Abstract
Rationale
Highly prevalent and severe sleep-disordered breathing caused by acute cervical spinal cord injury (quadriplegia) is associated with neurocognitive dysfunction and sleepiness and is likely to impair rehabilitation.
Objective
To determine whether 3 months of autotitrating CPAP would improve neurocognitive function, sleepiness, quality of life, anxiety and depression more than usual care in acute quadriplegia.
Methods and measurements
Multinational, randomised controlled trial (11 centres) from July 2009 to October 2015. The primary outcome was neurocognitive (attention and information processing as measure with the Paced Auditory Serial Addition Task). Daytime sleepiness (Karolinska Sleepiness Scale) was a priori identified as the most important secondary outcome.
Main results
1810 incident cases were screened. 332 underwent full, portable polysomnography, 273 of whom had an apnoea hypopnoea index greater than 10. 160 tolerated at least 4 hours of CPAP during a 3-day run-in and were randomised. 149 participants (134 men, age 46±34 years, 81±57 days postinjury) completed the trial. CPAP use averaged 2.9±2.3 hours per night with 21% fully ‘adherent’ (at least 4 hours use on 5 days per week). Intention-to-treat analyses revealed no significant differences between groups in the Paced Auditory Serial Addition Task (mean improvement of 2.28, 95% CI −7.09 to 11.6; p=0.63). Controlling for premorbid intelligence, age and obstructive sleep apnoea severity (group effect −1.15, 95% CI −10 to 7.7) did not alter this finding. Sleepiness was significantly improved by CPAP on intention-to-treat analysis (mean difference −1.26, 95% CI −2.2 to –0.32; p=0.01).
Conclusion
CPAP did not improve Paced Auditory Serial Addition Task scores but significantly reduced sleepiness after acute quadriplegia.
Trial registration number
ACTRN12605000799651.
Keywords: sleep apnoea
Key messages.
What is the key question?
Can autosetting CPAP treatment effectively treat the neurocognitive dysfunction and sleepiness that is secondary to the sleep-disordered breathing caused by acute cervical spinal cord injury (quadriplegia)?
What is the bottom line?
No significant difference was observed in the improvement in attention and information processing; however, sleepiness improved significantly with autosetting CPAP therapy between the treatment and usual care groups.
Why read on?
This rigorous and challenging study in a unique model of acute sleep-disordered breathing provides insights into the effect of CPAP on neuropsychological outcomes.
Introduction
Traumatic spinal cord injury is a rare but devastating event with an estimated average global incidence of 23 cases per million1 and prevalence from 236 to 1298 per million.2 The injury age distribution is bimodal: younger, predominantly men, involved in motor vehicle accidents and older people injured during a fall.1 In countries with better acute care, approximately 50% of injuries result in cervical spinal cord injury (quadriplegia/tetraplegia). Life expectancy in quadriplegia is reduced by 20%–30% overall compared with the general population; however, much of this reduction is due to peri-injury mortality, and if people survive the first year after injury, life expectancy is impacted less.3 As such, any prevalent secondary complication will be with a younger person for most of their life and will add to the comorbidities of ageing alongside the quadriplegia per se in an older person. Sleep-disordered breathing (SDB) is one such secondary complication.
SDB, predominantly obstructive sleep apnoea (OSA), can be caused acutely by cervical spinal cord injury4–6 and is up to five times more prevalent in chronic quadriplegia7–16 compared with the general population.17 18 The pathophysiological basis for SDB remain unclear in quadriplegia,19 although both ‘spinal specific’ issues such as a higher nasal resistance,20 21 ventilatory control instability,22 23 altered upper airway reflex responses24 and an increased upper airway lateral wall size,25 coupled with a high rate of ‘general population’ risks in chronic quadriplegia such as obesity, ageing and a male predominance all contribute.14
The most effective treatment for SDB is nasal CPAP, but CPAP adherence is challenging in the non-disabled and more so in quadriplegia, with success rates of 20%–50%.13 26 27 Left untreated, SDB significantly impairs neurocognitive function,28 29 increases sleepiness13 14 16 30 and reduces quality of life16 27 in people with quadriplegia. Untreated, incident SDB is highly likely to adversely impact on learning and skill acquisition during rehabilitation after cervical cord injury. We therefore undertook a randomised controlled trial to address the question of whether CPAP therapy to treat SDB in incident cases of quadriplegia altered outcomes CPAP for Obstructive Sleep Apnoea in Quadriplegia (COSAQ). The primary hypothesis was that 3 months of autotitrating, CPAP would improve attention and information processing, as measured with the Paced Auditory Serial Addition Task (PASAT) more than usual care.
Methods
Study design and governance
The COSAQ study31 was an international, multicentre, randomised, parallel-group trial with blinded outcome assessment. All participants provided written or independently witnessed oral consent if unable to sign.
Participants and outcome measurements
All incident cases of traumatic quadriplegia (neurological lesion at first thoracic level or higher and all injury severities) were screened for inclusion across 11 spinal injury units in Australia, Canada, New Zealand and England. Potential participants were excluded if they had successfully used CPAP for SDB prior to injury, had a significant coexistent head injury (Glasgow Coma Scale of <8 at first assessment), hypercapnia partial pressure of carbon dioxide in arterial blood (PaCO2 >45 mm Hg at randomisation), predicted inpatient stay of less than 3 months, a condition likely to significantly limit CPAP use (eg, major psychoses and facial or base of skull fractures), not proficient in English or unable to provide informed consent.
All consented participants underwent a full, portable sleep study in their hospital beds (Compumedics SomtePSG, Abbottsford, Australia). Those with an apnoea hypopnoea index (AHI) >10 events per hour32 were trialled on an autotitrating CPAP (S8 and S9, Resmed Autoset, San Diego, California, USA) for up to three nights. All sites received training in and had available a range of patient interfaces (nasal pillows, oronasal and nasal masks, see online supplementary file). Participants who tolerated the treatment for at least 4 hours on one of three nights were randomised to receive CPAP immediately (CPAP plus usual care) or wait for 3 months (usual care control). Simple, external, computer-generated, blocked (block size=10) randomisation was performed centrally through the study database. Allocation concealment was maintained by obscuring the randomisation sequence from study site users.
thoraxjnl-2018-212319supp001.pdf (424.5KB, pdf)
The same assessor, blinded to allocated group, administered outcome measures at baseline and study completion. The tests included a battery previously demonstrated to reveal neurocognitive limitation in those with quadriplegia and OSA.28 29 The neurocognitive test battery focused on memory, learning, attention, information processing and executive function (PASAT (primary outcome measure), the Rey Auditory Verbal Learning Test (RAVLT), the Digit Span subtest of the Wechsler Adult Intelligence Scale IV, Symbol Digit Modalities Test and North American Adult Reading Test (NAART)).31 Sleep disorder symptoms and state sleepiness were assessed with the Basic Nordic Sleep Questionnaire (BNSQ)33 and the Karolinska Sleepiness Scale (KSS).34 Health-related quality of life was assessed using the Assessment of Quality of Life Scale35 and mental health and mood using the Hospital Anxiety and Depression Scale and the Profile of Mood States.29 Additional, non-blinded measures of sleepiness (KSS), autonomic dysfunction (diary), CPAP adherence (hours) and troubleshooting of any mask or device issues were made weekly. Spirometry (FVC and FEV1) was measured monthly. Participants underwent a repeat sleep study at trial conclusion on CPAP or repeat diagnostic as per randomised group.
