Abstract
Our aim is to study unanticipated cardiotoxicity associated with the use of anticancer targeted agents, a problem that remains poorly understood. Using diagnosis codes, we retrospectively identified patients with both hematologic malignancies (HM) and cardiovascular diseases (n = 820 patients). Cardiotoxicity was defined per published criteria. The targeted agents of interest included tyrosine kinase inhibitors, proteasome inhibitors, monoclonal antibodies, and immunomodulatory agents. Patients found with cardiotoxicity (n = 29) were compared with 70 case-matched reference subjects. Median time from targeted therapy exposure to cardiotoxicity was 132 days. A higher percentage of patients had prior exposure to anthracyclines in study versus reference group (65.5 vs. 42.8%, P = 0.04), however, did not stay significant in multivariate analysis. Two variables were significant predictors, prior of DVT/PE and Karnofsky score of ≥ 80% (P ≤ 0.011). Only 2 study group patients died of cardiac causes. Most cardiotoxicity patients (23/29) had remained stable or improved, while 21 patients received further chemotherapy. OS was lower in the study group (P = 0.018) versus the reference group. In conclusion, a small number patients with HM experience unanticipated cardiotoxicity with low related mortality. Risk of cardiotoxicity was significantly associated with history of DVT/PE. Most patients do well, but despite that, their OS is significantly poorer.
Keywords: Cardiotoxicity, Hematologic malignancies, Targeted therapy
Introduction
With advances in cancer therapeutics, the overall survival of cancer patients has improved significantly over the last few decades [1]. This has led to the recognition of adverse effects in surviving patients that impart important morbidity and mortality risks. Among the different adverse effects, cardiac complications such as heart failure, myocardial ischemia/infarction, and arrhythmias have generated special attention as they are the most debilitating, consume considerable additional health-care resources, and may negatively impact overall survival (OS) [2]. Anthracyclines have been used for several decades and are the best studied agents with known cardiovascular effects and relatively high incidence of heart failure [3, 4]. However, recent advancements in the “targeted” therapies have significantly changed the cancer treatment paradigm [5]. Targeted agents, unlike conventional chemotherapy, act against specific molecular targets and have been found to cause fewer overall adverse effects versus conventional chemotherapy in controlled clinical trials [2]. However, reports on cardiotoxicity in the real-world clinical setting are unexpectedly high [6]. The frequency and pathogenesis of cardiotoxicity from targeted agents are incompletely understood but are believed related to “on-target” and “off-target” effects of these novel agents [7].
The independent Cardiac Review and Evaluation Committee (CREC) was established in view of initial reports about trastuzumab-induced cardiac toxicity [8]. CREC defined cardiac dysfunction secondary to chemotherapy based on following criteria: (1) cardiomyopathy characterized by decreased left ventricular ejection fraction (LVEF) that was either global or more severe in the septum; (2) congestive heart failure (CHF) symptoms; (3) associated signs of CHF, including but not limited to S3 gallop, tachycardia, or both; and (4) LVEF decline of at least 5% to less than 55% with accompanying signs and/or symptoms of CHF, or a decline in LVEF of at least 10% to below 55% without accompanying signs and/or symptoms [8, 9]. Any one of the four criteria is sufficient to confirm the diagnosis. Cardiotoxicity from targeted therapies includes cardiomyopathy, as well as myocardial infarction/ischemia, myocarditis, pericarditis, and various arrhythmias. The reported incidence of cardiomyopathy due to targeted agents appears widely variable, ranging from 3 to 27%. [10–12]. Understanding the pathogenesis, identifying patients at risk, prevention, and treatment of cardiotoxicity, cardiac monitoring during and after chemotherapies are among the important knowledge gaps in this area creating difficult challenges for physicians. Moreover, the natural history of such complication and its long-term effects are not well known. Anthracyclines usually cause cardiotoxicity in a dose-dependent manner, and one can anticipate toxicity at certain cumulative dose, while no such causal relationship has been identified for targeted agents; therefore, unanticipated cardiotoxicity is the name used here and in the literature to refer to such cardiac complication. The aims of this study are to estimate the incidence of such unanticipated cardiotoxicity, understand its natural history and impact on overall survival, and identify risk factors for its development in patients with hematological malignancies (HM) treated with targeted agents.
Patients and Methods
In this study approved by our Institutional Review Board, the University of Florida (UF Health), the Faculty Practice Decision Support (PFDS) team, conducted a search using 113 codes of International Classification of Diseases, version 9 (ICD-9) for HM and 18 ICD-9 codes for cardiac diseases. This yielded a file that containing medical records of 820 adult patients (≥ 18 years old) that had both HM and cardiac abnormalities for a 10-year period (2005–2014). These diagnosis codes were identified using billing data, encounter diagnosis codes, problem lists, and medical history. At our institution, the electronic medical records (EPIC) were launched in December 2011; thus, all the data before then were loaded into EPIC retrospectively. These patients were treated at our institution, thus patients referred with these diagnosis codes for other problems or specific treatments are not included. Overall, the cohort was divided into disease categories as follows (n): myelodysplastic syndrome (46), multiple myeloma (183), lymphoma (290), AML/ALL (182), chronic lymphocytic leukemia (54), and chronic myelocytic leukemia (65). Of note, during the same study period, 3593 patients with HM were treated at UF Health and the 820 patients out of all those patients with HM (23%) identified for the current study represent the patients with cardiac ICDs of interest.
