Abstract
Cycloaddition reactions provide direct and convergent routes to cycloalkanes, making them privileged targets for the development of synthetic methods. Whereas six-membered rings are readily accessible from Diels–Alder reactions, cycloadditions that generate five-membered rings are comparatively limited in scope. Here, we report that dinickel complexes catalyze [4 + 1]-cycloaddition reactions of 1,3-dienes. The C1 partner is a vinylidene equivalent generated from the reductive activation of a 1,1-dichloroalkene in the presence of stoichiometric Zn. Intermolecular and intramolecular variants of the reaction are described, with high levels of asymmetric induction achieved in the intramolecular cycloadditions using a C2-symmetric chiral ligand that stabilizes a metal–metal bond.
One Sentence Summary:
Dinickel catalysts promote [4 + 1]-cycloadditions of vinylidenes and 1,3-dienes to form cyclopentenes.
Natural products display a variety of carbocyclic structures that are readily assembled within the active sites of cyclase enzymes but are challenging to prepare de novo in the laboratory (1). It is also common for synthetic molecules to incorporate ring systems in order to impose geometric constraints or to arrange functional components at well-defined positions in three-dimensional space. For these reasons, methods that provide convenient access to common cycloalkanes are of substantial value to synthetic chemists. Whereas six-membered rings may be prepared using the Diels–Alder reaction, no cycloaddition of equivalent generality is available for the synthesis of five-membered rings. Current leading approaches include transition metal-catalyzed [2 + 2 + 1]-cycloadditions, such as the Pauson–Khand reaction (2), and [3 + 2]-cycloadditions using trimethylenemethane equivalents (3).
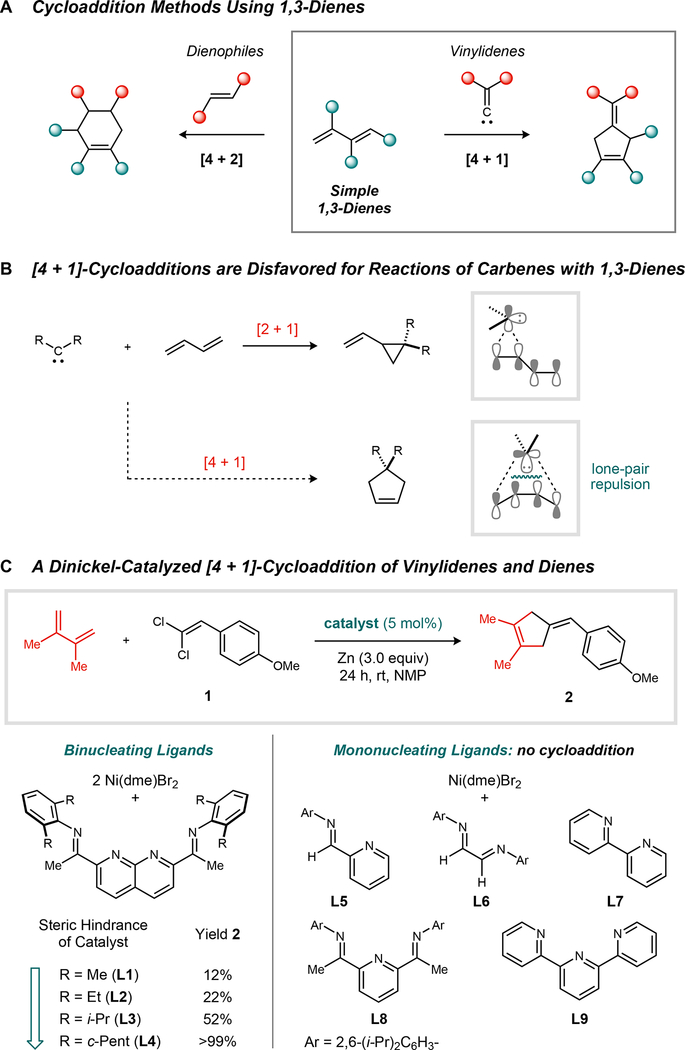
It is attractive to consider an alternative route to five-membered rings (4, 5) that would rely on a [4 + 1]-cycloaddition between a 1,3-diene and a suitable C1 partner (fig. 1A). A major impediment to realizing such a reaction by a concerted mechanism is the competing [2 + 1]-cycloaddition, which is often favored and generates vinylcyclopropanes as products (fig. 1B). Quantum mechanical models for the reaction between singlet methylene and 1,3-butadiene attribute this selectivity to excessive closed-shell repulsion between the carbene lone pair and the filled Ψ1 orbital of the diene in the symmetry-allowed transition state geometry (6–8). As a consequence, direct [4 + 1]-cycloadditions are exceedingly rare beyond specialized classes of substrates (9–11). A viable alternative is to carry out a sequential [2 + 1]-cycloaddition followed by a vinylcyclopropane 1,3-rearrangement; however, preparatively useful variants of this process require an activating substituent, an additional strain element, or a catalyst to accelerate the rearrangement step (12–17)—the rearrangement of the parent vinylcyclopropane molecule occurs at >300 °C and has an activation energy of approximately 50 kcal/mol (18, 19). In light of these challenges, transition metal-catalyzed [4 + 1]-cycloadditions have also been explored, culminating in the discovery of methods that allow for the addition of CO to various cumulene-containing dienes (20–23).
