Abstract
The enthesis, or interface between bone and soft tissues such as ligament and tendon, is prone to injury and often does not heal, even post-surgical intervention. Interface tissue engineering represents an integrative strategy for regenerating the native enthesis by functionally connecting soft and hard tissues and thereby improving clinical outcome. This review focuses on integrative and cell-instructive scaffold designs that target the healing of the two most commonly injured soft tissue-bone junctions: tendon-bone interface (e.g., rotator cuff) and ligament-bone interface (e.g., anterior cruciate ligament). The inherent connectivity between soft and hard tissues is instrumental for musculoskeletal motion and is therefore a key design criterion for soft tissue regeneration. To this end, scaffold design for soft tissue regeneration have progressed from single tissue systems to the emerging focus on pre-integrated and functional composite tissue units. Specifically, a multifaceted, bioinspired approach has been pursued wherein scaffolds are tailored to stimulate relevant cell responses using spatially patterned structural and chemical cues, growth factors, and/or mechanical stimulation. Moreover, current efforts to elucidate the essential scaffold design criteria via strategic biomimicry are emphasized as these will reduce complexity in composite tissue regeneration and ease the related burden for clinical translation. These innovative studies underscore the clinical relevance of engineering connective tissue integration and have broader impact in the formation of complex tissues and total joint regeneration.
Keywords: biomaterials, tendon, igament, tissue engineering, hard-to-soft-tissue repair
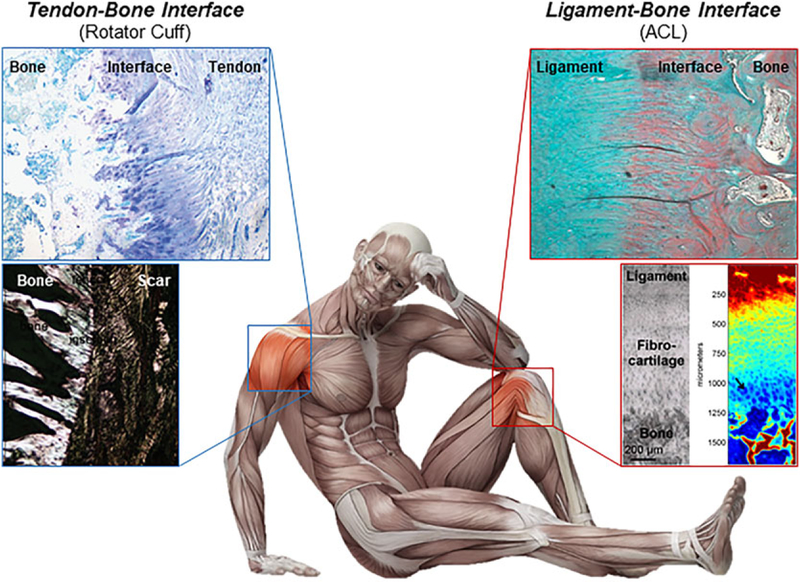
The musculoskeletal organ system is comprised of synchronized tissues or composite tissue units that collectively work to enable physiological motion via a coordinated kinetic chain. Contracting muscles generate forces which are transmitted through tendon to bone, while ligaments help to stabilize the joint and define the range of motion from bone to bone. By anchoring soft tissue to bone, the junctions, or interfaces between these disparate tissue types serve as key links throughout this action chain. As shown in Figure 1, the common examples of interfaces include the tendon-to-bone insertions found in the rotator cuff of the shoulder and the Achilles tendon of the ankle, as well as the two ligament-to-bone insertions of the anterior cruciate ligament (ACL), the primary joint stabilizer of the knee. It is noted that soft tissue-bone junctions present in major load-bearing joints are not a simple mixture of different cell types. Rather, they consist of multi-tissue regions characterized by well-defined spatial gradients in cell phenotype, matrix composition, and organization, leading to controlled changes in mechanical, and biological properties progressing from soft to hard tissues. This inherent matrix heterogeneity minimizes stress concentrations and enables complex load transfer between these disparate tissues.1–3
Figure 1.
Enthesis structure. The healthy tendon–bone interface consists of a functionally graded transition from tendon to the interface which progresses from non-mineralized fibrocartilage to mineralized fibrocartilage to bone (top left). The ligament-bone and tendon-bone are histologically similar but current treatments for tissue injury differ clinically.67 In contrast, a healing interface consists of scar tissue (bottom left).The ligament-bone interface shows a similar transition from ligament to bone (top right), with a spectroscopic map showing a gradient from high collagen (red) to low collagen (blue) (bottom right).18,22
Presently, there is a high incidence of musculoskeletal injuries and related disorders, with the number of injuries continuing to rise with age, significantly reducing the quality of life for millions of patients. For example, it is reported that one in two individuals in the US aged over 18, and three out of every four people aged over 65 will suffer from a musculoskeletal complaint, with the majority related to joint pain due to injury or arthritis.4 This has an estimated annual economic impact of $213 billion in costs of care, treatment, and lost wages.4 Moreover, with about 130,000 ACL-related reconstructions and 275,000 rotator cuff-related repairs reported annually in the United States,5,6 and many of these injuries are characterized by ligament or tendon rupture, often at the insertion site. The prevalence of soft tissue injuries and its tremendous societal and economic burden have motivated the design of functional and integrative strategies for treating soft tissue injuries in the field.