From the 820 patients, we identified 55 patients who received targeted agents and had one of the cardiac ICD codes. The targeted therapies included tyrosine kinase inhibitors (TKIs), proteasome inhibitors (PIs), monoclonal antibodies, hypomethylating agents, and immunomodulatory agents (IMiDs). We defined cardiac toxicity as left ventricular ejection fraction (LVEF) of < 50%, presence of arrhythmias, or ischemic cardiovascular (CV) events that appeared after starting the specific targeted therapy. Thus, cardiotoxicity was confirmed in 46 patients. Of these, 17 patients were excluded due to meeting criteria of cardiomyopathy used in this study, mainly abnormal LVEF. The remaining 29 patients (See study algorithm, Fig. 1) represent the cardiotoxicity study group. Based on the targeted therapy used in the study group, we simultaneously selected a case-matched reference group from the 820 patients in the data file who were exposed to the potentially cardiotoxic drugs of interest but did not have evidence for associated cardiac toxicity at the time of our research. Our target was to identify 2 control patients in each drug class to match each patient in the study group, thus we ended up identifying 70 patients to be used as the reference group.
Fig. 1.
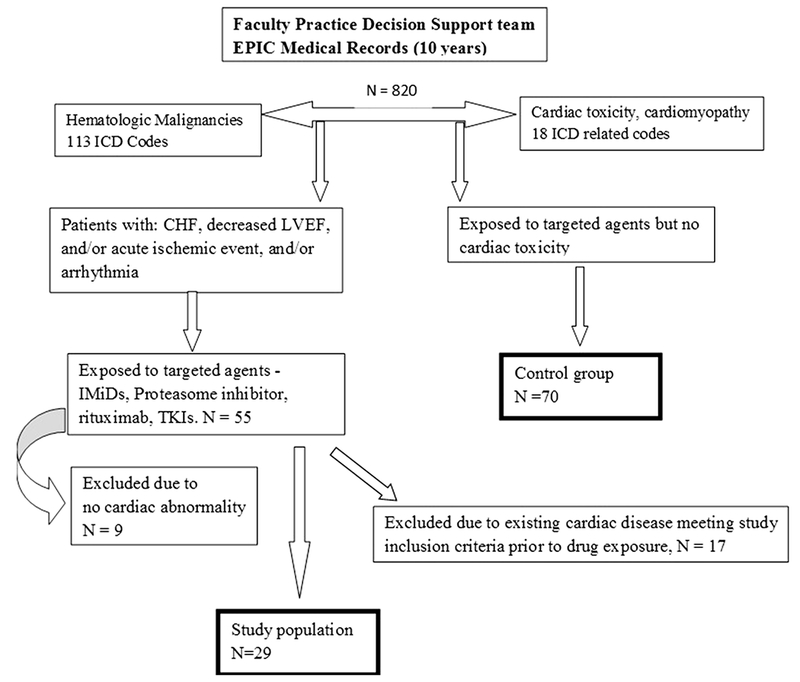
Schema showing selection of patients used in the study and reference groups
The following information was collected for both groups: age, gender, body mass index (BMI), Karnofsky performance status (KPS), cancer diagnosis, albumin, β2-microglobulin (B2M), C-reactive peptide (C-RP), B-type natriuretic peptide (BNP), troponin, creatinine, electrocardiography (EKG), cardiac echocardiography (ECHO), CV disease history and treatment, comorbidities (diabetes mellitus, hypertension, smoking, etc.), time to cardiac event, cancer progression-free (PFS) and overall survival (OS), chemotherapy before and after cardiac event.
Continuous variables were compared between those with cardiotoxicity (study group) versus control subjects (reference group) with t test and categorical variables were compared with Chi-square test. Multivariable logistic regression analysis was performed to estimate the odds ratios (ORs) and 95% confidence intervals (95% CI) of the potential risk factors for cardiotoxicity. Kaplan–Meier analysis and log-rank test were used to evaluate the effect of cardiotoxicity on the outcome of cancer therapy and its effect on PFS and OS. PFS is defined as the time from the start of induction therapy to documented relapse and is censored at the last clinic visit. OS is the time from diagnosis until patient death from any cause. All analyses were performed in SAS 9.4 (Cary, NC).