Fig. 1. Reaction development.
(A) Complementary cycloaddition routes to five- and six-membered rings from 1,3-dienes. (B) Pericyclic [4 + 1]-cycloadditions suffer from large electronic barriers due to repulsion between the carbene lone pair and the Ψ1 orbital of the 1,3-diene. (C) A dinickel-catalyzed reductive [4 + 1]-cycloaddition of 1,1-dichloroalkenes and 1,3-dienes. NMP, N-methyl-2-pyrrolidone; rt, room temperature.
We recently reported a dinickel catalyst that promotes the [2 + 1]-cycloaddition of vinylidenes and alkenes to form methylenecyclopropane products (24). 1,1-Dichloroalkenes, which are conveniently prepared in a single step from the corresponding aldehyde or ketone, serve as vinylidene precursors (25), and Zn is used as a stoichiometric reductant. Experiments using stereochemically labelled alkenes were suggestive of a stepwise mechanism for cyclopropane formation. The intermediacy of a metallacycle generated from the addition of a Ni2(C=CHR) species to the alkene could account for this observation. Subsequent C–C reductive elimination would then close the three-membered ring. We reasoned that such a process might be adapted to 1,3-dienes as a means of circumventing the electronic constraints of the pericyclic [4 + 1]-cycloaddition pathway. In this scenario, the partitioning between vinylcyclopropane and cyclopentene products would be dictated by the relative facility of the two possible C–C reductive eliminations. Here, we report that dinickel catalysts induce highly selective [4 + 1]-cycloadditions of vinylidenes and simple 1,3-dienes, providing a direct synthetic entry into polysubstituted cyclopentenes.
The Ni2 catalyst 3 was previously shown to promote the reductive methylenecyclopropanation reaction and thus served as a starting point for our investigations of a model [4 + 1]-cycloaddition (fig. 1C). In an initial survey of reaction parameters, the use of a polar solvent such as N-methyl-2-pyrrolidone was found to promote high conversions of the 1,1-dichloroalkene. The catalyst generated from Ni(1,2-dimethoxyethane)Br2 (10 mol%) and L3 (5 mol%) afforded cyclopentene product 2 in up to 52% yield. The steric profile of the catalyst proved to be a critical determinant of reaction efficiency. When the i-Pr substituents of the flanking N-aryl groups were replaced with Me or Et (L1 or L2, respectively), the yield of 2 decreased to <22%. Conversely, the more hindered cyclopentyl-substituted ligand (L4) provided a near-quantitative yield of 2. There was no competing [2 + 1]-cycloaddition to generate a vinylcyclopropane, nor did the catalyst isomerize the skipped diene of product 2 into conjugation. The importance of the dinuclear catalyst structure was examined by comparing the efficiency of catalysts generated using related mononucleating ligands (L5–L9). In no case did we observe significant yields of cyclopentene 2 using a mononickel catalyst.