Given the complex cellular and matrix makeup of the interface and due to the inherently poor healing capacity of ligaments and tendons, regeneration of the enthesis has been particularly challenging, even with modern surgical repair techniques. For instance, in the case of rotator cuff repair, overall failure rates remain near 21%.7 Arthroscopic surgical techniques such as double-row and suture-bridge constructs have been refined to improve the time-zero biomechanical properties of the repair, but these new techniques have not led to enduring clinical results.8,9 A recent meta-analysis of re-tear rates following rotator cuff surgery found that for the worst category of tears, those larger than 5 cm, re-tears occurred in 40–78% of patients by at least a 1 year follow-up.7 Biological healing or true repair off these injuries remains elusive as disorganized scar tissue that is structurally and compositionally inferior to native tendon dominate the repair at the tendon-bone junction, ultimately leading to high failure rates and poor long-term outcomes.10,11 Similarly for ACL reconstruction, the mechanical fixation of autologous semitendinosus grafts fails to preserve or re-establish the enthesis anatomically, without which both joint stability and long-term repair outcome are compromised.12–15
For the past decade, the apparent lack of integrative treatment options for either tendon or ligament injuries have fueled the growing interest in interface tissue engineering and integrative soft tissue repair strategies. This review will highlight current advances in interface tissue engineering, specifically the design of cell-instructive scaffolds for the regeneration of the two most commonly injured soft tissue–bone junctions: The tendon-bone interface of the supraspinatus tendon of the rotator cuff and the ACL to bone interface in the knee. Histologically, the insertion sites of the supraspinatus tendon and the ACL with bone are similar,16 comprised of four structurally contiguous but compositionally distinct regions, beginning with the soft tissue, be it tendon or ligament, then non-mineralized fibrocartilage, mineralized fibrocartilage, and finally bone (Figure 1). Elongated fibroblasts interspersed between parallel collagen fibers reside in the soft tissue, with fibrochondrocytes organized along the long axis of the collagen fiber, populating the non-calcified fibrocartilage region. Hypertrophic chondrocytes are observed in the mineralized fibrocartilage, and are also arranged in columns, while osteoblasts, osteocytes, and osteoclasts are present in bone. The interface is characterized by a proteoglycan-rich matrix that also contains types I and II collagen, with a sharp increase in mineral content progressing from calcified fibrocartilage to bone.17–19 On average, the fibrocartilage regions spans 300–800 mm in width depending on the species.18,20–22 The mineral gradient across a narrow tissue region decreases tissue strain while increases stiffness across the interface regions,2,23,24 effectively reducing stress concentrations and allowing load transfer from soft tissue to bone.25,26
Successful biological fixation depends primarily on the soft tissue graft’s capability to achieve extended and functional integration with host bone. From a biomimetic standpoint, to enable soft tissue-to-bone healing, the ideal interface regeneration strategy must recapitulate the multi-tissue organization of the enthesis or insertion site. Furthermore, the scaffold must be able to support the phenotypic expression of distinct cell populations, as well as embodying a spatial gradient in matrix composition and microstructure similar to that of the native interface. Third, the degradation and mechanical properties of the interface scaffold must be tailored to maintain a balance in new tissue growth with continue to sustain physiological loading. This review will focus on current efforts in tendon–bone and ligament–bone interface regeneration with an emphasis on cell-based and biomaterial-based strategies (Tables 1 and 2). Biomimetic scaffolds with functional spatial variations in structure and composition mimicking those of the native enthesis will be highlighted. Treatment of ACL ruptures or rotator cuff tears differ surgically (e.g., ACL reconstruction vs. rotator cuff repair), thus to ease clinical adaptation, graft design must take into account compatibility with current surgical procedures. The optimal interface scaffold for ligament–bone integration will therefore differ from those for tendon, therefore both approaches will be reviewed separately here. This review will conclude with a summary and reflections on future directions in interface tissue engineering and composite tissue regeneration for functional soft tissue healing.
Table 1.