All testing was performed at the participants’ bedside. Respiratory function, questionnaire and other subjective data were collected at the same time in the mid-afternoon for each subject to control for possible circadian influences. Sleep studies commenced at participants’ usual sleep time.
Sample size estimation
A sample size of 150 subjects was required to detect a mean difference of 18±33 in the primary outcome (PASAT) at the 0.05 level with 0.8 power, assuming non-adherence to CPAP at 15%. This sample was also adequate to detect a difference of 1 unit in the key secondary outcome of sleepiness (KSS number required=44).31 Further details are available online.
Analyses
The primary analysis was an intention to treat, between randomised group comparison based on random allocation. The per protocol analysis was based on CPAP usage where ‘adherent’ was classified as recorded device use of at least 4 hours on at least five out of seven (70%) nights between randomisation and final neuropsychological assessment.36 37 Data were analysed in Stata V.13. The primary analysis used Student’s t-tests to assess between-group differences in change over the 3-month trial in neuropsychological function and sleepiness. Age and premorbid intelligence were controlled for at baseline in the linear regression modelling of the effect of CPAP on change in neuropsychological function (see online supplementary file).29 The effect of CPAP use on change in sleepiness in each week of the study was assessed using a mixed model linear regression, controlling for baseline sleepiness and with participant as a random effect.
Repeated measure analyses of variance assessed between-group differences in change in respiratory function over time. Between-group differences in medications (grouped as baclofen; benzodiazepines, opiates and other potentially sedative medications) were assessed with a logistic regression model controlling for baseline use.
Results
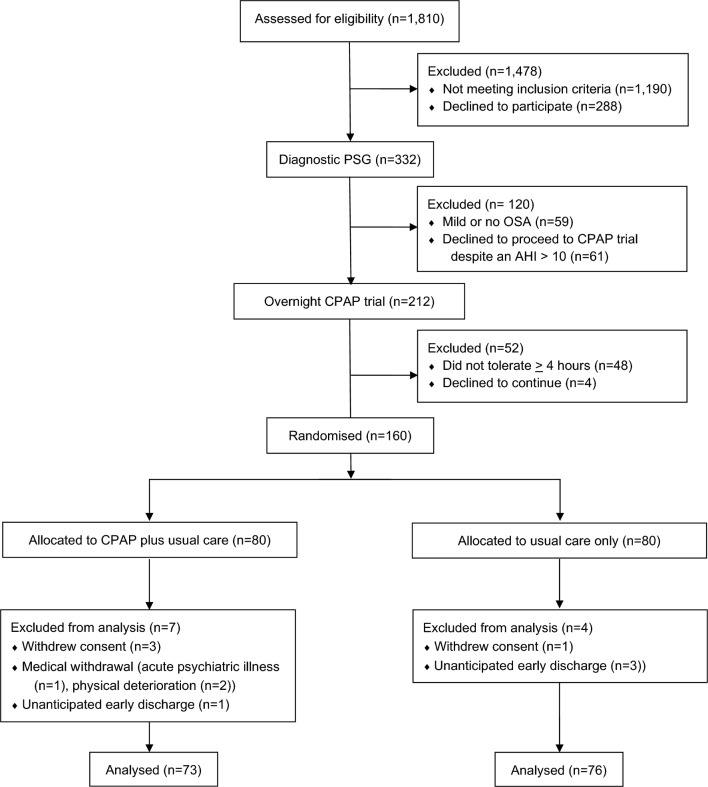
A total of 1810 consecutive incident cases of quadriplegia were screened for study inclusion between July 2009 and October 2015. Three hundred and thirty-two people met the inclusion criteria and underwent full, portable polysomnography. Of these, 59 (18%) had mild or no SDB (AHI ≤10), 273 (82%) had an AHI >10 (OSA group) and 212 progressed to a three-night autotitrating CPAP trial. Respiratory events during the baseline sleep studies were predominantly hypopnoeas with progressively more obstructive, rather than central or mixed apnoeas, as the AHI increased (table 1). Eighty-one per cent of participants had a greater percentage of obstructive than central apnoeas. Four people (1.2%) had a greater percentage of their respiratory events that were classified as central apnoeas rather than hypopnoeas. Eight participants were excluded due to hypercapnia (see figure 1 and online supplementary file for participant details).
Table 1.
Respiratory event types and proportion from the baseline sleep studies (prerandomisation, n=332)
| OSA severity | Proportion of events (%) | |||
| Hypopnoea | Apnoea | |||
| Central | Obstructive | Mixed | ||
| AHI <10 | 94.5 | 2.6 | 2.5 | 0.05 |
| AHI 10–30 | 82.7 | 3.7 | 13.3 | 0.3 |
| AHI >30 | 52.2 | 4.8 | 39.4 | 3.6 |
| All AHI | 83.4 | 3.2 | 11.8 | 1.5 |
AHI, apnoea hypopnoea index; OSA, obstructive sleep apnoea.
Figure 1.
Participant flow through the study. AHI, apnoea hypopnoea index; OSA, obstructive sleep apnoea.
One hundred and sixty-four participants tolerated at least 4 hours of CPAP during run-in, four withdrew and 160 were randomised. One hundred and forty-nine completed the study (figure 1 and table 2). Participants were 90% male, slightly overweight (mean=25.2 kg/m2, SD=5.0) and had a restrictive ventilatory deficit (VC; 2.9 L, 0.95, % predicted 58.9%, 24.6; FEV1 2.3 L/s, 0.78, % predicted 58.2%, 23.4). The average age was 47 (15) and the age distribution was bimodal (online supplementary figure E1) in line with changes in injury demographics.1
Table 2.