Results
Pertinent characteristics of patients in both groups are summarized in Table 1. There were no statistically significant differences in age, sex, race, or body mass index (BMI): although not numerically, a higher percentage of patients in the study group were men compared with reference subjects (P = 0.081). As shown in Table 1, disease type and drug class were similarly distributed in both groups (P = 0.271 and 0.306, respectively). There was a significantly higher number of patients (P = 0.04) who had prior exposure to anthracyclines in the study group (65.5%) versus reference group (43%) (see Table 1). Yet, none of these patients met criteria for the usually dose-dependent anthracyclines-induced cardiomyopathy.
Table 1.
Patient characteristics of study group (n = 29), and reference group (n = 70)
| Characteristics | Study group n (%) | Reference group n (%) | P value |
|---|---|---|---|
| Age, years, median (range) | 64 (27–88) | 68.5 (27–89) | 0.1811 |
| Male gender | 18 (62) | 30 (43) | 0.0817 |
| Race | 0.6917 | ||
| White | 22 (76) | 49 (70) | |
| AA | 4 (14) | 9 (13) | |
| Other | 3 (10) | 12 (17) | |
| BMI, median (range) | 29.06 (15.3–37.6) | 28 (17.1–46.7) | 0.2017 |
| Malignancy type | 0.2718 | ||
| Multiple myeloma | 15 (52) | 29 (41.43) | |
| Lymphoma/CLL | 10 (34) | 21 (30.00) | |
| Leukemia/MDS | 4 (14) | 19 (27.14) | |
| Targeted drug class | 0.3064 | ||
| Anti-CD20 (rituximab) | 10 (35) | 27 (38.5) | |
| Multi-targeted TKI | 3 (10) | 13 (19) | |
| Immunomodulator | 4 (14) | 3 (4) | |
| Proteasome inhibitor | 12 (41) | 27 (38.5) | |
| Anthracyclines exposure | 19 (65.5) | 30 (43) | 0.04 |
BMI body mass index (kg/m2), CLL chronic lymphocytic leukemia, MDS myelodysplastic syndrome, TKI tyrosine kinase inhibitor
About 3.5% (29 of 820) of patients with HM who also had a diagnosis of heart failure/cardiomyopathy experienced unanticipated cardiotoxicity due to targeted anticancer agents over the 10-year study period. The distribution of patients according to the type of drug exposure appears in Table 2 and is compared to the total number patients receiving the drug within the whole group of 820 patients. Carfilzomib is the most recently FDA approved drug in the group, which explains the small number of patients included. However, although it seems that cardiotoxicity is a prominent side effect of carfilzomib [12], but the true incidence of cardiotoxicity for any of these drugs cannot be established based on our single-center design.
Table 2.
Drugs causing cardiomyopathy in study group in comparison with their use in the entire group of patients with hematologic malignancies and cardiac ICD codes
| Drug used | Study group N = 29 | Reference group N = 70 | All patients N = 820 |
|---|---|---|---|
| IMiDs: Thal/Len/Pom | 0/2/2 | 0/4/3 | 9/42/8 |
| Bortezomib | 9 | 21 | 51 |
| Carfilzomib | 3 | 3 | 9 |
| TKIs | 3 | 13 | 35 |
| Rituximab | 10 | 26 | 52 |
| Total | 29 | 70 | 206 |
IMiDs immunomodulators, Thal thalidomide, Len lenalidomide, Pom pomalidomide, TKIs tyrosine kinase inhibitors
Median time from exposure to cardiac event was 132 days (range 1–1176). Among the study group, 19 patients had no prior cardiac disease, while 6 patients had a history of coronary artery disease, and 4 patients had prior history of arrhythmias. Nine patients had elevation in cardiac troponin, and 4 of these were diagnosed with non-ST elevation myocardial infarction (NSTEMI) at the time of cardiotoxicity. Among the remainder of the patients, troponin was thought to be elevated due to various reasons, such as cardiomyopathy, demand ischemia due to profound anemia, or arrhythmia. A total of 8 patients developed new EKG changes after starting targeted agents, the majority were atrial fibrillation, while one had developed Mobitz type-1 second-degree atrioventricular block. A total of 27 patients developed reduced LVEF and were diagnosed with cardiomyopathy, while 2 had NSTEMI and one of these developed signs and symptoms of heart failure with normal LVEF.
Whether rituximab causes cardiomyopathy is controversial [13–16]. We analyzed the 10 patients who developed cardiomyopathy after receiving rituximab in more details (see Table 3). Only minimal (50 mg/m2) or no concomitant anthracyclines were found in 5 patients who had no other explanation for cardiotoxicity. The reminder received less than maximal dose of anthracyclines. One patient had reduced LVEF (35%) prior to rituximab, 4 had prior cardiac abnormalities, and 3 had no comorbidities. Of note, 3 patients received further treatment with rituximab after the diagnosis of cardiomyopathy but without negative effect in 2 of the patients (number 2 and 9 in Table 3).
Table 3.