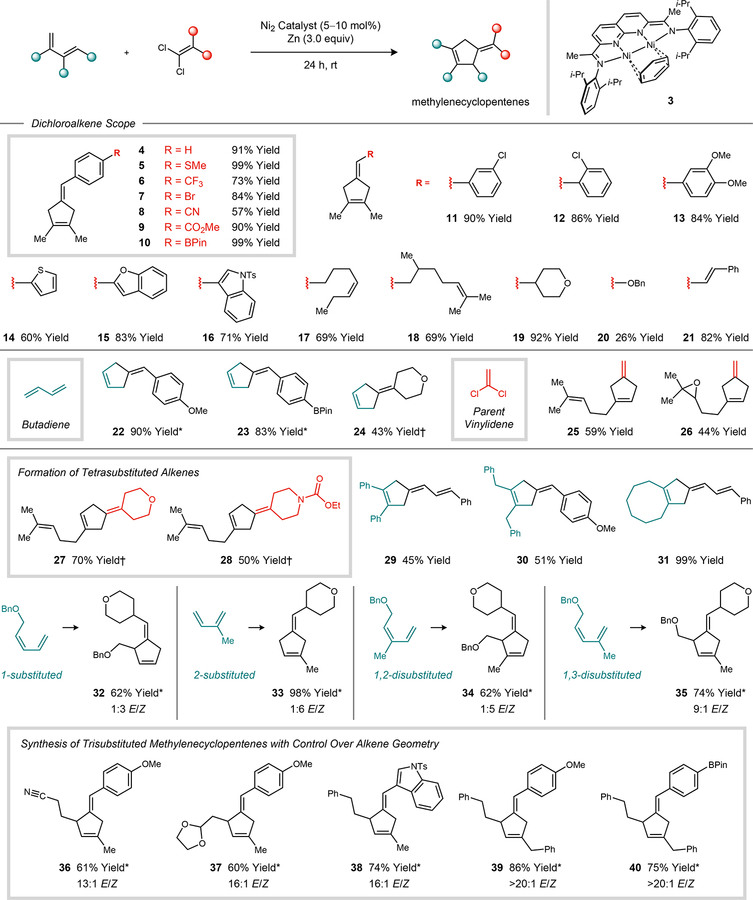
The substrate scope of the reductive [4 + 1]-cycloaddition reaction is summarized in Fig. 2. A variety of common functional groups are tolerated, including thioethers, trifluoromethyl groups, nitriles, esters, ethers, protected amines, epoxides, acetals, and boronate esters. Products containing exocyclic vinyl ethers (20) are accessible, albeit in moderate yield due to competing reductive decomposition of the alkoxy-substituted 1,1-dichloroalkene. Aryl bromides (7), which are often employed in Ni-catalyzed cross-coupling reactions, are left untouched in the cycloaddition due to the comparatively rapid oxidative addition of the 1,1-dichloroalkene by the catalyst. Butadiene is a viable substrate (22–24), and the relatively unhindered alkene in the product is not susceptible to a secondary methylenecyclopropanation. 1,1-Dichloroethylene provides a source of the parent vinylidene fragment (25–26), yielding cyclopentene products with no substituents on the exocyclic methylene. Products containing tetrasubstituted alkenes are generated using ketone-derived 1,1-dichloroalkenes (27–28). Representative 1-substituted (32), 2-substituted (33), 1,2-disubstituted (34), and 1,3-disusbstituted (35) dienes were found to react efficiently with a model 1,1-dichloroalkene. In the case of 1,3-disubstituted dienes (35–40) the cycloadditions proceeded with high E selectivity (9:1 to >20:1 ratio of stereoisomers).
Fig. 2. Reaction scope.
Reactions were conducted on a 0.2 mmol scale, and isolated yields were determined following purification. Standard reaction conditions: 1,1-dichloroalkene (1.0 equiv), 1,3-diene (2.0 to 3.0 equiv), Zn (3.0 equiv), Ni(dme)Br2 (10 to 20 mol%), L4 (5 to 10 mol%). See Supplementary material for experimental details. *Using catalyst 3. †Using Ni(dme)Br2/L10.
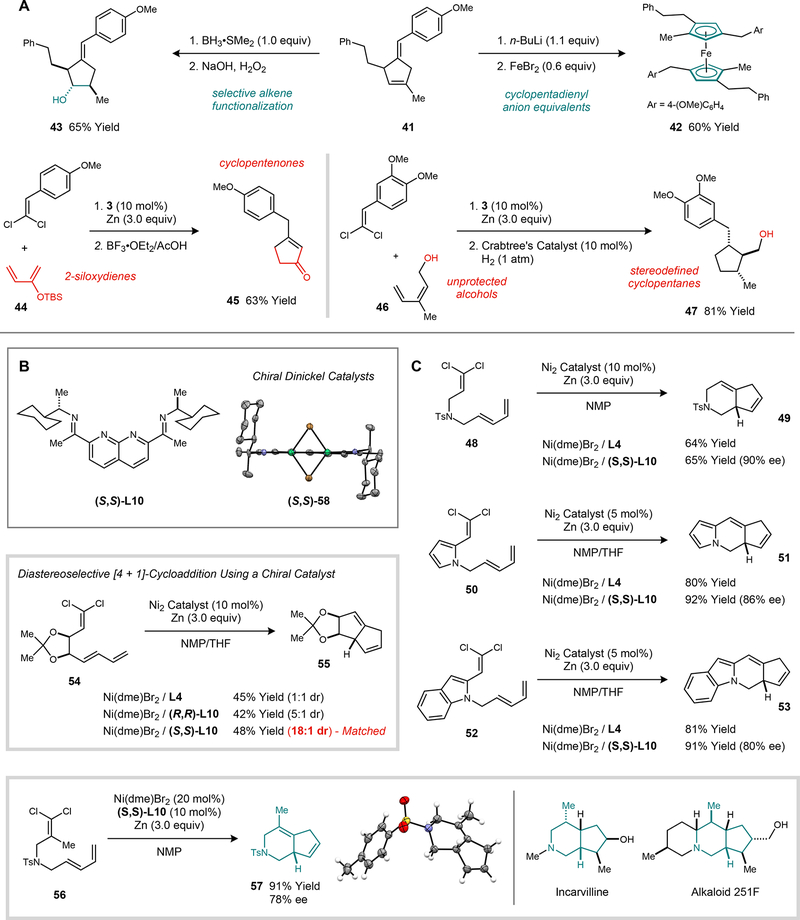
In order to explore the synthetic utility of this method, the 4-methylene-1-cyclopentene products were converted into other classes of cyclopentane derivatives commonly featured in organic and organometallic compounds (fig. 3A). The direct products of the [4 + 1]-cycloaddition possess the same degree of unsaturation as a cyclopentadiene motif. Accordingly, deprotonation of 41 with n-BuLi afforded a cyclopentadienyl anion equivalent that was quenched with FeBr2 (0.6 equiv) to yield a hexasubstituted ferrocene (42). Additionally, the two trisubstituted alkenes in 41 could be readily differentiated in a reaction with BH3•SMe2, which adds to the ring alkene but leaves the exocyclic alkene intact. Next, the 2-siloxy substituted diene 44 was subjected to the standard catalytic cycloaddition conditions to afford a silyl enol ether product. Subsequent silyl deprotection and alkene isomerization yielded cyclopentenone 45. Finally, the disubstituted diene 46, containing a free alcohol, proved to be a viable substrate for the cycloaddition. A directed hydrogenation of both double bonds in the resulting cycloadduct afforded saturated trisubstituted cyclopentane 47.