Participant characteristics at baseline
| CPAP (n=73) Mean (SD) or count (%) |
Usual care (n=76) Mean (SD) or count (%) |
|
| Age, years | 46.32 (15.59) | 47.03 (14.87) |
| Sex | ||
| Male | 67 (91.78) | 67 (88.16) |
| Injury characteristics | ||
| AIS A | 29 (39.73) | 26 (34.67) |
| Lesion level | ||
| C2–C4 | 40 (54.79) | 34 (44.72) |
| C5–T1 | 33 (45.21) | 42 (55.26) |
| Time from injury to sleep study, days (median (IQR)) | 66 (49–90) | 80 (60–123) |
| Body mass index, kg/m2 | 25.01 (4.77) | 25.47 (5.15) |
| Number likely to have preinjury OSA* | 20/71 (28.6) | 20/73 (27.4) |
| Karolinska Sleepiness Scale | 4.86 (2.32) | 4.24 (2.03) |
| NAART | 31.01 (12.18) | 33.53 (13.39) |
| Vital capacity, L | 2.83 (0.86) | 2.91 (1.04) |
| FEV1, L/s | 2.24 (0.68) | 2.27 (0.88) |
| Baseline sleep study indices | ||
| Apnoea hypopnoea index (median (IQR)) | 42.7 (22.7–69.3) | 41.1 (24.4–55.2) |
| Arousal index (median (IQR)) | 23.9 (16.4–35.7) | 25.1 (13.8–38.4) |
| Sleep efficiency | 67.12 (16.60) | 66.41 (18.72) |
| % total sleep time with SpO2 <90% | 10.30 (16.20) | 9.49 (17.69) |
| Medications | ||
| Baclofen | 27 (37) | 34 (45) |
| Benzodiazepines | 24 (33) | 30 (40) |
| Opiates | 49 (67) | 55 (72) |
| Other potentially sedating medications | 60 (82) | 64 (84) |
Data are provided as count (n) or mean (SD) as appropriate.
*Likelihood based on the Multivariate Apnoea Prediction Index.57 Note that with medications, participants could be included across multiple categories.
AIS A, American Spinal Injuries Association Impairment Scale A (motor and sensory complete lesion); C2–C4, second to fourth cervical cord lesion; C5–T1, fifth cervical to first thoracic cord lesion; NAART, North American Adult Reading Test; OSA, obstructive sleep apnoea.
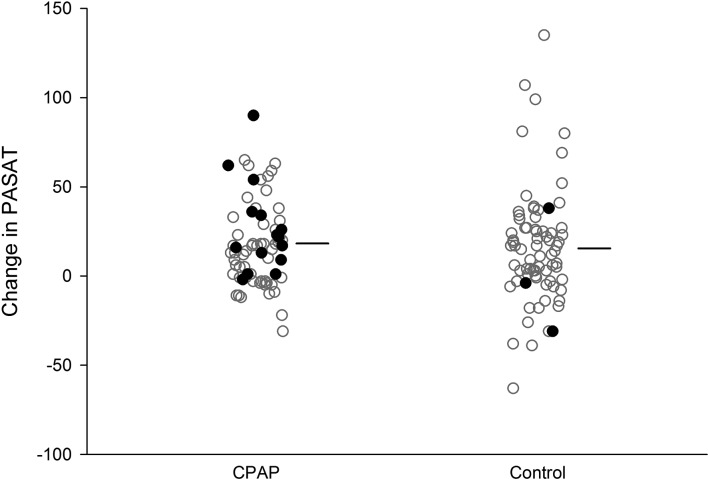
Overall CPAP use averaged 2.9 (SD=2.3) hours per night with 15 (21%) of those randomised to autotitrating CPAP classified as fully ‘adherent’ (online supplementary figure E2). The average 95th centile CPAP was 9.29 (2.56) cmH2O. Final sleep studies on autotitrating CPAP demonstrated overall good control of OSA in those randomised to CPAP; oxygen desaturation index fell from 13.0 (1.8–44.5, median interquartile range) at baseline to 0.0 (0.0–0.7) at the repeat CPAP study (p<0.01; table E2). Three participants had a residual oxygen desaturation index of greater than five events per hour. Six usual care participants were crossed over to autotitrating CPAP treatment by their clinical care teams. Of these, three were adherent on the per protocol analyses (figure 2).
Figure 2.
Individual participant and average change in the primary outcome measure (PASAT). Individual participants represented by dots. Solid dots represent those participants classified as adherent overall and thus included in the per protocol analyses. Three of six participants randomised to control and provided CPAP by their clinical care teams were adherent with CPAP. Horizontal lines represent the average change in the primary outcome measure. PASAT, Paced Auditory Serial Addition Task.
Study participants improved on the PASAT by an average of 17.0 (SD=28.1) over the 3 months; however, no significant difference was observed between groups in the intention-to-treat analysis (p=0.63, mean difference in improvement of 2.28, 95% CI −7.09 to 11.6; table 3). Results were similar after controlling for premorbid intelligence, age and OSA severity (group effect estimate of −1.15, 95% CI −10 to 7.7; table E2). In the per protocol analysis in the 18 adherent participants, the magnitude of effect was greater but not statistically significant (p=0.38, 6.30, 95% CI −7.75 to 20.34: table E3 and figure 2). Performance on the PASAT was poor at baseline compared with both non-disabled control data (15%–33% worse) and other clinical populations (table E4).38 All participants improved, although scores remained 7%–25% worse than controls.
Table 3.
Change scores and comparisons between participants randomised to usual care or CPAP
| Baseline mean (SD) | Final mean (SD) | Average within group change scores (95% CIs) | Between allocated group differences | |||||
| CPAP | Usual care | CPAP | Usual care | CPAP | Usual care | Mean (95% CI) | P values | |
| PASAT* | 99.17 (45.26) |
99.69 (46.28) |
117.66 (45.71) |
116.92 (47.17) |
18.19
(12.67 to 23.71) |
15.91
(8.40 to 23.41) |
2.29 (−7.09 to 11.66) |
0.63 |
| KSS† | 4.86 (2.32) |
4.24 (2.03) |
3.51 (1.82) |
4.14 (2.02) |
−1.36
(−1.99 to −0.72) |
−0.09 (−0.79 to 0.61) |
−1.26
(−2.20, to −0.32) |
0.01 |