Studies Involving Biomaterials for Ligament-Bone Regeneration
| Study | Study Type | Source/Model | Design | Outcomes |
|---|---|---|---|---|
| Ma et al.38 | In vivo model; MCL | Female Fischer rats | A scaffoldless method to engineer 3D bone–ligament –bone (BLB) construct from rat bone marrow stem cells in vitro | The bone region of 3D BLB constructs integrated well with the native bone, whereas the ligament region showed the presence of aligned, type I collagen, and elastin |
| Cooper et al.32 | In vitro testing; In vivo model; ACL | New Zealand White rabbits | A 3D braided-design scaffolds comprised of Poly-DTE and PLLA seeded with primary rabbit ACL cells | Scaffolds achieved tissue infiltration throughout the scaffold and extensive collagen tissue infiltration after 12 weeks |
| Spalazzi et al.24 | In vitro testing; explant model | Human-derived osteoblast-like cells and bovine-derived fibroblasts and ostroblasts | To mimic the native ACL-to-bone interface, the tri-phase scaffold consisted of: (i) PLGA mesh for ligament; (ii) microspheres for interface; and (iii) sintered PLGA with glass for bone | Tri-culture resulted in distinct cellular regions and extracellular matrix deposition to be maintained. Cell proliferation, migration and phenotypic matrix production were observed. |
| Wang et al.68 | In vivo model; achilles tendon for ligament-bone regeneration | Rabbit-derived chondrocytes, osteoblasts, and fibroblasts; rabbit model | Decellularized rabbit tendons with genetically modified osteoblasts and chondrocytes | A gradient in matrix properties across the bone– interface–ligament scaffold was observed, along with tissue specific gene expression and matrix formation |
| Phillips et al.47 | In vitro testing; subcutaneous pouches in rat model | Primary fibroblasts derived from Wistar rats; rat model | Biomaterial-mediated spatial distribution of retroviral genes to induce differentiation of primary dermal fibroblasts | Gene expression, mineralized matrix deposition, osteoblastic differentiation, and graded distribution of mineral deposition were maintained |
| Kimura et al.69 | In vivo model; ACL | New Zealand white rabbits | A combination of PLLA scaffold with a gelatin hydrogel for release of bFGF, and wrapped in collagen for ligament regeneration | bFGF-controlled release with the scaffold resulted in enhanced mechanical strength of the regenerated ACL tissue, and production of type III and type I collagen was also increased |
| Paxton et al.36 | In vitro model | Primary fibroblasts derived from rat Achilles tendon | PEG-DA hydrogels loaded with HA and a RGD peptide to mimic intact ligaments | By adding HA/RGD to a PEG-hydrogel, it increases mechanical strength, ability to attach cells, and capacity to integrate with biological materials via an functional interface |
Table 2.
Studies Involving Biomaterials for Tendon-Bone Regeneration
| Study | Study Type | Source/Model | Design | Outcomes |
|---|---|---|---|---|
| Santoni et al.70 | Chronic rotator cuff rupture model | Sheep model | Non-resorbable polyurethane scaffold mesh | Scaffold mesh provides greater biomechanical strength in the critical healing period as compared to traditional suture anchor repair |
| Dickerson et al.58 | In vitro testing; In vivo model; rotator cuff | Human adult adipose stem cells; sheep model | Cancellous bone scaffold with one end being rigid and minerealized, while the other being flexible and non-mineralized, to mimic interface structure | The scaffold integrated with host tissue, facilitated organized collagenous tissue, reduced enthesophytes, and produce a four-zone fibrocartilageenous interface |
| Moffat et al.71 | In vitro testing | Human-derived fibroblasts | PLGA nanofiber in the aligned vs. unaligned orientation with cultured fibroblasts | Biomechanical properties of aligned nanofiber scaffolds were significantly higher than those of unaligned fibers, and nanofiber organization has a substantial effect on matrix properties and cellular response |
| Liu et al.57 | In vitro testing | Rat-derived AD-MSCs | Nanofiber-based scaffold coated with a graded mineral to mimic the mineral composition of the native tendon-to-bone insertion | A graded mineral content on the surface of a nanofiber scaffold is capable of inducing differentiation of ASCs in a graded manner into osteoblasts for enthesis repair |
| Li et al.56 | In vitro testing | Mouse calvaria-derived, preosteoblastic cells | A linear gradient of calcium phosphate across a electrospun nonwoven nanofibers | The gradient in mineral content resulted in a gradient in the stiffness and influenced behavior of seeded cells |
| Ladd et al.65 | In vitro testing | C2C12 myoblasts and NIH3T3 fibroblasts | A dual scaffold with regional mechanical property differences by co-electrospinning to create a scaffold with 3 regions | Spatial variations in mechanical properties observed, and culture of C2C12 myoblasts and NIH3T3 fibroblasts accommodated cell attachment and myotube formation |