Fig. 3. Synthetic applications of the catalytic [4 + 1]-cycloaddition reaction.
A [4 + 1]-Cycloaddition approaches to the synthesis of cyclopentenones, stereodefined cyclopentanes, and cyclopentadienyl metal complexes. B Chiral dinickel catalyst using a C2-symmetric ligand. C Intramolecular and asymmetric [4 + 1]-cycloaddition reactions (reaction times: 12 to 24 h; reaction temperatures: rt to 50 °C).
Intramolecular [4 + 1]-cycloadditions were carried out using substrates in which the two reacting partners were connected by a two- or three-atom tether (fig. 3C). For example, substrate 56 reacts under standard catalytic conditions to provide 5,6-bicyclic amine 57, which is a substructure found in several terpene alkaloid natural products (26). Heteroarenes may be incorporated into the tether without loss in reaction efficiency (50 and 52). The intramolecular cycloaddition is also amenable to the formation of 5,5-bicyclic systems (55).
We next turned our attention to demonstrating a catalytic asymmetric variant of the [4 + 1]-cycloaddition reaction. The imine substituents of the naphthyridine–diimine ligand presented the most straightforward opportunity for the incorporation of chirality, and a series of chiral catalysts was prepared by condensing commercially available α-secondary amines with 2,7-diacetyl-1,8-naphthyridine. The ligand derived from (S)-1-cyclohexylethylamine ((S,S)-L10) afforded enantiomeric excesses of up to 90% for the intramolecular [4 + 1]-cycloaddition reaction (fig. 3B). Additionally, substrate 54, which was prepared in enantiomerically enriched form from a carbohydrate precursor, reacted with high diastereoselectivity (18:1 dr) using (S,S)-L10.
In order to examine the chiral environment presented by the (S,S)-L10 ligand, its corresponding [NDI]Ni2Br2 complex ((S,S)-58) was prepared by a comproportionation route using Ni(COD)2 (1.0 equiv) and Ni(dme)Br2 (1.0 equiv). The pre-synthesized dinickel complex (S,S)-58 exhibits the same enantioselectivity as the catalyst generated in situ using Ni(dme)Br2 and (S,S)-L10 for the cycloaddition of 48. In the solid state, catalyst (S,S)-58 adopts a C2-symmetric geometry (fig. 3B). The stereogenic N-(1-cyclohexylethyl) substituents are in a 1,3-allylic strain-minimized orientation, which places the C–H bond in an eclipsing geometry relative to the C=N bond of the imine. This conformation projects the larger cyclohexyl groups and smaller methyl groups in opposing quadrants of the substrate binding pocket, suggesting a steric rationale for the high degree of asymmetric induction imparted by this catalyst design.