| RAVLT immediate recall* | 6.45 (2.27) |
6.12 (1.93) |
7.49 (2.22) |
7.03 (2.50) |
1.04
(0.49 to 1.59) |
0.91
(0.49 to 1.33) |
0.13 (−0.55 to 0.81) |
0.70 |
| RAVLT total recall* | 47.62 (12.27) |
46.95 (11.30) |
50.53 (11.56) |
49.93 (12.51) |
2.92
(0.68 to 5.16) |
2.99
(0.97 to 5.00) |
−0.07 (−3.05 to 2.91) |
0.96 |
| RAVLT learning* | 5.00 (1.89) |
5.41 (2.05) |
4.15 (2.34) |
4.53 (2.31) |
−0.85
(−1.53 to −0.17) |
− 0.88
(−1.50 to −0.26) |
0.03 (−0.88 to 0.94) |
0.94 |
| RAVLT short term memory* | 9.51 (3.35) |
9.45 (3.83) |
9.99 (3.13) |
10.38 (3.55) |
0.51 (−0.22 to 1.25) |
0.99 (0.32 to 1.65) |
−0.47 (−1.45 to 0.51) |
0.34 |
| RAVLT delayed recall* | 8.77 (3.87) |
8.97 (4.00) |
9.68 (3.70) |
10.09 (3.58) |
0.94
(0.27 to 1.62) |
1.08
(0.40 to 1.76) |
−0.14 (−1.09 to 0.81) |
0.77 |
| RAVLT forgetting† | 2.69 (2.45) |
2.53 (2.43) |
1.96 (2.14) |
1.47 (2.20) |
−0.75
(−1.40 to −0.10) |
−1.05
(−1.64 to –0.47) |
0.30 (−0.56 to 1.17) |
0.49 |
| RAVLT recognition* | 12.70 (2.43) |
13.51 (1.51) |
13.55 (1.64) |
13.63 (1.63) |
0.73
(0.20 to 1.27) |
0.12 (−0.30 to 0.54) |
0.61 (−0.06 to 1.29) |
0.07 |
| SDMT* | 42.21 (11.04) |
42.19 (14.10) |
46.14 (11.74) |
43.62 (15.10) |
3.73
(1.52 to 5.93) |
1.43 (−0.94 to 3.81) |
2.30 (−0.93 to 5.52) |
0.16 |
| Digit Span forwards* | 9.78 (2.17) |
9.32 (2.35) |
10.14 (2.27) |
9.53 (2.43) |
0.36 (−0.16 to 0.87) |
0.21 (−0.23 to 0.65) |
0.15 (−0.52 to 0.81) |
0.67 |
| Digit Span backwards* | 8.05 (2.05) |
7.91 (2.18) |
8.55 (2.68) |
8.13 (2.58) |
0.49
(0.02 to 0.97) |
0.22 (−0.27 to 0.71) |
0.27 (−0.41 to 0.95) |
0.43 |
| HADS anxiety† | 5.57 (3.96) |
5.25 (3.84) |
4.42 (3.42) |
4.95 (3.25) |
−1.10
(−1.92 to −0.28) |
−0.30 (−1.11 to 0.50) |
−0.79 (−1.93 to 0.34) |
0.17 |
| HADS depression† | 6.28 (3.78) |
5.72 (4.07) |
5.16 (3.42) |
5.22 (3.80) |
−1.06
(−1.75 to −0.36) |
−0.65 (−1.61 to 0.31) |
−0.40 (−1.58 to 0.78) |
0.50 |
| POMS tension/anxiety† | 9.21 (7.00) |
8.22 (6.01) |
8.16 (6.42) |
6.45 (5.49) |
−1.04 (−2.38 to 0.30) |
−1.81
(−2.95 to –0.67) |
0.77 (−0.97 to 2.51) |
0.38 |
| POMS depression† | 11.90 (10.40) |
11.68 (10.94) |
9.84 (10.53) |
8.81 (10.11) |
−2.07 (−4.27 to 0.13) |
−2.96
(−5.19 to –0.73) |
0.89 (−2.21 to 4.00) |
0.57 |
| POMS anger† | 7.59 (6.28) |
7.15 (6.14) |
8.53 (7.31) |
6.61 (5.50) |
0.95 (−0.48 to 2.37) |
−0.61 (−1.91 to 0.69) |
1.55 (−0.36 to 3.47) |
0.11 |
| POMS vigour* | 16.11 (6.46) |
16.76 (6.17) |
16.96 (6.64) |
17.77 (5.60) |
0.85 (−0.70 to 2.40) |
1.14 (−0.34 to 2.61) |
−0.29 (−2.41 to 1.84) |
0.79 |
| POMS fatigue† | 10.75 (6.30) |
10.57 (6.03) |
9.66 (5.93) |
9.00 (5.82) |
−1.10 (−2.37 to 0.17) |
−1.64
(−3.01 to –0.26) |
0.54 (−1.32 to 2.40) |
0.57 |
| POMS confusion† | 9.08 (5.30) |
7.62 (4.26) |
7.92 (5.20) |
7.27 (3.99) |
−1.16 (−2.45 to 0.13) |
−0.42 (−1.29 to 0.45) |
−0.75 (−2.29 to 0.80) |
0.34 |
| POMS total† | 32.42 (32.81) |
28.47 (31.38) |
27.15 (35.67) |
20.37 (29.17) |
−5.27 (−12.22 to 1.67) |
−8.57
(−14.93 to –2.21) |
3.29 (−6.04 to 12.63) |
0.49 |
| AQoL illness* | 0.15 (0.20) |
0.16 (0.21) |
0.36 (0.30) |
0.35 (0.29) |
0.20
(0.13 to 0.27) |
0.18
(0.13 to 0.24) |
0.02 (−0.07 to 0.11) |
0.65 |
| AQoL relationships* | 0.70 (0.26) |
0.71 (0.25) |
0.76 (0.17) |
0.76 (0.18) |
0.06 (−0.003 to 0.12) |
0.04 (−0.02 to 0.11) |
0.01 (−0.07 to 0.10) |
0.74 |
| AQoL sensory* | 0.95 (0.07) |
0.94 (0.10) |
0.95 (0.08) |
0.93 (0.09) |
−0.01 (−0.03 to 0.01) |
−0.001 (−0.02 to 0.01) |
−0.01 (−0.03 to 0.02) |
0.51 |
| AQoL mental health* | 0.81 (0.16) |
0.81 (0.17) |
0.83 (0.14) |
0.84 (0.12) |
0.02 (−0.02 to 0.07) |
0.03 (−0.01 to 0.07) |
−0.01 (−0.06 to 0.05) |
0.83 |
| AQoL total health utility* | 0.14 (0.14) |
0.15 (0.16) |
0.29 (0.23) |
0.27 (0.19) |
0.15
(0.09 to 0.20) |
0.11
(0.08 to 0.15) |
0.03 (−0.03 to 0.10) |
0.32 |
| BNSQ† | 42.31 (11.18) |
42.07 (11.28) |
36.71 (10.66) |
38.82 (11.58) |
−6.44
(−9.27 to 3.62) |
−3.58
(−6.42 to –0.74) |
2.86 (−1.11 to 6.84) |
0.16 |
Bold indicates significant within-group improvement from baseline. P values refer to between-group comparisons of change scores.
*Higher test scores represent better functioning.
†Lower test scores represent better functioning.
AQoL, Assessment of Quality of Life; BNSQ, Basic Nordic Sleep Questionnaire; Digit Span, Digit Span subtest of the Wechsler Adult Intelligence Scale IV; HADS, Hospital Anxiety and Depression Scale; KSS, Karolinska Sleepiness Scale; PASAT, Paced Auditory Serial Addition Task; KSS, Karolinska Sleepiness Scale; POMS, Profile of Mood States; RAVLT, Rey Auditory Verbal Learning Test; SDMT, Symbol Digit Modalities Test.