| Larkin et al.64 | In vivo model; rotator cuff | Rat tendon fibroblasts; rat model | 3-D muscle constructs were cultured with engineered tendon constructs, or adult/ fetal rat tendon | The tendon-bone interface was withstood tensile loading beyond the physiological strain range and paxillin expression was increased |
INTEGRATIVE GRAFT DESIGN FOR LIGAMENT REGENERATION
Ligaments bridge bone to bone, stabilizing joints and facilitating musculoskeletal motion. Thus the functional ligament comprises of compositionally distinct and structurally continuous regions: Bone– interface–ligament–interface–bone. Anatomically, ligaments attach to bone via either indirect insertions where aligned collagen fibers insert into bone (i.e., no fibrocartilage), or through more complex direct insertions which consist of a fibrocartilaginous interface that is further divided in non-calcified and calcified regions.3,22,26,27 A structurally and/or compositionally heterogeneous scaffold design is therefore necessary to recapitulate the composite tissue structure across the ligament–bone junction. Specifically, the studies highlighted in this section will focus on integrative scaffold design for the regeneration of indirect insertions as they are found in the commonly injured ligaments such as the ACL. In general, a scaffold with pre-designed changes in mechanical properties progressing from ligament to the bone is required to ensure mechanical competence under the physiological tension, torsion expected at the ligament/interface regions and then compression relevant for the interface and bone regions. Moreover, the scaffold would ideally support attachment, growth, and differentiation of relevant cell types, enable heterotypic cellular interactions, promote matrix heterogeneity, and be biodegradable to make space for growth of new tissue. In particular, the composite ligament scaffold should support the formation of an organized matrix that is comprised of collagen III and I, while in addition to collagen I and II, the fibrocartilage interface is characterized by glycosaminoglycans (GAG) and a mineralized collagen I matrix is expected for the bone region. Furthermore, interconnectivity and functional integration of ligament–interface–bone phases, as well as compatibility with current ACL reconstruction or repair surgery are other key criteria for composite scaffold design.
Historically, the design of ACL grafts have focused largely on the ligament proper,28,29 while recent efforts have shifted to forming multi-tissue units consisting of integrated ligament–bone, bone–ligament–bone, or ligament–interface–bone regions. For bone–ligament– bone designs, Bourke et al.30 was the first to report on a scaffold consisting of polydesamino tyrosyl-tyrosine ethyl ester carbonate or polylactide (PLA) fibers embedded in polymethyl methacrylate plugs. This composite scaffold was shown to promote collagen ingrowth in a rat subcutaneous model, with physiologically comparable graft strength retained after 30 weeks in vivo. Cooper et al. designed a braided ACL graft consisting of PLGA yarns, with the two distal regions arranged with denser fibers to enable bone formation.31–33 Testing in a rabbit ACL reconstruction model resulted in extensive collagen production after 12 weeks. More recently, Altman et al. reported on a silk bone–ligament–bone graft with denser knit regions at graft ends for bony attachment,34,35 with oriented cells in a crimped ligament-like matrix observed after 12 months testing in a caprine ACL reconstruction model. Growth factors have also been used to enhance graft-bone integration, where a braided PLA-collagen graft with basic fibro-blast growth factor-releasing gelatin at the ends supported the formation of ligament- and bone-like matrix and augmented tensile properties compared to ligament phase-only controls. Combining calcium phosphate and a RGD peptide in a polyethylene glycol (PEG) hydrogel, Paxton et al. observed that the incorporation of hydroxyapatite (HA) with the two ends embedded in fibrin gel forms a bone–ligament– bone construct.36,37 The addition of HA increased overall hydrogel mechanical properties and cell adhesion, albeit functional integration of the ends with fibrin was limited. Adopting a cell-based strategy, Ma et al.38 formed bone–ligament–bone constructs by co-culturing mesenchymal stem cell (MSC)-derived bone constructs with a MSC-derived ligament monolayer rolled in between. The composite graft was first tested in a rat MCL model and after eight weeks, the construct integrated with the native bone and an aligned, crimped, collagen I, and elastin matrix was seen in the ligament phase. Moving to a large animal model of ACL reconstruction, graft integration within bone tunnel and a visible interface region was found between the bone and ligament that structurally resembled fibrocartilage.39 It is clear that these pioneering bone–ligament–bone designs are physiologically more relevant when compared to ligament-only designs, with biomimetic multi-tissue regeneration translating to greater in vivo functionality.