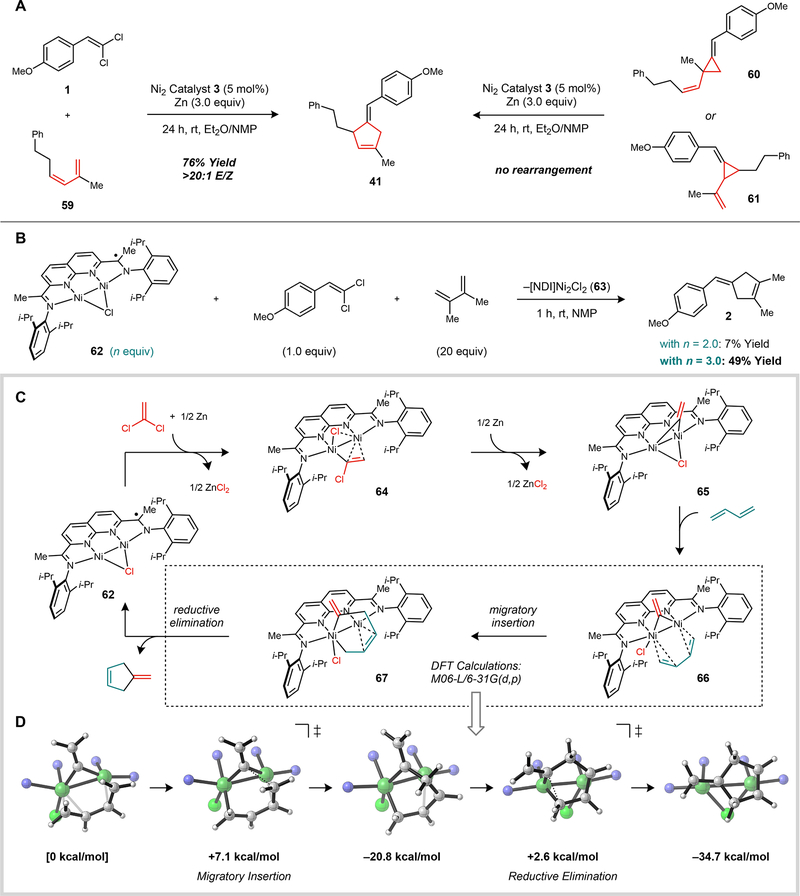
Our initial mechanistic studies were aimed at distinguishing between a direct [4 + 1]-cycloaddition pathway and a tandem [2 + 1]-cycloaddition followed by a vinylcyclopropane 1,3-rearrangement (fig. 4A). To address this question, we synthesized vinylcyclopropanes 60 and 61 (mixture of stereoisomers), which would correspond to the putative intermediates of this stepwise process. Under standard catalytic conditions, the [4 + 1]-cycloaddition between 1,1-dichloroalkene 1 and diene 59 proceeded efficiently to form 41 in 76% yield. Neither vinylcyclopropane regioisomer was observed to form at partial conversions. When separately prepared 60 or 61 was subjected to the same reaction conditions, there was no detectable rearrangement after 24 h of reaction time at room temperature.
Fig. 4. Mechanistic investigations.
A Distinguishing between direct [4 + 1]-cycloaddition and tandem [2 + 1]-cycloaddition/1,3-rearrangement mechanisms. B Stoichiometric [4 + 1]-cycloaddition using an isolable low-valent [NDI]Ni2Cl complex. C Proposed catalytic mechanism. D DFT models for the stepwise migratory insertion–reductive elimination pathway. Energies are relative to that of 66, and all structures are fully optimized at the M06-L/6–31G(d,p) level of DFT (S = 1/2 spin state).
We next sought to determine whether Zn was playing a necessary role in the formation of the [4 + 1]-cycloadducts or simply serving as a terminal reductant. A single turnover experiment was carried out using the isolable [NDI]Ni2Cl complex (62), which is the most reduced form of the catalyst that is accessible at Zn potentials (fig. 4B). When [NDI]Ni2Cl complex 62 (3.0 equiv) was added to a solution containing 1,1-dichloroalkene 1 (1.0 equiv) and excess 2,3-dimethylbutadiene, it was rapidly oxidized to the green paramagnetic [NDI]Ni2Cl2 complex 63, and the cycloaddition product 2 was obtained in 49% yield. Decreasing the amount of [NDI]Ni2Cl (62) to 2.0 equiv leads to a precipitous decrease in the yield of 2 (7%). This reaction stoichiometry suggests that the high-yielding pathway for the [4 + 1]-cycloaddition is only accessible when two additional equivalents of the [NDI]Ni2Cl complex are present to serve as Cl abstractors.