Rituximab exposure prior to cardiomyopathy diagnosis (the event) in 10 patients
| Age (years) | Rituximab cycles prior to event | Total anthracycline dose (mg/m2) | Prior LVEF % | Post-Chemo LVEF % | Other chemotherapy | Comorbidities | Alive/dead (repeat LVEF) |
|---|---|---|---|---|---|---|---|
| 67 | 3 (CHOP) | 150 | 60–65 | 35 | RT | None | D (CABG) |
| 60 | 1 (hyper-CVAD) | 50 | 60 | 35 | IVAC-R | Liver transplant | A (45%) |
| 88 | 2 (BR) | 0 | 60 | 35–40 | Bendamustine | CAD/CABG | D |
| 78 | 5 (COPE) | 0 | 35 | 20 | None | NSCLC/chemo | D |
| 63 | 3 (CHOP) | 150 | 60 | 20 | R-COPEx3, MTX, BRx1, RT | None | D |
| 57 | 6 (CHOP) | 300 | 55 | 30 | BRx1, ASCT | None | D (40%) |
| 49 | 4 | 0 | 40 | 20 | None | HTN, A.Fib | D |
| 62 | 2 (CHOP) + 4 (BR) | 100 | 65 | 30 | Fludara, | COPD, infusional reaction. | D (40–45%) |
| 31 | 6 (COPE) | 0 | 40–60 | 35 | RICEx3 | AI, Pacemaker | A (45%) |
| 75 | 9 (CHOP, ICE) | 400 | 60 | 10–15 | ESHAPx1 | A.Fib | D (35%) |
A.Fib atrial fibrillation, ASCT autologous stem cell transplantation, AI aortic insufficiency, D dead, A alive, RT radiation therapy, CAD coronary artery disease, CABG coronary artery bypass grafting, Fludara fludarabine, MTX methotrexate, NSCLC non-small cell lung cancer, CHOP cyclophosphamide, doxorubicin, vincristine, and prednisone, Hyper-CVAD cyclophosphamide, vincristine, doxorubicin, dexamethasone, BR bendamustine and rituximab, COPE cyclophosphamide, vincristine, prednisone, etoposide, RICE rituximab, Ifosfamide, carboplatin, etoposide, IVAC-R etoposide, cytarabine, ifosfamide, rituximab, ESHAP etoposide, solumedrol, high dose cytarabine, cisplatin
A summary of the different variables obtained and compared between groups to identify predisposing risk factors appears in Table 4. Interestingly, conventional risk factors for CVD such as age, sex, hypertension, diabetes mellitus, hyperlipidemia, obesity, and smoking were similarly distributed without statistical significance between groups. Only two variables remained significant in multivariable analysis, including history of deep venous thrombosis/pulmonary embolism (DVT/PE; OR 4.88, 95% CI 1.44–16.54, P = 0.011), and KPS of ≥ 80% (OR 3.99, 95% CI 1.51–10.6, P = 0.005). Prior exposure to anthracyclines was not significantly different between two groups on multivariable analysis (OR 2.06, 95% CI 0.79–5.36, P = 0.13).
Table 4.
Comparison of potential risk factors for cardiotoxicity in study and control groups
| Potential risk factors | Study group n (%) | Reference group n (%) | P value |
|---|---|---|---|
| Prior heart disease | 10 (35) | 34 (49) | 0.1992 |
| Smoking | 15 (52) | 30 (43) | 0.42 |
| Renal failure | 9 (31) | 32 (46) | 0.1772 |
| Liver abnormalities | 7 (24) | 9 (13) | 0.1652 |
| P450_drug | 14 (48) | 30 (43) | 0.621 |
| Anthracycline exposure | 19 (66) | 30 (43) | 0.04** |
| Diabetes | 6 (21) | 22 (31) | 0.2803 |
| Hypertension | 20 (69) | 49(70) | 0.9188 |
| Prior arrhythmia | 5 (17) | 20 (29) | 0.2376 |
| Prior stem cell transplant | 8 (28) | 24 (34) | 0.5166 |
| Prior stroke or TIA | 1 (4) | 4 (6) | 0.2196 |
| Hyperlipidemia | 7 (24) | 24 (34) | 0.3218 |
| DVT/PE | 8 (28) | 7 (10) | 0.0263** |
| Karnofsky performance scale ≥ 80% | 15 (51) | 18 (25) | 0.0365** |
DVT/PE deep vein thrombosis and/or pulmonary embolism, TIA transient ischemic attack
Significantly different
With median follow-up from diagnosis of cardiotoxicity of 27 months (range 1–120), 17 patients in the study group died, but only 2 of CVD causes (6.8%). Repeat echocardiograms showed further reduction in LVEF in 4 patients, while LVEF remained stable or improved in 23 patients (79%), and 21 patients when on to receive further chemotherapy. Survival analysis (Fig. 2) showed significantly worse OS for patients with cardiotoxicity when compared with the reference group (P = 0.018; Hazard ratio 2.615, 95% CI 1.377–4.966). Median OS with the primary cancer was 60 and 120 months for those with and without cardiac toxicity, respectively. These findings are especially important since the cancer median PFS was 36 months for both groups (P = 0.41, Fig. 2).