At present, the major clinical barrier for translating the bone–ligament–bone design is that the fibrocartilaginous interface between the ligament and bone regions is not consistently regenerated, especially within the time frame needed to ensure the multi-tissue connectivity that is required for supporting physiological loading. During joint loading, the enthesis plays a critical role in facilitating the transfer of complex loads (compressive and tensile) and easing stress concentrations between the ligament and bone.2,25,40 Mitigating the significant mismatch in mechanical properties between soft and hard tissues, the transition from non-calcified to calcified fibrocartilage enables a gradual transition in matrix stiffness and in turn, shields the ligament from excessive deformation at high strains.2,16,41 For the ACL, an exponential increase in mineral content across the calcified fibrocartilage region that is maintained with age has been observed.18,42 Hence, functional ligament graft design must ensure interface regeneration by embodying multi-tissue motifs which pre-integrates the soft and hard tissues. To this end, Spalazzi et al. pioneered the design of a ligament–interface–bone scaffold, formed by sintering a PLGA mesh-based ligament phase with a PLGA microsphere-based interface phase and a polymer-bioactive glass (BG) composite microsphere-based bone phase.24,43 The phases were joined by solid state sintering at the glass transition temperature of PLGA that ensured the three phases were integrated structurally at the molecular scale, thus preventing phase delamination seen in traditional stratified designs. Optimizing scaffold design to impart spatial control in cell distribution have also ensured that tri-culture of fibroblasts, chondrocytes, and osteoblasts on the tri-phasic scaffold led to the formation of interconnected ligament-, fibrocartilage-, and bone-like matrices in the respective phases both in vitro and in vivo. Another unique aspect of the design is the graded mechanical properties across the scaffold, with the highest elastic modulus and yield strength in the bone phase, mimicking the properties of the native enthesis. Building on the promising findings, Subramony et al.44 designed a poly-ε-caprolactone (PCL) nanofiber-based bone–interface– ligament–interface–bone graft, and optimized the graft for induction of MSCs into fibroblast-, fibrochondrocyte-, and osteoblast-like cells on the relevant graft phases. Preliminary in vivo evaluation in a rat ACL reconstruction model revealed accelerated formation of mineralized tissue within the bone tunnels, accompanied by greater graft mechanical properties compared to single-phased controls. Together, these results emphasize that biomimetic, composite graft design with biomimetic connectivity built-in between soft and hard tissue are essential for functional and integrative ligament regeneration.
To better mimic the compositional and structural gradient of the interface as well as to potentially accelerate integration, interface scaffolds with spatial gradients of mineral content and/or growth factors have also been explored. Samavedi et al. investigated inducing osteoblastic differentiation in a spatially graded manner using scaffolds with mineral gradients.45,46 The scaffold was fabricated by electrospinning a two-polymer solution containing HA particles with offset spinnerets, producing an HA-graded scaffold. The gradient scaffolds induced a spatial gradient in the expression of osteogenic markers by bone marrow stromal cells. Other studies have focused on biochemical gradients across the scaffold to induce graded calcification. Phillips et al.47 established a concentration gradient of osteogenic transcription factors (Runx2/Cbfa1) on collagen scaffolds which was shown to guide the formation of a matrix with a mineral gradient by fibroblasts. These exciting studies underscore the promise of gradient scaffolds for biomimetic interface regeneration, with the potential to fully emulate the complex micro- and nano-scale organization of the native tissue transitions. Unlike stratified scaffolds, gradient designs exhibit more gradual, continuous transitions in composition, and mechanical properties. On the other hand, the stepwise increase in mineral content between phases of stratified scaffolds better approximates the exponential increase in mineral content across the interface regions.18 Presently, fabricating gradient scaffolds at physiologically relevant scales remains challenging. It is also possible that as the scaffold degrades and is remodeled by host cells, physiologically relevant composition gradients will likely emerge in vivo.
Overall, it is clear that integrative graft design remains a frontier challenge for functional ligament regeneration, and consideration of tissue connectivity in graft design and methods for promoting interface regeneration will be essential. By pre-engineering the ligament-bone interface ex vivo, one can focus on the less challenging task of osteointegration in vivo. Moreover, in order to determine the optimal design for interface tissue engineering, it is important to conduct additional studies that systematically compare gradient scaffolds with stratified designs in preclinical models.
INTEGRATIVE GRAFT DESIGN FOR TENDON REGENERATION
The functional tendon connects muscle to bone and major tendons in the body, such as the rotator cuff, and inserts into bone via a fibrocartilaginous transition with graded nonmineralized and mineralized zones.1 Similar to the ACL, the relatively compliant interface is half as strong as the tendon in tension and also serves to minimize stress concentrations.21,48–50 However tendons are not subjected to torsion,51 and both injury characteristics and surgical repair methods differ between these two types of soft tissues. The optimal scaffold for tendon integration should incorporate compositional and structural heterogeneity, thus enabling phase-specific changes in supporting interface cell populations while exhibiting a gradation in both tensile and compressive mechanical properties.