A proposed catalytic mechanism based on these observations is shown in fig. 4C. Initial oxidative addition of the 1,1-dichloroalkene by the [NDI]Ni2Cl complex would generate a high-valent species that can undergo one-electron reduction by Zn to generate the chloroalkenyl complex 64. A second reduction event, followed by C–Cl oxidative addition, then forms a reactive Ni2(vinylidene)(Cl) species (65). The pathway for cycloaddition from this intermediate was evaluated using computational methods (fig. 4D). According to DFT models, the diene first coordinates symmetrically across the Ni–Ni bond in an η4-fashion (66). This geometry permits the two Ni centers to stabilize the incipient allyl fragment as the diene undergoes migratory insertion (activation barrier = 7.1 kcal/mol). The resulting metallacycle 67 is structurally related to a Ni2 azametallacycle that we previously characterized from a vinylaziridine ring-opening reaction (27). The final C–C reductive elimination is calculated to have a barrier of 23.4 kcal/mol and generates the product complex, which is thermodynamically favored over intermediate 67 by 13.9 kcal/mol. A salient feature of the Ni2 active site is the ability of the two metals to cooperatively stabilize the π-systems in the reacting vinylidene and diene fragments as they undergo C–C bond formation. Collectively, these studies demonstrate that dinickel catalysts can take advantage of these unique interactions to provide an efficient pathway for cycloadditions that are challenging to achieve under thermal conditions.
Supplementary Material
Acknowledgments:
We thank John Harwood, Huaping Mo, Sudipta Pal, Qingqing Qi, Talia Steiman, and Shiqing Xu for experimental assistance. We thank Matthias Zeller for assistance with collecting and analyzing X-ray diffraction data.
Funding: Financial support is provided by the NIH (R35 GM124791). C.U. is an Alfred P. Sloan Foundation Fellow. X-ray diffraction data were collected using an instrument funded by the NSF (CHE-1625543);
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre, under reference numbers CCDC 1888129, 1888130, 1888131. All other data are available in the main text or the supplementary materials.
Supplementary Materials:
Materials and Methods
Figures S1–S13
Tables S1–S7
NMR Spectra
References (28–47)
References and Notes:
- 1.Maimone TJ, Baran PS, Modern synthetic efforts toward biologically active terpenes. Nat. Chem. Biol 3, 396 (2007); doi: 10.1038/nchembio.2007.1 [DOI] [PubMed] [Google Scholar]
- 2.Gibson SE, Mainolfi N, The intermolecular Pauson–Khand reaction. Angew. Chem., Int. Ed 44, 3022–3037 (2005); doi: 10.1002/anie.200462235 [DOI] [PubMed] [Google Scholar]
- 3.Trost BM, [3+2] Cycloaddition approaches to five-membered rings via trimethylenemethane and its equivalents [New Synthetic Methods (55)]. Angew. Chem., Int. Ed 25, 1–20 (1986); doi: 10.1002/anie.198600013 [DOI] [Google Scholar]
- 4.Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J, Formal [4+1] annulation reactions in the synthesis of carbocyclic and heterocyclic systems. Chem. Rev 115, 5301–5365 (2015); doi: 10.1021/cr5006974 [DOI] [PubMed] [Google Scholar]
- 5.Barluenga J, López S, Flórez J, [3+2] and [4+1] Cycloaddition reactions of Fischer alkoxy(alkenyl)carbene complexes with electronically neutral 1,3-dienes. Angew. Chem., Int. Ed 42, 231–233 (2003); doi: 10.1002/anie.200390087 [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto H, Hoffmann R, Molecular orbital study of the addition of singlet methylene to butadiene. J. Phys. Chem 78, 1167–1173 (1974); doi: 10.1021/j100605a007 [DOI] [Google Scholar]
- 7.Schoeller WW, Yurtsever E, The synchronous 1,4-addition of methylene to cis-butadiene. J. Am. Chem. Soc 100, 7548–7550 (1978); doi: 10.1021/ja00492a018 [DOI] [Google Scholar]
- 8.Evanseck JD, Mareda J, Houk KN, Theoretical investigation of intramolecular singlet carbene 1,4- and 1,2-cycloadditions. J. Am. Chem. Soc 112, 73–80 (1990); doi: 10.1021/ja00157a012 [DOI] [Google Scholar]