Fig. 2.
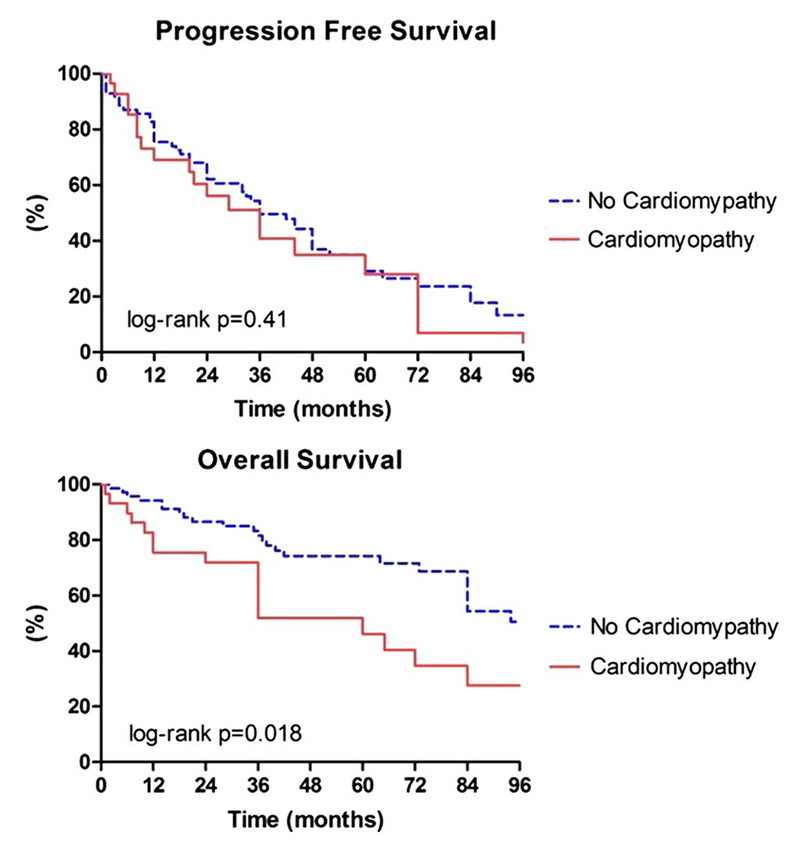
Kaplan–Meier curves for PFS and OS in the study and reference groups. Log-rank tests showed no difference in PFS (upper panel) between groups (P = 0.41), while OS (lower panel) was significantly shorter in the study group (P = 0.018) versus the reference group
Discussion
Overall use of targeted anticancer agents is increasing in most centers. These newer agents inhibit specific molecular components in cells and are believed to cause fewer serious adverse effects when compared to conventional chemotherapy. With the widespread use of newer anticancer therapies and parallel improvement in cancer patient survival, treating physicians are in uncharted territory relative to unknown adverse effects from these newer therapies. Among them, cardiotoxicity is of major concern in contemporary oncology, with many unresolved issues such as the frequency, mechanism of toxicity, risk factors, and effects on overall outcome.
Here, we present results of a retrospective analysis of patients with HM treated with targeted agents who developed cardiac toxicity in a real-world setting. Our findings suggest that only a small number of patients with HM experienced unanticipated cardiac toxicity associated with various targeted anticancer agents. These agents included rituximab, multi-targeted TKIs (imatinib, dasatinib, nilotinib), immunomodulators (lenalidomide, pomalidomide), and proteasome inhibitors (bortezomib, carfilzomib). Various studies have suggested conflicting results regarding the risk of cardiotoxicity secondary to these agents [17–20]. For instance, in a meta-analysis [11], the incidences of all-grade and high-grade cardiotoxicity associated with bortezomib were 3.8% (95% CI 2.6–5.6%) and 2.3% (1.6–3.5%), respectively. However, the same meta-analysis also suggested that the patients treated with bortezomib did not have an increase in the risk of all-grade (OR 1.15, 95% CI 0.82–1.62, P = 0.41) and high-grade (OR 1.13, 95% CI 0.58–2.24, P = 0.72) cardiotoxicity compared with patients treated with control medication. Carfilzomib, which is an irreversible inhibitor of proteasome, has been reported to cause a slightly higher rate of cardiotoxicity than bortezomib (7–11%) [12]. The damaging effects of bortezomib and carfilzomib are likely related to inhibition of proteasomal-dependent ongoing sarcomeric protein turnover in cardiomyocytes, but an endothelial mechanism has also been suggested [21].