Sleepiness measured by the KSS was significantly improved (lower scores are less sleepy) by autotitrating CPAP on intention-to-treat analysis (table 3; p<0.01, mean difference in improvement of −1.26, 95% CI −2.2 to –0.32), after controlling for baseline predictors (−0.65, 95% CI −1.3 to –0.01; table E2) and in each week in which participants were classified as adherent (online supplementary figure E2; p=0.004, −0.45, 95% CI −0.77 to –0.14). The mean improvement was marginally larger on per protocol (table E3), but the difference was no longer statistically significant (p=0.07; −1.34, 95% CI −2.80 to 0.12). The BNSQ measure of sleepiness was non-significantly improved by CPAP on intention to treat (p=0.16; −2.86, 95% CI −0.11 to 6.84) but significantly improved after controlling for baseline predictors (−3.42, 95% CI −6.67 to –0.16; table E2) and on per protocol (p=0.003; −9.09, 95% CI −3.2 to –15; table E3). The POMS Fatigue score improved overall (−1.37, 95% CI −2.2 to –0.44) but was not different between groups (p=0.57, table 3).
Ten of the 27 outcome measures (table 3) significantly improved within group over time. The intention-to-treat, linear regression models and the per protocol comparisons, found no between-group differences in any of the outcome measures other than sleepiness (KSS). Linear regression modelling of change in neuropsychological function after controlling for the baseline values of age, premorbid intelligence and AHI found that being younger was associated with significantly increased overall improvement in the PASAT, the Symbol Digit Modality Test and RAVLT ‘delayed recall’ and ‘forgetting’ domains (table E2). A higher premorbid intelligence score (NAART) was also associated with increased overall improvement in the PASAT, sleepiness on the BNSQ, the Symbol Digit Modality Test and on RAVLT ‘immediate recall’ and ‘total recall’ (table E2).
The use of pain and antispasmodic medications that were potentially sedating was high at both baseline and at study conclusion (tables 1 and 4). Baclofen prescription increased at study conclusion but proportionally less in the CPAP group (52% vs 75%, p=0.03).
Table 4.
Baseline and end-study (3 months) usage of potentially sedating medications
| Medications | CPAP, n (%) | Usual care, n (%) | P values | ||
| Baseline | Final | Baseline | Final | ||
| Baclofen | 27 (37) | 38 (52) | 34 (45) | 54 (71) | 0.03 |
| Benzodiazepines | 24 (33) | 22 (30) | 30 (40) | 21 (28) | 0.43 |
| Opiates | 49 (67) | 43 (60) | 55 (72) | 48 (63) | 0.74 |
| Other potentially sedating medications | 60 (82) | 64 (88) | 64 (84) | 68 (89) | 0.76 |
P values represent effect of group allocation in the presence or absence of these medications at the final assessment. Differences were assessed with a logistic regression model that controlled for individuals’ baseline use and group allocation.
No differences were observed between randomisation groups in the frequency of autonomic dysreflexia events per week (p=0.37; −0.17, 95% CI −0.55 to –0.21), serious adverse events or measures of heart rate variability (see supplementary data).
Discussion
This study confirmed that SDB is highly prevalent (82% of those tested with full polysomnography (PSG)) and that neurocognitive performance is substantially impaired after an acute cervical spinal cord injury. Treatment with autotitrating CPAP did not improve PASAT scores more than the improvement seen over time on both intention-to-treat and per protocol analyses. As previously reported, neurocognitive impairment was associated with the severity of SDB at baseline.29 The autotitrating CPAP effectively treated SDB when people were able to tolerate the therapy and the SDB persisted in those untreated with autotitrating CPAP. Subjective sleepiness was significantly improved by autotitrating CPAP on the intention-to-treat analyses despite the low overall adherence.
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) study, a large randomised controlled trial of CPAP versus sham-CPAP in 1098 able-bodied participants,39 found a small improvement in a subset of a battery of neurocognitive tests after 2 months of active therapy. The APPLES authors speculated that the relatively high measured intelligence of their sample, and an associated cognitive reserve that was potentially resistant to the detrimental effects of SDB,40 may have masked any opportunity for improved neurocognitive function with CPAP. The NAART-estimated average intelligence in our study was 23% lower than general population data (33.8/61 and 41.4/61, respectively; online supplementary etable 5)41 and thus our sample exhibited less cognitive reserve and should have provided more opportunity for improvement as suggested by the APPLES authors. Furthermore, the SDB treated in the current study was acutely caused by the cervical injury5 and as such potentially spared the less reversible, structural neural and neuropsychological changes seen in the able bodied with long-standing SDB.40 Despite controlling for cognitive reserve, age and SDB severity as suggested in a recent review,40 the current study suggests that treating SDB does not improve neurocognitive dysfunction overall, and specifically attention and information processing as measured by the PASAT, in acute quadriplegia.
A number of controlled trials of CPAP for SDB have demonstrated improvements in sleepiness with treatment.39 42 43 The minimally clinical important difference in the KSS has not been defined; however, the 1.26 unit relative reduction in sleepiness with CPAP observed on the intention-to-treat analysis compares favourably with the reported difference of 1.35 between people with and without moderate SDB44 and the approximate 1.5 unit increase in KSS observed after a night of CPAP withdrawal in people successfully treated for SDB.45 Improved sleepiness was also observed on both the KSS and the BNSQ total scores after controlling for baseline confounders. Additionally, the analyses of weekly KSS reports compared with CPAP use or not in the preceding week suggest that effective treatment directly reduced sleepiness, although as these sleepiness weekly data were self-reported and thus not blinded to group, a placebo response cannot be excluded. The sleepiness changes over multiple measures suggest that the improvement with autotitrating CPAP was both statistically and clinically significant.
Potentially sedating and ventilatory control modifying medications are used commonly in SCI care and could confound measures of neuropsychological function and sleepiness. There was no change in the proportions of benzodiazepine, opiate or other potentially sedating medications over time except for baclofen. Baclofen, a gamma aminobutyric acid-B agonist, is a centrally acting, antispasmodic agent and muscle relaxant used to treat spasticity in quadriplegia that has been shown to have a sedative effect but not to worsen OSA in a non-disabled sample.46 The majority of studies in SCI have similarly found no effect of baclofen on the AHI.13 14 29 30 Baclofen use increased by approximately 40% in the CPAP group and 60% in the usual care arm (online supplementary etable 6). In people with quadriplegia, periodic leg movements are common,6 worsened by SDB47 and have been treated with baclofen as ‘spasticity and spasm’.48 The smaller increase in baclofen in the CPAP group could partially explain the between-group difference in sleepiness, or alternatively, less spasm associated with OSA treatment may have reduced baclofen prescription by clinicians.
The current study confirmed substantial neurocognitive impairment (online supplementary etable 5) in a sample where significant acute brain injury was excluded29 and demonstrated clinically important improvement in PASAT scores in both trial arms. Approximately one-third of test battery scores improved significantly within group (table 2), and thus while resolution of comorbid mild traumatic brain injury49 and/or a learning effect cannot be excluded, there was no statistically significant group mean difference on any domain other than sleepiness. Recent research has revealed that isolated SCI is associated with generalised cortical neuroinflammation,50 and we speculate that resolution of inflammation may partially explain the overall improvement in attention and information processing. Further research exploring this hypothesis and its effect on rehabilitation may be warranted.