- 9.Turkenburg LAM, de Worlf WH, Bickelhaupt F, 1,4-Addition of dichlorocarbene to 1,2-bismethylenecycloheptane. Tetrahedron Lett. 23, 769–770 (1982); doi: 10.1016/S0040-4039(00)86944-0 [DOI] [Google Scholar]
- 10.Sugita H, Mizuno K, Mori T, Isagawa K, Otsuji Y, Unusual mode of addition of 1,2‐alkadienylidene carbenes to 1,3‐dienes: 1,4 addition to rigid and flexible 1,3‐dienes. Angew. Chem., Int. Ed 30, 984–986 (1991); doi: 10.1002/anie.199109841 [DOI] [Google Scholar]
- 11.Boisvert L, Beaumier F, Spino C, Evidence for a concerted [4 + 1]-cycloaddition between electron-rich carbenes and electron-deficient dienes. Org. Lett 9, 5361–5363 (2007); doi: 10.1021/ol702172t [DOI] [PubMed] [Google Scholar]
- 12.Coscia RW, Lambert TH, Development of a formal [4 + 1] cycloaddition: Pd(OAc)2-catalyzed intramolecular cyclopropanation of 1,3-dienyl β-keto esters and MgI2-promoted vinylcyclopropane−cyclopentene rearrangement. J. Am. Chem. Soc 131, 2496–2498 (2009); doi: 10.1021/ja809226x [DOI] [PubMed] [Google Scholar]
- 13.Danheiser RL, Martinez-Davila C, Auchus RJ, Kadonaga JT, Stereoselective synthesis of cyclopentene derivatives from 1,3-dienes. J. Am. Chem. Soc 103, 2443–2446 (1981); doi: 10.1021/ja00399a067 [DOI] [Google Scholar]
- 14.Danheiser RL, Bronson JJ, Okano K, Carbanion-accelerated vinylcyclopropane rearrangement. Application in a general, stereocontrolled annulation approach to cyclopentene derivatives. J. Am. Chem. Soc 107, 4579–4581 (1985); doi: 10.1021/ja00301a051 [DOI] [Google Scholar]
- 15.Hudlicky T, Koszyk FF, Kutchan TM, Sheth JP, Cyclopentene annulation via intramolecular addition of diazoketones to 1,3-dienes. Applications to the synthesis of cyclopentanoid terpenes. J. Org. Chem 45, 5020–5027 (1980); doi: 10.1021/jo01313a003 [DOI] [Google Scholar]
- 16.Beaumier F, Dupuis M, Spino C, Legault CY, Formal intramolecular (4 + 1)-cycloaddition of dialkoxycarbenes: control of the stereoselectivity and a mechanistic portrait. J. Am. Chem. Soc 134, 5938–5953 (2012); doi: 10.1021/ja211927b [DOI] [PubMed] [Google Scholar]
- 17.Zuo G, Louie J, Highly active nickel catalysts for the isomerization of unactivated vinyl cyclopropanes to cyclopentenes. Angew. Chem., Int. Ed 43, 2277–2279 (2004); doi: 10.1002/anie.200353469 [DOI] [PubMed] [Google Scholar]
- 18.Overberger CG, Borchert AE, Novel thermal rearrangements accompanying acetate pyrolysis in small ring systems. J. Am. Chem. Soc 82, 1007–1008 (1960); doi: 10.1021/ja01489a069 [DOI] [Google Scholar]
- 19.Vogel E, Kleine kohlenstoff‐ringe. Angew. Chem 72, 4–26 (1960); doi: 10.1002/ange.19600720103 [DOI] [Google Scholar]
- 20.Murakami M, Itami K, Ito Y, Rhodium-catalyzed asymmetric [4 + 1] cycloaddition. J. Am. Chem. Soc 119, 2950–2951 (1997); doi: 10.1021/ja964259m [DOI] [Google Scholar]
- 21.Eaton BE, Rollman B, Kaduk JA, The first catalytic iron-mediated [4 + 1] cyclopentenone assembly: stereoselective synthesis of 2,5-dialkylidenecyclo-3-pentenones. J. Am. Chem. Soc 114, 6245–6246 (1992); doi: 10.1021/ja00041a052 [DOI] [Google Scholar]
- 22.Sigman MS, Eaton BE, Catalytic iron-mediated [4 + 1] cycloaddition of diallenes with carbon monoxide. J. Am. Chem. Soc 118, 11783–11788 (1996); doi: 10.1021/ja962908o [DOI] [Google Scholar]
- 23.Murakami M, Itami K, Ito Y, A study on rhodium – vinylallene complexes leading to a new reaction, rhodium‐catalyzed carbonylative [4 + 1]cycloaddition. Angew. Chem., Int. Ed 34, 2691–2694 (1996); doi: 10.1002/anie.199526911 [DOI] [Google Scholar]
- 24.Pal S, Zhou Y-Y, Uyeda C, Catalytic reductive vinylidene transfer reactions. J. Am. Chem. Soc 139, 11686–11689 (2017); doi: 10.1021/jacs.7b05901 [DOI] [PubMed] [Google Scholar]
- 25.Iyoda M, Mizusuna A, Oda M, A novel one-step nickel-mediated synthesis of C6Ar6 conjugated hydrocarbons, highly substituted and overcrowded fulvenes and 3,4-dimethylenecyclobutenes. Chem. Lett 17, 149–152 (1988); doi: 10.1246/cl.1988.149 [DOI] [Google Scholar]
- 26.Kam T-S, Lim K-H, in The alkaloids: chemistry and biology, Cordell GA, Ed. (Academic Press, 2008), vol. 66, pp. 1–111. [DOI] [PubMed] [Google Scholar]