The most common tendon injury occurs at the rotator cuff, with clinical treatment centered upon mechanically reattaching the torn tendon back to bone. This approach is however prone to failure as the native fibrocartilaginous insertion is not reformed to provide biological fixation of tendon to bone. To improve fixation, Chang et al.52 and Sundar et al.,53 have shown that placing demineralized bone or periosteum between the tendon and bone helps to improve mechanical function and induce fibrocartilage formation. However, the native graded interface structure is not consistently restored, and tissue harvesting or additional graft processing proves to be a clinical limitation. Focused on restoring the fibrocartilaginous tendon–bone interface, Moffat et al.54 designed a biphasic scaffold, emulating the non-calcified and calcified region of the interface by electrospinning PLGA atop PLGA-HA fibers during fabrication. In vivo evaluations found that distinct interface regeneration was only observed using the biphasic scaffold design, while controls scaffolds of either single phase showed partial or incomplete regeneration. When tested in both small and large rotator cuff repair models as an inter-positional graft, a fibrocartilage-like interface formed between tendon and bone with a calcified matrix observed only on Phase-B. Greater maturation and collagen organization were evident at the neo-interface after a bone marrow aspirate was added to the scaffold immediately before implantation. It was concluded that the mineral-free top layer facilitated organized integration with tendon and promoted fibrocartilage regeneration, while the mineral-containing Phase-B layer enabled osteointegration and calcified fibrocartilage deposition.
Other interface scaffold designs have incorporated mineral gradients to mimic the reported mineral gradient at the tendon–bone junction.55 By immersion in a simulated body fluid (SBF) with high mineral ion concentration, Li et al.56 developed a PLGA-based nanofiber scaffold with a calcium phosphate gradient along the fiber surface. The linear mineral distribution imparted a stiffness gradient that regulated MC3T3 activity. By increasing bicarbonate ion concentration in the SBF, denser mineral coatings could be formed on the fibers which enhanced mechanical properties that based on immuno-histochemical analysis, could induce osteogenic differentiation of adipose-derived MSCs spatially.57 Also working with SBF soaking, Dickerson et al.58 designed a flexible, regionally-demineralized cancellous bone scaffold, harvested from the vertebral bodies of steers, with one end being rigid and mineralized and the other end being flexible and non-mineralized. The scaffold integrated with the host tissue, enabled the development of organized collagen-like tissue, and a fibrocartilage-like transition was formed when tested in an ovine tendon repair model. Cui et al. grafted PLA fibers with carboxyl, hydroxyl, and amino groups that served as induction sites for calcification, and produced meshes with a HA gradient that spatially regulated pre-osteoblasts (MC3T3) growth, collagen deposition, and differentiation.59,60 These studies underscore the promise of gradient scaffolds for interface tissue engineering and the next challenge is to form physiologically relevant gradient profiles with micro- and nano-scale gradients that emulate the native tendon–bone enthesis. It is clear that the cellular response and differentiation capacity is influenced by exposing stem cells to a spatial gradient of induction cues, and further research is needed to better understand the mechanism by which these gradient scaffolds regulate cell differentiation and tissue regeneration.
While most tendon–bone injuries occur either at the insertion or the tendon proper, muscle atrophy, and detachment are also associated with tendon degeneration. Reestablishment of this interface, which distributes mechanical loads between skeletal muscle and bone, is important for restoration of function after injury.61 From a structure-function perspective, the muscle–tendon interface has drastically different mechanical properties compared to muscle or tendon. In terms of its physiological properties, tendon has an elastic modulus almost three orders of magnitude greater than that of muscle.62 The interdigitating interface, consisting of fibroblast-laden tissue on elastic muscle fibers connected to dense collagenous tendon fibers, results in an almost 10-fold increase in tendon-muscle contact surface area and thus distributes stresses over a wide area.63 Hence, an ideal scaffold should also present a multi-phased design for emulating the varying mechanical properties between the tendon and bone regions. To this end, Larkin et al.64 co-cultured skeletal muscle and engineered tendon constructs in vitro to form a muscle–interface–tendon construct, resulting in a robust muscle–tendon interface that remained intact when force was applied or generated. Moreover, the interface showed increased expression and localization of paxillin, and was also analogous to the protein expression patterns and structural characteristics of neonatal interfaces in vivo. Recently, Ladd et al.65 designed a scaffold with continuous muscle–interface–tendon regions by co-electrospinning PCL-collagen and PLA-collagen onto opposite ends of an electrospinning mandrel. This composite tissue graft supported both myoblast and fibroblast adhesion, with a strain profile similar to the native muscle–tendon interface.
SUMMARY AND CONCLUSIONS
Current scaffold design approaches in integrative ligament and tendon repair are a reflection of the prevalence of soft tissue injuries and adaptation to current clinical practice, and it is clear that composite grafts (graded and gradient) are needed to recapitulate native soft tissue function and reestablish tissue connectivity. To this end, interface tissue engineering represents an attractive approach to regenerate the soft tissue-to-bone interface, facilitating functional tissue-to-tissue integration and improving long term clinical outcome. Enthesis regeneration is particularly challenging due to the composite structure of the native interface and the interdependence between its mechanical properties, function, organization, and structure. The studies highlighted here and others have demonstrated that the early focus on single tissue grafts for soft tissue repair is insufficient given the inherent dependence of soft tissue functionality on tissue connectivity. By pre-engineering the anatomical interfaces ex vivo in the design of composite tissue graft (bone–interface–ligament–interface–bone, tendon–interface–bone, or muscle– interface–tendon), both host integration and physiologic loading can be achieved in vivo. In addition, regional scaffold cues and heterotypic cellular interactions can be used to direct cell fate in the absence of differentiation media, making graded or gradient designs particularly attractive for spatially directing cell fate and ensuring region-specific matrix elaboration. Moreover, it is noted that design requirements vary by tissue type and must take into consideration existing surgical practices for soft tissue repair. For example, while multi-tissue units are a must for total ligament reconstruction, an interface scaffold that enables organized fibrocartilaginous interface formation is sufficient for rotator cuff repair.