In common with most pragmatic randomised clinical controlled trials, this study had a number of limitations. In particular, the average use of autotitrating CPAP was low at 2.9 hours per night. The reasons for this poor adherence in people with quadriplegia are multifactorial (as in the non-disabled), but the most commonly reported complaints in quadriplegia are nasal congestion, an inability to fall asleep with the mask on and no perceived benefit or noticeable change in symptoms.27 51 CPAP remains the first-line therapy for SDB, although there is increasing recognition that alternate therapies are needed. Emerging evidence suggests that SDB in quadriplegia may result from different combinations of phenotypic factors than in the non-disabled population22–25 52 53 and that some of these factors, in particular a markedly raised nasal resistance,20 21 may require alternative treatment targets.
Autotitrating CPAP usage, while low, was sufficient to improve sleepiness. Similar CPAP usage has been demonstrated in populations such as aged care (median use of 1:52 hours per night at 3 months)43 where a comparable improvement in sleepiness without any differential improvement in cognitive function was observed. The usage observed in the current study is also similar to the recent Sleep Apnoea Cardiovascular Endpoints (SAVE) trial of 2717 people where CPAP for OSA was used on average for 3.3 hours per night and a 10.4% improvement in sleepiness was observed.42 The aforementioned APPLES study reported average adherence of 4.2 hours in the active arm,39 with similar sleepiness changes, particularly in those with more severe baseline OSA. Despite the low adherence overall, per protocol analyses examining ‘good users’ did not suggest any improvement in attention and information processing favouring treatment. In contrast, the sleepiness improvement with CPAP was significant in both the primary comparisons and in the analysis relating sleepiness to usage in the prior week. The per protocol analysis of sleepiness demonstrated a larger effect size favouring autotitrating CPAP. We speculate this failed to achieve statistical significance (online supplementary etable 4; p=0.07), due to the low numbers who attained our conservative measure of adherence.
While the majority of respiratory events in the current study were hypopnoeas or obstructive apnoeas32 and daytime hypercapnia was rare, bilevel positive airway pressure (PAP) rather than autotitrating CPAP may be hypothesised to have been a ‘better tolerated’ or ‘more effective’ therapy. Sleep-onset hypoventilation15 23 and central sleep apnoea15 53 have been described in a series of smaller, laboratory-based studies; however, larger and, particularly population-based, samples, have consistently reported predominant OSA in quadriplegia.6 7 14 30 54–56 Occasional central sleep apnoea is observed in larger trials, but as in the current inception cohort, it appears to be the exception not the rule. There is no published direct comparison of adherence with bilevel PAP versus CPAP therapy in quadriplegia and as such it is not possible to answer whether CPAP or bilevel PAP is ‘best’ in quadriplegia. A recent case series in spinal cord injury (74% quadriplegia) described bilevel PAP to treat OSA and bilevel PAP with volume-assured pressure support to treat OSA and sleep (but not necessarily daytime) hypoventilation.56 If those lost to follow-up in that paper are assumed to be non-adherent, then overall PAP use of 28% at 3 months is similar to the 21% observed in our experiment. As such, we believe it is difficult to assert that bilevel PAP would have materially affected adherence and outcomes, although this is speculation that warrants further investigation.
As recently reviewed,40 the relationship between SDB including OSA, neurocognitive impairments and CPAP is complex. Groups of people with OSA are neurocognitively impaired compared with non-OSA controls but not all those with OSA demonstrate neurocognitive dysfunction. Comorbid conditions, ageing, cognitive reserve and other factors confound the effect of SDB on neurocognition, and there is no clear ‘dose’ of CPAP that is consistently associated with improvement. It remains possible that the average CPAP use in the current study was insufficient to reverse the SDB associated, neurocognitive impairments observed at baseline.29 APPLES demonstrated small improvements in executive function but only in those most sleepy at baseline,39 whereas the previously mentioned aged-care trial with comparable CPAP use to the current study found no effect on cognitive function.43 The Bucks et al review concluded that it remains unclear whether CPAP can reverse neurocognitive dysfunction. The current study findings in a sensitised quadriplegia model with both less cognitive reserve and acute SDB supports this uncertainty.
This study found that treatment of OSA with CPAP in acute quadriplegia did not modify neuropsychological improvement as measured with the PASAT but was associated with improved sleepiness. In a non-disabled population, improving sleepiness is seen as a worthwhile goal of treatment, and worse sleepiness is linearly associated with poorer health status in quadriplegia.16 As such, we suggest that treating sleepiness from SDB is important in quadriplegia and that exploration of novel therapies for those who are intolerant of CPAP deserves further investigation.
Footnotes
Contributors: All authors contributed to drafting the work or revising it critically for important intellectual content; provided final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All named authors made substantial contributions to the conception and design of the work and the acquisition, analysis and interpretation of data for the work. The COSAQ Collaborative author group made substantial contributions to the acquisition and interpretation of data for the work.
Funding: The project was primarily funded by the Transport Accident Commission (Victoria, Australia) as an element of the Sleep Health in Quadriplegia research programme (DP158) and also supported by the National Health and Medical Research Council (Australia, APP1080020) and the Rick Hansen Foundation (Canada). Auto-setting continuous positive airway pressure devices were provided as in-kind support by ResMed (San Diego. USA).
Competing interests: DJB, NA and AT report a grant from the Rick Hansen Foundation during the conduct of the study. DM, PAC, BL, IG, DJB, DB and NA report a grant from the National Health and Medical Research Council during the conduct of the study. RJP, PK, DJB and DB report a grant from the Transport Accident Commission during the conduct of the study. All authors received non-financial support from ResMed during the conduct of the study. Outside the submitted work PAC has an appointment to an endowed academic chair at the University of Sydney that was created from ResMed funding. He has received research support from ResMed, SomnoMed and Zephyr Sleep Technologies. He is a consultant/adviser to Zephyr Sleep Technologies and ResMed (Narval). He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004).
Patient consent: Obtained.
Ethics approval: The trial was approved by human research ethics committees at each site (Austin Health Human Research Ethics Committee (lead)).