- 27.Rounds HR, Zeller M, Uyeda C, Dinuclear pathways for the activation of strained three-membered rings. Organometallics 37, 545–550 (2018); doi: 10.1021/acs.organomet.7b00862 [DOI] [Google Scholar]
- 28.Zhou Y-Y, Hartline DR, Steiman TJ, Fanwick PE, Uyeda C Dinuclear nickel complexes in five states of oxidation using a redox-active ligand. Inorg. Chem, 53, 11770–11777 (2014); doi: 10.1021/ic5020785. [DOI] [PubMed] [Google Scholar]
- 29.Corp Rigaku., The Woodlands, Texas, USA. [Google Scholar]
- 30.Otwinowski Z, Minor W, Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997); doi:0.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 31.Bruker (2016). Apex3 v2016.9–0, Saint V8.34A, SAINT V8.37A, Bruker AXS Inc: Madison (WI), USA, 2013/2014. [Google Scholar]
- 32.SHELXTL suite of programs, Version 6.14, 2000–2003, Bruker Advanced X-ray Solutions, Bruker AXS Inc, Madison, Wisconsin: USA. [Google Scholar]
- 33.Sheldrick GM, A short history of SHELX. Acta Crystallogr A. 64, 112–122 (2008); doi: 10.1107/S0108767307043930 [DOI] [PubMed] [Google Scholar]
- 34.GM Sheldrick. University of Göttingen, Germany, 2018. [Google Scholar]
- 35.GM Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem. 71, 3–8 (2015); doi: 10.1107/S2053229614024218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hübschle CB, Sheldrick GM, Dittrich B, ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr 44, 1281–1284 (2011); doi: 10.1107/S0021889811043202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monfette S, Turner ZR, Semproni SP, Chirik PJ, Enantiopure C1-symmetric bis(imino)pyridine cobalt complexes for asymmetric alkene hydrogenation. J. Am. Chem. Soc 134, 4561–4564 (2012); doi: 10.1021/ja300503k [DOI] [PubMed] [Google Scholar]
- 38.Burton G, Elder JS, M Fell SC, Stachulski AV, Acetonitrile: an excellent solvent for the 1,1-dichloromethylenation of certain ketones. Tetrahedron Lett. 29, 3003–3006 (1988); doi: 10.1016/0040-4039(88)85072-X25 [DOI] [Google Scholar]
- 39.Poornachandran M, Raghunathan R. Synthesis of pyrrolo[3,4-b]pyrroles and perhydrothiazolo[3′,4′−2,3]pyrrolo[4,5-c]pyrroles. Tetrahedron 64, 6461–6474 (2008); doi: 10.1016/j.tet.2008.04.063 [DOI] [Google Scholar]
- 40.Shing TKM, Wong AWH, Li H, Liu ZF, Chan PKS. Conformationally locked bicyclo[4.3.0]nonane carbanucleosides: synthesis and bio-evaluation. Org. Biomol. Chem 12, 9439–9445 (2014); doi: 10.1039/c4ob01763c [DOI] [PubMed] [Google Scholar]
- 41.Nagarapu L, Karnakanti S, Bantu R. Total synthesis of sapinofuranone A from D-ribose. Tetrahedron 68, 5829–5832 (2012); doi: 10.1016/j.tet.2012.05.012 [DOI] [Google Scholar]
- 42.Korkis SE, Burns DJ, Lam HW. Rhodium-catalyzed oxidative C−H allylation of benzamides with 1,3-dienes by allyl-to-allyl 1,4-Rh(III) migration. J. Am. Chem. Soc 138, 12252–12257(2016); doi: 10.1021/jacs.6b06884 [DOI] [PubMed] [Google Scholar]
- 43.Kliman LT, Mlynarski SN, Ferris GE, Morken JP. Catalytic enantioselective 1,2‐ diboration of 1,3‐dienes: versatile reagents for stereoselective allylation. Angew. Chem. Int. Ed 51, 521–524 (2012); doi: 10.1002/anie.201105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simaan S, Masarwa A, Zohar E, Stanger A, Bertus P, Marek I. Cyclopropenylcarbinol derivatives as new versatile intermediates in organic synthesis: application to the formation of enantiomerically pure alkylidenecyclopropane derivatives. Chem. Eur. J 15, 8449–8464 (2009); doi: 10.1002/chem.200901074 [DOI] [PubMed] [Google Scholar]
- 45.Cahiez G, Duplais C, Buendia J. Manganese-catalyzed oxidative cross-coupling of Grignard reagents with oxygen as an oxidant. Angew. Chem. Int. Ed 48, 6731–6734 (2009); doi: 10.1002/anie.200902188 [DOI] [PubMed] [Google Scholar]
- 46.Gaussian 09, Revision A.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, and Fox DJ, Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 47.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account 120, 215–241 (2008); doi: 10.1007/s00214-007-0310-x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.