In terms of future directions, in-depth understanding of both the enthesis structure-function relationship and the biological processes that drive interface development, regeneration, and homeostasis remain much needed. Further investigation is required to understand how the boundaries between various tissues types (such as the four regions of the enthesis) are formed, maintained, and regenerated after injury. While there are multiple factors at play during enthesis development and healing, the cues that direct this complex process remain largely unknown. Additionally, identifying the signals that initiate cell homing, modulate the immune response, or drive stem cell differentiation will be essential for ensuring functional healing and expedited tissue integration. Similar to other tissue engineered grafts, a translation gap remains between promising approaches in the laboratory and successful implementation in the clinical setting. Many of the technologies discussed above have only been evaluated in the preclinical setting.66 While encouraging, small or large animal results do not necessarily guarantee similar performance when used in humans. Moreover, adaptation of these integrative design for use with arthroscopy surgery represents another translational challenge.
In conclusion, regeneration of musculoskeletal interfaces is a pre-requisite for functional and integrative soft tissue healing. Taking advantage of the current tissue engineering toolkit to develop complex scaffolds for composite tissue regeneration, designing multi-unit grafts is one of the most promising approaches for interface tissue engineering. The advent of high resolution 3D-printing will make it possible to engineer multi-layered, multi-cellular tissue composites with finely controlled and physiologically relevant spatial distributions of cells, minerals, and biofactors. Given the inherent complexity in scaffold design, strategic biomimcry must be applied to identify the most salient and relevant design criteria for composite tissue engineering, avoiding over-engineering and reducing the burden for clinical translation. Success of the many exciting efforts in interface tissue engineering will not only drive the development of novel fixation devices for effective treatment of soft tissue injuries, but also be instrumental in engineering complex tissues and total joint regeneration.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (HHL, R01-AR055280), the DoD CDMRP (HHL&WNL, W81XWH-15-1-0685), the SEAS Translational Fellows Program (SP), and the New York State Stem Cell ESSC Board (NYSTEM Training Grant—Postdoctoral Fellowship for SP).
Grant sponsor: National Institute for Health Research; Grant number: R01-AR055280; Grant sponsor: U.S. Department of Defense; Grant number: W81XWH-15-1-0685; Grant sponsor: SEAS Translational Fellows Program; Grant sponsor: New York State Stem Cell ESSC Board (NYSTEM Training Grant).
REFERENCES
- 1.Benjamin M, Evans EJ, Copp L. 1986. The histology of tendon attachments to bone in man. J Anat 149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Moffat KL, Sun W-HS, Pena PE, et al. 2008. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci 105: 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo SL, Gomez MA, Seguchi Y, et al. 1983. Measurement of mechanical properties of ligament substance from a boneligament-bone preparation. J Orthop Res 1:22–29. [DOI] [PubMed] [Google Scholar]
- 4.2014. United States Bone and Joint Initiative: The burden of musculoskeletal diseases in the United States (BMUS), 3rd Ed. Rosemont, Illinois: United States Bone and Joint Initiative. [Google Scholar]
- 5.Jain NB, Higgins LD, Losina E, et al. 2014. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet Disord 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buller LT, Best MJ, Baraga MG, et al. 2015. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med 3:2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hein J, Reilly JM, Chae J, et al. 2015. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy 31:2274–2281. [DOI] [PubMed] [Google Scholar]
- 8.Park MC, Elattrache NS, Ahmad CS, et al. 2006. “Transosseous-equivalent” rotator cuff repair technique. Arthroscopy 22:1360.e1361–1360.e1365. [DOI] [PubMed] [Google Scholar]
- 9.Park MC, ElAttrache NS, Tibone JE, et al. 2007. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg 16:461–468. [DOI] [PubMed] [Google Scholar]
- 10.Freedman BR, Fryhofer GW, Salka NS, et al. 2017. Mechanical, histological, and functional properties remain inferior in conservatively treated Achilles tendons in rodents: long term evaluation. J Biomech 56:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galatz LM, Ball CM, Teefey SA, et al. 2004. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86-a:219–224. [DOI] [PubMed] [Google Scholar]
- 12.Friedman MJ, Sherman OH, Fox JM, et al. 1985. Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop Relat Res 9–14. [PubMed]