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: The COSAQ Collaborative authors (group authorship) are: Rick Acland (Burwood Spinal Unit, Burwood Hospital, Canterbury District Health Board, Christchurch, New Zealand), Janette L Alexander (Victorian Spinal Cord Service, Austin Hospital, Heidelberg, Australia), Amber E Backwell (University of British Columbia, Department of Physical Medicine & Rehabilitation, GF Strong Rehab Centre, Vancouver, Canada), Lauren Booker (Institute for Breathing and Sleep, Austin Health, Heidelberg, Australia), Joy R Chowdhury (The Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry, UK), Amanda Davies (Queen Elizabeth Hospital, Birmingham NHS Foundation Trust, Birmingham, UK), Brett Duce (Princess Alexandra Hospital, Ipswich Road, Woolloongabba, Queensland, Australia), Rebecca Dytor (The Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry Shropshire, UK), Nurit Fox (University of British Columbia, Faculty of Medicine, Vancouver, Canada), AJ Hirsch Allen (University of British Columbia, Faculty of Medicine, Vancouver, Canada), Denise M Hislop (Auckland Spinal Rehabilitation, Otahuhu, Auckland), Rachel Jones (Princess Alexandra Hospital, Ipswich Road, Woolloongabba, Queensland, Australia), Teresa Jones (The Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry Shropshire, UK), Carol Li (Prince of Wales Hospital, Barker Street, Randwick, Australia), Meghan Leigh (Icon Cancer Foundation, South Brisbane, Australia), Sarah Leighton (Princess Royal Spinal Cord Injuries Centre, Northern General Hospital, Sheffield, UK), Louise MacLellan (Victorian Spinal Cord Service, Austin Hospital, Heidelberg, Australia), Victoria Middleton (Princess Royal Spinal Cord Injuries Centre, Northern General Hospital, Sheffield, UK), Melinda S Millard (Victorian Spinal Cord Service, Austin Hospital, 145 Studley Rd, Heidelberg, Australia), Lianne Nier (Spinal Rehabilitation, Royal North Shore Hospital, St. Leonard’s, Australia), Lynne O’Keeffe (Auckland Spinal Rehabilitation, Otahuhu, Auckland), Aheed Osman (The Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry Shropshire, UK), Jessica Patti (Institute for Breathing and Sleep, Austin Health, Heidelberg, Australia), Valerie Pick (Princess Alexandra Hospital, Ipswich Road, Woolloongabba, Queensland, Australia), Warren R Ruehland (Institute for Breathing and Sleep, Austin Health, Heidelberg, Australia), Jo Spong (Institute for Breathing and Sleep, Austin Health, Heidelberg, Australia; College of Science, Health and Engineering, La Trobe Rural Health School, La Trobe University, Bendigo, Australia), Kate Sutherland (Department of Respiratory and Sleep Medicine, Royal North Shore, St. Leonard’s, Australia), Amber M Van Lit (Auckland Spinal Rehabilitation, Otahuhu, Auckland) and Catherine Whittall (The Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry, UK).
Contributor Information
the COSAQ Collaborative:
Rick Acland, Janette L Alexander, Amber E Backwell, Lauren Booker, Joy R Chowdhury, Amanda Davies, Brett Duce, Rebecca Dytor, Nurit Fox, Aj Hirsch Allen, Denise M Hislop, Rachel Jones, Teresa Jones, Carol Li, Meghan Leigh, Sarah Leighton, Louise Maclellan, Victoria Middleton, Melinda S Millard, Lianne Nier, Lynne O’keeffe, Aheed Osman, Jessica Patti, Valerie Pick, Warren R Ruehland, Jo Spong, Kate Sutherland, Amber M Van lit, and Catherine Whittall
References
- 1. Lee BB, Cripps RA, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014;52:110–6. 10.1038/sc.2012.158 [DOI] [PubMed] [Google Scholar]
- 2. Furlan JC, Sakakibara BM, Miller WC, et al. Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci 2013;40:456–64. 10.1017/S0317167100014530 [DOI] [PubMed] [Google Scholar]
- 3. Middleton JW, Dayton A, Walsh J, et al. Life expectancy after spinal cord injury: a 50-year study. Spinal Cord 2012;50:803–11. 10.1038/sc.2012.55 [DOI] [PubMed] [Google Scholar]
- 4. Tran K, Hukins C, Geraghty T, et al. Sleep-disordered breathing in spinal cord-injured patients: a short-term longitudinal study. Respirology 2010;15:272–6. 10.1111/j.1440-1843.2009.01669.x [DOI] [PubMed] [Google Scholar]
- 5. Berlowitz DJ, Brown DJ, Campbell DA, et al. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil 2005;86:1193–9. 10.1016/j.apmr.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 6. Proserpio P, Lanza A, Sambusida K, et al. Sleep apnea and periodic leg movements in the first year after spinal cord injury. Sleep Med 2015;16:59–66. 10.1016/j.sleep.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 7. Short DJ, Stradling JR, Williams SJ. Prevalence of sleep apnoea in patients over 40 years of age with spinal cord lesions. J Neurol Neurosurg Psychiatry 1992;55:1032–6. 10.1136/jnnp.55.11.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McEvoy RD, Mykytyn I, Sajkov D, et al. Sleep apnoea in patients with quadriplegia. Thorax 1995;50:613–9. 10.1136/thx.50.6.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biering-Sørensen F, Biering-Sørensen M, Hilden J. Reproducibility of Nordic Sleep Questionnaire in spinal cord injured. Paraplegia 1994;32:780–6. 10.1038/sc.1994.124 [DOI] [PubMed] [Google Scholar]
- 10. Burns SP, Little JW, Hussey JD, et al. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil 2000;81:1334–9. 10.1053/apmr.2000.9398 [DOI] [PubMed] [Google Scholar]
- 11. Levi R, Hultling C, Nash MS, et al. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia 1995;33:308–15. 10.1038/sc.1995.70 [DOI] [PubMed] [Google Scholar]
- 12. Star AM, Osterman AL. Sleep apnea syndrome after spinal cord injury. Report of a case and literature review. Spine 1988;13:116–7. [DOI] [PubMed] [Google Scholar]
- 13. Stockhammer E, Tobon A, Michel F, et al. Characteristics of sleep apnea syndrome in tetraplegic patients. Spinal Cord 2002;40:286–94. 10.1038/sj.sc.3101301 [DOI] [PubMed] [Google Scholar]
- 14. Berlowitz DJ, Spong J, Gordon I, et al. Relationships between objective sleep indices and symptoms in a community sample of people with tetraplegia. Arch Phys Med Rehabil 2012;93:1246–52. 10.1016/j.apmr.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 15. Sankari A, Bascom A, Oomman S, et al. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 2014;10:65–72. 10.5664/jcsm.3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spong J, Graco M, Brown DJ, et al. Subjective sleep disturbances and quality of life in chronic tetraplegia. Spinal Cord 2015;53:636–40. 10.1038/sc.2015.68 [DOI] [PubMed] [Google Scholar]