Cardiotoxicity induced by rituximab, independent of anthracycline use, remains controversial [15, 16]. In our study, among 10 patients who developed cardiotoxicity with rituximab, 4 did not have concomitant or prior exposure to anthracyclines. CD20 functions as a calcium channel in the cell membranes, but is not known to be expressed in cardiomyocytes. However, some CVD complications, mainly arrhythmias, are mentioned in the Drug Package Insert with recommendations to “Discontinue infusions for serious or life-threatening cardiac arrhythmias.” Furthermore, it is recommended to “Perform cardiac monitoring during and after all infusions of RITUXAN for patients who develop clinically significant arrhythmias, or who have a history of arrhythmia or angina”. Cardiac dysrhythmias have been reported in 8% of patients treated with rituximab [15]. Other complications such as myocardial infarction [22], ventricular tachycardia [23], and possible Takotsubo cardiomyopathy [24] have also been reported with rituximab infusion. Cardiotoxicity associated with CHOP ± rituximab is well known and well published; however, at least one study reported no difference in cardiotoxicity between R-CHOP and CHOP [14], while another showed higher incidence of cardiac events with R-CHOP (47% for R-CHOP, vs. 35% for CHOP) which mainly was due to higher incidence of grade 1 events in R-CHOP treatment, with 8% patients developing grade 3 or 4 [25]. Despite the suggestive findings in our study group for a causative relationship between cardiomyopathy and rituximab, 3 of the patients continued to receive rituximab in combination with other chemotherapy, and 2 of them were alive at the time of this report, and with slight improvement in LVEF. Thus, we recommend continued caution with use of rituximab as mentioned in the Package Insert.
Similarly, cardiotoxicity due to TKIs have also shown inconsistent results in prior studies. In a case series, 10 patients were reported to develop significant left ventricular dysfunction at 7.2 ± 5.4 months of therapy with imatinib [26]. The incidence of congestive heart failure (CHF) reported with dasatinib ranges from 2 to 4% [27]. Apart from cardiomyopathy, targeted agents have also been shown to increase the arrhythmogenic potential in cancer patients. In one study, lenalidomide provoked cardiac arrhythmia such as atrial fibrillation (18 vs. 11% in the placebo group) [28]. In our study, among 29 patients in the study group, 5 developed new-onset atrial fibrillation after starting targeted therapy (rituximab in 3 patients and bortezomib in 2).
It remains largely unclear what predisposes some patients and not others to the development of cardiac toxicity. Although not consistently, presence of prior CVD, advanced age, and some CVD traditional risk factors such as diabetes mellitus, hypertension, hyperlipidemia, smoking have been suggested to increase risk in some reports and reviews [10, 29]. Interestingly, our study did not identify an association with any traditional risk factors. Prior exposure to anthracyclines is the single most predisposing factor with the strongest evidence in trastuzumab-induced cardiomyopathy [10]. In our study, higher number of patients had prior exposure to anthracyclines in the study versus reference group (65.5 vs. 43%, P = 0.04); however, this was not a significant risk factor in multivariable analysis. Others [30] found the combination of doxorubicin, with either bortezomib or carfilzomib, may produce additive cardiotoxicity, which is similar to known effects of trastuzumab potentiating anthracycline toxicity. As the majority of cancer patients are usually treated with more than one chemotherapeutic agent concurrently, this additive effect on cardiotoxicity can lead to unanticipated higher rates of toxicity.
Our study suggests that cardiotoxicity risk was significantly higher in patients with history of DVT/PE (OR 4.88, 95% CI 1.44–16.54, P = 0.011). Others [31] reported the antiangiogenic drugs such as thalidomide, lenalidomide, bevacizumab increase likelihood of venous thromboembolic complications. This is likely a result of inhibiting endothelial growth factor (VEGF)/VEGF receptor signaling and increased platelet aggregation. Animal studies have also demonstrated that angiogenesis plays a key role in the normal adaptive response to a pressure load, and pressure overload in presence of antiangiogenic agents results in reduction in myocardial capillary density, contractile dysfunction, myocardial fibrosis, and eventually decompensated CHF [32].
Surprisingly, our analysis also indicates that patients with cardiac toxicity had higher KPS. In our opinion, the possible explanation for such an association is that patients with higher-performance status would have had exposure to higher doses of chemotherapeutic agents in various combinations compared with those whose performance status is poor at baseline.
Cardiomyopathy from targeted agents is due to cellular dysfunction and thus is known to be more reversible (type II cardiomyopathy), as opposed to irreversible cell loss caused from repeat exposure to anthracyclines (type I cardiomyopathy). Our study results support this pattern and what has been reviewed in the literature [29], and hence > 79% of the patients in our study had stable or improved echocardiogram findings after stopping causative agents and 72% of patients safely received additional chemotherapy and/or autologous stem cell transplantation. Although, the majority of our patients had stable or improved cardiac function on subsequent echocardiography, mortality from cardiac causes was 6.8%. However, OS for the study group was significantly worse compared to the reference group. Such worse OS could be secondary to the added morbidity, interruption in anticancer therapy, and more cautious use of chemotherapeutic agents with fewer and limited options.
