmunoconjugate (right).
| Pretargeting with huA33-TCO and 177Lu-DOTA-PEG7-Tz (24 h interval) | 177Lu-CHX-A˝-DTPA-huA33 | |
|---|---|---|
| Organ/Tissue | Absorbed Dose (mGy/MBq) | Absorbed Dose (mGy/MBq) |
| Adrenals | 0.056 | 0.034 |
| Brain | 0.055 | 0.029 |
| Breasts | 0.053 | 0.028 |
| Gallbladder Wall | 0.06 | 0.035 |
| LLI Wall | 0.059 | 0.041 |
| Small Intestine | 0.066 | 0.082 |
| Stomach Wall | 0.057 | 0.048 |
| ULI Wall | 0.058 | 0.038 |
| Heart Wall | 0.065 | 0.076 |
| Kidneys | 0.055 | 0.168 |
| Liver | 0.039 | 0.156 |
| Lungs | 0.043 | 0.126 |
| Muscle | 0.036 | 0.129 |
| Ovaries | 0.057 | 0.034 |
| Pancreas | 0.056 | 0.035 |
| Red Marrow | 0.059 | 0.105 |
| Osteogenic Cells | 0.292 | 0.651 |
| Skin | 0.052 | 0.028 |
| Spleen | 0.035 | 0.131 |
| Testes | 0.054 | 0.030 |
| Thymus | 0.055 | 0.032 |
| Thyroid | 0.055 | 0.032 |
| Urinary Bladder Wall | 0.056 | 0.033 |
| Uterus | 0.057 | 0.034 |
| Total Body | 0.070 | 0.097 |
| Effective Dose (mSv/MBq) | 0.054 | 0.068 |
In the preceding pages, we have described the successful demonstration of the pretargeted radioimmunotherapy of mice bearing human colorectal carcinoma tumors using a strategy based on the in vivo ligation of 177Lu-DOTA-PEG7-Tz and huA33-TCO. A pronounced dose-dependent therapeutic effect was observed. Moreover, the data reveal modest dosimetric improvements over traditional RIT with a directly-labeled radioimmunoconjugate. We believe these data strongly suggest that this Tz/TCO-based approach to PRIT can be employed successfully with other tumor types as well. Future investigations will focus primarily on enhancing the dosimetric benefits of PRIT through the use of Tz-bearing clearing agents, fractionated dosing schedules, and shorter-lived therapeutic radionuclides such as 211At. While the use of clearing agents to accelerate the removal of unbound antibody from circulation is a particularly enticing and often effective option, it is important to note that clearing agents are not without their drawbacks. Indeed, the introduction of yet another component to the already complex PRIT schema can create new complications, particularly with respect to logistics and regulatory approval. Ultimately, however, we are hopeful and cautiously optimistic that the optimization of this approach to PRIT will lead to the creation of a safe and effective approach to the targeted radiotherapy of patients in the clinic.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the generous financial support of the National Institutes of Health (4R00CA178205-02 and R01CA204167), the TeamConnor Childhood Cancer Foundation, and the National Institute on Minority Health and Health Disparities (G12MD007599). Services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH grants R24 CA83084 and P30 CA08748
ABBREVIATIONS
- RIT
radioimmunotherapy
- PRIT
pretargeted radioimmunotherapy
- IEDDA
inverse electron demand Diels-Alder
- Tz
tetrazine
- TCO
trans-cyclooctene
- iTLC
instant thin layer chromatography
- %ID/g
percent injected dose per gram
- p.i.
post-injection
- NHS
N-hydroxysuccinimide
Footnotes
ASSOCIATED CONTENT
Supporting Information: Reagents and general procedures; detailed experimental methods for chemical syntheses, radiosyntheses, and in vivo experiments; dosimetry calculations; PET imaging protocols; tables of biodistribution data, both activity concentrations and tumor-to-tissue activity concentration ratios; and an additional longitudinal therapy study. This material is available free of charge via the Internet at http://pubs.acs.org.
All animals were treated according to the guidelines approved by the Research Animal Resource Center and Institutional Animal Care and Use Committee at Memorial Sloan Kettering Cancer Center.