- 17. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–8. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–14. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuller DD. How does spinal cord injury lead to obstructive sleep apnoea? J Physiol 2018;596:2633 10.1113/JP276162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wijesuriya NS, Lewis C, Butler JE, et al. High nasal resistance is stable over time but poorly perceived in people with tetraplegia and obstructive sleep apnoea. Respir Physiol Neurobiol 2017;235:27–33. 10.1016/j.resp.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 21. Gainche L, Berlowitz DJ, LeGuen M, et al. Nasal resistance is elevated in people with tetraplegia and is reduced by topical sympathomimetic administration. J Clin Sleep Med 2016;12:1487–92. 10.5664/jcsm.6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sankari A, Bascom AT, Riehani A, et al. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. J Appl Physiol 2015;119:1183–93. 10.1152/japplphysiol.00088.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bascom AT, Sankari A, Goshgarian HG, et al. Sleep onset hypoventilation in chronic spinal cord injury. Physiol Rep 2015;3:e12490 10.14814/phy2.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wijesuriya NS, Gainche L, Jordan AS, et al. Genioglossus reflex responses to negative upper airway pressure are altered in people with tetraplegia and obstructive sleep apnoea. J Physiol 2018;596:2853–64. 10.1113/JP275222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Donoghue FJ, Meaklim H, Bilston L, et al. Magnetic resonance imaging of the upper airway in patients with quadriplegia and obstructive sleep apnea. J Sleep Res 2018;27 10.1111/jsr.12616 [DOI] [PubMed] [Google Scholar]
- 26. Burns SP, Rad MY, Bryant S, et al. Long-term treatment of sleep apnea in persons with spinal cord injury. Am J Phys Med Rehabil 2005;84:620–6. 10.1097/01.phm.0000171008.69453.b9 [DOI] [PubMed] [Google Scholar]
- 27. Berlowitz DJ, Spong J, Pierce RJ, et al. The feasibility of using auto-titrating continuous positive airway pressure to treat obstructive sleep apnoea after acute tetraplegia. Spinal Cord 2009;47:868–73. 10.1038/sc.2009.56 [DOI] [PubMed] [Google Scholar]
- 28. Sajkov D, Marshall R, Walker P, et al. Sleep apnoea related hypoxia is associated with cognitive disturbances in patients with tetraplegia. Spinal Cord 1998;36:231–9. 10.1038/sj.sc.3100563 [DOI] [PubMed] [Google Scholar]
- 29. Schembri R, Spong J, Graco M, et al. Neuropsychological function in patients with acute tetraplegia and sleep disordered breathing. Sleep 2017;40 10.1093/sleep/zsw037 [DOI] [PubMed] [Google Scholar]
- 30. Leduc BE, Dagher JH, Mayer P, et al. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil 2007;88:333–7. 10.1016/j.apmr.2006.12.025 [DOI] [PubMed] [Google Scholar]
- 31. Berlowitz DJ, Ayas N, Barnes M, et al. Auto-titrating continuous positive airway pressure treatment for obstructive sleep apnoea after acute quadriplegia (COSAQ): study protocol for a randomized controlled trial. Trials 2013;14:181 10.1186/1745-6215-14-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- 33. Biering-Sørensen F, Biering-Sørensen M. Sleep disturbances in the spinal cord injured: an epidemiological questionnaire investigation, including a normal population. Spinal Cord 2001;39:505–13. 10.1038/sj.sc.3101197 [DOI] [PubMed] [Google Scholar]
- 34. Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep 1994;17:236–41. 10.1093/sleep/17.3.236 [DOI] [PubMed] [Google Scholar]
- 35. Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999;8:209–24. 10.1023/A:1008815005736 [DOI] [PubMed] [Google Scholar]
- 36. Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30:711–9. 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5:173–8. 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 2006;21:53–76. 10.1016/j.acn.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 39. Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593–602. 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bucks RS, Olaithe M, Rosenzweig I, et al. Reviewing the relationship between OSA and cognition: Where do we go from here? Respirology 2017;22:1253–61. 10.1111/resp.13140 [DOI] [PubMed] [Google Scholar]
- 41. Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol 2002;24:1123–37. 10.1076/jcen.24.8.1123.8375 [DOI] [PubMed] [Google Scholar]
- 42. McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med 2016;375:919–31. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 43. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med 2014;2:804–12. 10.1016/S2213-2600(14)70172-9 [DOI] [PubMed] [Google Scholar]
- 44. Wong KK, Marshall NS, Grunstein RR, et al. Comparing the neurocognitive effects of 40 h sustained wakefulness in patients with untreated OSA and healthy controls. J Sleep Res 2008;17:322–30. 10.1111/j.1365-2869.2008.00665.x [DOI] [PubMed] [Google Scholar]
- 45. Filtness AJ, Reyner LA, Horne JA. One night’s CPAP withdrawal in otherwise compliant OSA patients: marked driving impairment but good awareness of increased sleepiness. Sleep Breath 2012;16:865–71. 10.1007/s11325-011-0588-8 [DOI] [PubMed] [Google Scholar]
- 46. Finnimore AJ, Roebuck M, Sajkov D, et al. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J 1995;8:230–4. 10.1183/09031936.95.08020230 [DOI] [PubMed] [Google Scholar]
- 47. Peters AEJ, van Silfhout L, Graco M, et al. Periodic limb movements in tetraplegia. J Spinal Cord Med 2018;41:318–25. 10.1080/10790268.2017.1320874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levy J, Lansaman T, Ferrapie A-L, et al. Spasticity or periodic limb movements? Lessons from a not so uncommon case report. Ann Phys Rehabil Med 2017;60:e10 10.1016/j.rehab.2017.07.086 [DOI] [PubMed] [Google Scholar]
- 49. Craig A, Guest R, Tran Y, et al. Cognitive Impairment and Mood States after Spinal Cord Injury. J Neurotrauma 2017;34:1156–63. 10.1089/neu.2016.4632 [DOI] [PubMed] [Google Scholar]
- 50. Jure I, Labombarda F. Spinal cord injury drives chronic brain changes. Neural Regen Res 2017;12:1044–7. 10.4103/1673-5374.211177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Guen MC, Cistulli PA, Berlowitz DJ. Continuous positive airway pressure requirements in patients with tetraplegia and obstructive sleep apnoea. Spinal Cord 2012;50:832–5. 10.1038/sc.2012.57 [DOI] [PubMed] [Google Scholar]
- 52. Sankari A, Bascom AT, Badr MS. Upper airway mechanics in chronic spinal cord injury during sleep. J Appl Physiol 2014;116:1390–5. 10.1152/japplphysiol.00139.2014 [DOI] [PubMed] [Google Scholar]
- 53. Sankari A, Bascom AT, Chowdhuri S, et al. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol 2014;116:345–53. 10.1152/japplphysiol.00731.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klefbeck B, Sternhag M, Weinberg J, et al. Obstructive sleep apneas in relation to severity of cervical spinal cord injury. Spinal Cord 1998;36:621–8. 10.1038/sj.sc.3100675 [DOI] [PubMed] [Google Scholar]
- 55. Graco M, Schembri R, Cross S, et al. Diagnostic accuracy of a two-stage model for detecting obstructive sleep apnoea in chronic tetraplegia. Thorax 2018;73:864–71. 10.1136/thoraxjnl-2017-211131 [DOI] [PubMed] [Google Scholar]
- 56. Brown JP, Bauman KA, Kurili A, et al. Positive airway pressure therapy for sleep-disordered breathing confers short-term benefits to patients with spinal cord injury despite widely ranging patterns of use. Spinal Cord 2018;56:777–89. 10.1038/s41393-018-0077-z [DOI] [PubMed] [Google Scholar]
- 57. Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep 1995;18:158–66. 10.1093/sleep/18.3.158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2018-212319supp001.pdf (424.5KB, pdf)