Since this targeted therapy-related cardiomyopathy is largely unanticipated and likely not dose dependent, it has been difficult to develop a preventative approach similar to that used with anthracyclines and trastuzumab. Since it is unpredictable and affects only small proportion of patients who mostly lack traditional CVD risk factors, genetic variability and predisposition is a strong possibility that should be investigated. A broad pharmacogenomic approach may identify such predisposing genetic variations in the targets. Such studies have been done in different types of cardiomyopathies such as idiopathic dilated cardiomyopathy, peri-partum cardiomyopathy, cardiomyopathy among hematopoietic cell transplant survivors, and stress-related cardiomyopathy [33–36].
There are some important limitations to our study that should be noted. All our findings are observational and not the result of randomized controlled trial, thus no implications about causality can be made. Furthermore, our findings are from a single-center, non-NCI experience and should not be generalized to other populations. Finally, these findings should be considered hypothesis generating and require confirmation.
In summary, we report here about a small cohort of HM patients who experienced targeted therapy-associated cardiomyopathy over a 10-year period. Our results support the reversible nature of such complication in the majority of patients, although the OS of these patients appears worse in comparison with similar reference group of patients treated with same targeted therapies. We have identified DVT/PE as a potential predictive risk factor for development of cardiotoxicity. We suggest that optimal management of targeted therapy-induced cardiotoxicity requires collaborative efforts between oncologists/hematologists and cardiologists. Furthermore, there is an urgent need for additional research to better identify risk factors and biomarkers. These should assist in individualizing treatment regimens without the risk of potentially detrimental toxicities. Moreover, further studies can be useful toward finding better tools in identifying myocardial damage at the early stages of cardiac toxicities.
Acknowledgements
Dr. Pepine receives support from the NIH-NHLBI-5 UM1 HL087366-10; 1R01HL132448-01; 5 R01 HL033610-29; 5 R01 HL056921-19; and NCATS-University of Florida Clinical and Translational Science UL1TR001427; PCORnet—OneFlorida Clinical Research Consortium CDRN-1501-26692 and the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine.
References
- 1.Siegel RL, Miller KD, & Jemal A (2016). Cancer statistics. CA: A Cancer Journal for Clinicians, 66, 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, & Ai D (2016). Cardiotoxicity associated with targeted cancer therapies. Molecular and Clinical Oncology, 4, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisberg CA, & Sawyer DB (2010). Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Current Hypertension Reports, 12, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Ashraf A, Singh R, Rahim A, Rostom W, Hussain M, et al. (2017). Incidence, time of occurrence and response to heart failure therapy in patients with anthracycline cardiotoxicity. Internal Medicine Journal, 47, 104–109. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. (2001). Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New England Journal of Medicine, 344, 1031–1037. [DOI] [PubMed] [Google Scholar]
- 6.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, & Noonan DM (2010). Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. Journal of the National Cancer Institute, 102, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen MH, Kerkelä R, & Force T (2008). Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation, 118, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. (2002). Cardiac dysfunction in the trastuzumab clinical trials experience. Journal of Clinical Oncology, 20, 1215–1221. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. (2012). ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Annals of Oncology, 23(Suppl 7), vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 10.Adão R, de Keulenaer G, Leite-Moreira A, & Brás-Silva C (2013). Cardiotoxicity associated with cancer therapy: Pathophysiology and prevention strategies. Revista Portuguesa de Cardiologia, 32, 395–409. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Y, Yin J, Wei J, & Shang Z (2014). Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: A systematic review and meta-analysis. PLoS ONE, 9, e87671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lendvai N, Hilden P, Devlin S, Landau H, Hassoun H, Lesokhin AM, et al. (2014). A phase 2 single-center study of carfilzomib 56 mg/m2 with or without low-dose dexamethasone in relapsed multiple myeloma. Blood, 124, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervera Grau JM, Esquerdo Galiana G, Belso Candela A, Llorca Ferrándiz C, Juárez Marroquí A, & Maciá, Escalante S. (2008). Complete atrioventricular block induced by rituximab in monotherapy in an aged patient with non-Hodgkin’s diffuse large B-cell lymphoma. Clinical and Translational Oncology, 10, 298–299. [DOI] [PubMed] [Google Scholar]