REFERENCES
- 1.Sharkey RM; Goldenberg DM Cancer radioimmunotherapy. Immunotherapy 2011, 3, (3), 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karacay H; Brard PY; Sharkey RM; Chang CH; Rossi EA; McBride WJ; Ragland DR; Horak ID; Goldenberg DM Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin. Cancer Res 2005, 11, (21), 7879–7885. [DOI] [PubMed] [Google Scholar]
- 3.Repp R; van Ojik HH; Valerius T; Groenewegen G; Wieland G; Oetzel C; Stockmeyer B; Becker W; Eisenhut M; Steininger H; Deo YM; Blijham GH; Kalden JR; van de Winkel JG; Gramatzki M Phase I clinical trial of the bispecific antibody mdx-h210 (anti-fcgammari x anti-her-2/neu) in combination with filgrastim (g-csf) for treatment of advanced breast cancer. Br. J. Cancer 2003, 89, (12), 2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraeber-Bodéré F; Rousseau C; Bodet-Milin C; Ferrer L; Faivre-Chauvet A; Campion L; Vuillez J-P; Devillers A; Chang C-H; Goldenberg DM; Chatal J-F; Barbet J Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J. Nucl. Med 2006, 47, (2), 247–255. [PubMed] [Google Scholar]
- 5.Rossin R; Renart Verkerk P; van den Bosch SM; Vulders RCM; Verel I; Lub J; Robillard MS In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed 2010, 49, (19), 3375–3378. [DOI] [PubMed] [Google Scholar]
- 6.Rossin R; Läppchen T; van den Bosch SM; Laforest R; Robillard MS Diels–alder reaction for tumor pretargeting: In vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med 2013, 54, (11), 1989–1995. [DOI] [PubMed] [Google Scholar]
- 7.Houghton JL; Membreno R; Abdel-Atti D; Cunanan KM; Carlin S; Scholz WW; Zanzonico PB; Lewis JS; Zeglis BM Establishment of the in vivo efficacy of pretargeted radioimmunotherapy utilizing inverse electron demand Diels-Alder click chemistry. Mol. Cancer Ther 2017, 16, (1), 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeglis BM; Brand C; Abdel-Atti D; Carnazza KE; Cook BE; Carlin S; Reiner T; Lewis JS Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand pharmacokinetics. Mol. Pharmaceutics 2015, 12, (10), 3575–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeglis BM; Sevak KK; Reiner T; Mohindra P; Carlin SD; Zanzonico P; Weissleder R; Lewis JS A pretargeted PET imaging strategy based on bioorthogonal Diels–Alder click chemistry. J. Nucl. Med 2013, 54, (8), 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath JK; White SJ; Johnstone CN; Catimel B; Simpson RJ; Moritz RL; Tu G-F; Ji H; Whitehead RH; Groenen LC; Scott AM; Ritter G; Cohen L; Welt S; Old LJ; Nice EC; Burgess AW The human a33 antigen is a transmembrane glycoprotein and a novel member of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. U.S.A 1997, 94, (2), 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welt S; Scott AM; Divgi CR; Kemeny NE; Finn RD; Daghighian F; Germain JS; Richards EC; Larson SM; Old LJ Phase I/II study of iodine 125-labeled monoclonal antibody a33 in patients with advanced colon cancer. J. Clin. Oncol 1996, 14, (6), 1787–1797. [DOI] [PubMed] [Google Scholar]
- 12.Scott AM; Lee F-T; Jones R; Hopkins W; MacGregor D; Cebon JS; Hannah A; Chong G; U P; Papenfuss A; Rigopoulos A; Sturrock S; Murphy R; Wirth V; Murone C; Smyth FE; Knight S; Welt S; Ritter G; Richards E; Nice EC; Burgess AW; Old LJ A phase I trial of humanized monoclonal antibody a33 in patients with colorectal carcinoma: Biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin. Cancer Res 2005, 11, (13), 4810–4817. [DOI] [PubMed] [Google Scholar]
- 13.Stabin MG; Sparks RB; Crowe E Olinda/exm: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med 2005, 46, (6), 1023–1027. [PubMed] [Google Scholar]
- 14.Larson SM; Carrasquillo JA; Cheung NK; Press OW Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, (6), 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.