- 14.Kilickap S, Yavuz B, Aksoy S, Sahiner L, Dincer M, Harputluoglu H, et al. (2008). Addition of rituximab to chop does not increase the risk of cardiotoxicity in patients with non-Hodgkin’s lymphoma. Medical Oncology, 25, 437–442. [DOI] [PubMed] [Google Scholar]
- 15.Foran JM, Rohatiner AZ, Cunningham D, Popescu RA, Solal-Celigny P, Ghielmini M, et al. (2000). European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. Journal of Clinical Oncology, 18, 317–324. [DOI] [PubMed] [Google Scholar]
- 16.Xue K, Gu JJ, Zhang Q, Liu X, Wang J, Li XQ, et al. (2016). Cardiotoxicity as indicated by LVEF and troponin T sensitivity following two anthracycline-based regimens in lymphoma: Results from a randomized prospective clinical trial. Oncotarget, 7, 32519–32531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. (2016). Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. The Lancet Oncology, 17, 27–38. [DOI] [PubMed] [Google Scholar]
- 18.Danhof S, Schreder M, Rasche L, Strifler S, Einsele H, & Knop S (2016). ‘Real-life’ experience of preapproval carfilzomib-based therapy in myeloma—Analysis of cardiac toxicity and predisposing factors. European Journal of Haematology, 97, 25–32. [DOI] [PubMed] [Google Scholar]
- 19.Grandin EW, Ky B, Cornell RF, Carver J, & Lenihan DJ (2015). Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. Journal of Cardiac Failure, 21, 138–144. [DOI] [PubMed] [Google Scholar]
- 20.Reneau JC, Asante D, van Houten H, Sangaralingham LR, Buadi FK, Lerman A, et al. (2017). Cardiotoxicity riskm with bortezomib versus lenalidomide for treatment of multiple myeloma: A propensity matched study of 1790 patients. American Journal of Hematology, 92, E15–E17. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal A, Luthi J, Belohlavek M, Kortüm KM, Mookadam F, Mayo A, et al. (2016). Carfilzomib and the cardiorenal system in myeloma: An endothelial effect? Blood Cancer Journal, 6, e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arunprasath P, Gobu P, Dubashi B, Satheesh S, & Balachander J (2011). Rituximab induced myocardial infarction: A fatal drug reaction. Journal of Cancer Research and Therapeutics, 7, 346–348. [DOI] [PubMed] [Google Scholar]
- 23.Arai Y, Tadokoro J, & Mitani K (2005). Ventricular tachycardia associated with infusion of rituximab in mantle cell lymphoma. American Journal of Hematology, 78, 317–318. [DOI] [PubMed] [Google Scholar]
- 24.Ng KH, Dearden C, & Gruber P (2015). Rituximab-induced Takotsubo syndrome: More cardiotoxic than it appears? BMJ Case Reports. doi: 10.1136/bcr-2014-208203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. (2002). CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-Bcell lymphoma. New England Journal of Medicine, 346, 235–242. [DOI] [PubMed] [Google Scholar]
- 26.Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. (2006). Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nature Medicine, 12, 908–916. [DOI] [PubMed] [Google Scholar]
- 27.Yeh ET, & Bickford CL (2009). Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. Journal of the American College of Cardiology, 53, 2231–2247. [DOI] [PubMed] [Google Scholar]
- 28.Trněný M, Lamy T, Walewski J, Belada D, Mayer J, Radford J, et al. (2016). Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): A phase 2, randomised, multicentre trial. The Lancet Oncology, 17, 319–331. [DOI] [PubMed] [Google Scholar]
- 29.Sethi TK, Basdag B, Bhatia N, Moslehi J, & Reddy NM (2017). Beyond anthracyclines: Preemptive management of cardiovascular toxicity in the era of targeted agents for hematologic malignancies. Current Hematologic Malignancy Reports, 12, 257–267. [DOI] [PubMed] [Google Scholar]
- 30.Hasinoff BB, Patel D, & Wu X (2017). Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovascular Toxicology, 17, 237–250. [DOI] [PubMed] [Google Scholar]
- 31.Khorana AA, Francis CW, Culakova E, Kuderer NM, & Lyman GH (2007). Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of Thrombosis and Haemostasis, 5, 632–634. [DOI] [PubMed] [Google Scholar]
- 32.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. (2005). Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. The Journal of Clinical Investigation, 115, 2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. (2016). Shared genetic predisposition in peripartum and dilated cardiomyopathies. New England Journal of Medicine, 374, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leger KJ, Cushing-Haugen K, Hansen JA, Fan W, Leisenring WM, Martin PJ, et al. (2016). Clinical and genetic determinants of cardiomyopathy risk among hematopoietic cell transplantation survivors. Biology of Blood and Marrow Transplantation, 22, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nef HM, Möllmann H, Troidl C, Kostin S, Böttger T, Voss S, et al. (2008). Expression profiling of cardiac genes in Tako-Tsubo cardiomyopathy: Insight into a new cardiac entity. Journal of Molecular and Cellular Cardiology, 44, 395–404. [DOI] [PubMed] [Google Scholar]
- 36.Asakura M, & Kitakaze M (2009). Global gene expression profiling in the failing myocardium. Circulation Journal, 73, 1568–1576. [DOI] [PubMed] [Google Scholar]