- 13.Kurosaka M, Yoshiya S, Andrish JT. 1987. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med 15:225–229. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DB, Daniel DM, Biden E. 1986. Soft tissue fixation to bone. Am J Sports Med 14:398–403. [DOI] [PubMed] [Google Scholar]
- 15.Rodeo SA, Arnoczky SP, Torzilli PA, et al. 1993. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am 75:1795–1803. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin M, Toumi H, Ralphs JR, et al. 2006. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 208:471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu D, Mosher CZ, Boushell MK, et al. 2015. Engineering complex orthopaedic tissues via strategic biomimicry. Ann Biomed Eng 43:697–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spalazzi JP, Boskey AL, Pleshko N, et al. 2013. Quantitative mapping of matrix content and distribution across the ligament-to-bone insertion. PLoS ONE 8:e74349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelzer E, Blitz E, Killian ML, et al. 2014. Tendon-to-bone attachment: from development to maturity. birth defects research part C. Birth Defects Research Part C. Embryo Today 102:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milz S, Rufai A, Buettner A, et al. 2002. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat 200:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomopoulos S, Williams GR, Gimbel JA, et al. 2003. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 21:413–419. [DOI] [PubMed] [Google Scholar]
- 22.Wang IE, Mitroo S, Chen FH, et al. 2006. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res 24:1745–1755. [DOI] [PubMed] [Google Scholar]
- 23.Lipner J, Liu W, Liu Y, et al. 2014. The mechanics of PLGA nanofiber scaffolds with biomimetic gradients in mineral for tendon-to-bone repair. J Mech Behav Biomed Mater 40:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spalazzi JP, Doty SB, Moffat KL, et al. 2006. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng 12: 3497–3508. [DOI] [PubMed] [Google Scholar]
- 25.Matyas JR, Anton MG, Shrive NG, et al. 1995. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. J Biomech 28:147–157. [DOI] [PubMed] [Google Scholar]
- 26.Woo SL-Y, Buckwalter JA. 1988. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987 J Orthop Res 6:907–931. [DOI] [PubMed] [Google Scholar]
- 27.Woo SL, Buckwalter JA. 1988. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987 J Orthop Res 6:907–931. [DOI] [PubMed] [Google Scholar]
- 28.Vunjak-Novakovic G, Altman G, Horan R, et al. 2004. Tissue engineering of ligaments. Annu Rev Biomed Eng 6:131–156. [DOI] [PubMed] [Google Scholar]
- 29.Weitzel PP, Richmond JC, Altman GH, et al. 2002. Future direction of the treatment of ACL ruptures. Orthop Clin North Am 33:653–661. [DOI] [PubMed] [Google Scholar]
- 30.Bourke SL, Kohn J, Dunn MG. 2004. Preliminary development of a novel resorbable synthetic polymer fiber scaffold for anterior cruciate ligament reconstruction. Tissue Eng 10:43–52. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JA, Lu HH, Ko FK, et al. 2005. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials 26:1523–1532. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JA, Sahota JS, Gorum WJ, et al. 2007. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci 104:3049–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu HH, Cooper JA Jr, Manuel S, et al. 2005. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials 26:4805–4816. [DOI] [PubMed] [Google Scholar]
- 34.Altman GH, Horan RL, Weitzel P, et al. 2008. The use of long-term bioresorbable scaffolds for anterior cruciate ligament repair. J Am Acad Orthop Surg 16:177–187. [DOI] [PubMed] [Google Scholar]
- 35.Horan RL, Toponarski I, Boepple HE, et al. 2009. Design and characterization of a scaffold for anterior cruciate ligament engineering. J Knee Surg 22:82–92. [DOI] [PubMed] [Google Scholar]
- 36.Paxton JZ, Donnelly K, Keatch RP, et al. 2009. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A 15:1201–1209. [DOI] [PubMed] [Google Scholar]
- 37.Paxton JZ, Grover LM, Baar K. 2010. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A 16:3515–3525. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Goble K, Smietana M, et al. 2009. Morphological and functional characteristics of three-dimensional engineered boneligament-bone constructs following implantation. J Biomech Eng 131:101017. [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Smietana MJ, Kostrominova TY, et al. 2012. Three-dimensional engineered bone-ligament-bone constructs for anterior cruciate ligament replacement. Tissue Eng Part A 18:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spalazzi JP, Gallina J, Fung-Kee-Fung SD, et al. 2006. Elastographic imaging of strain distribution in the anterior cruciate ligament and at the ligament-bone insertions. J Orthop Res 24:2001–2010. [DOI] [PubMed] [Google Scholar]
- 41.Hems T, Tillmann B. 2000. Tendon entheses of the human masticatory muscles. Anat Embryol 202:201–208. [DOI] [PubMed] [Google Scholar]
- 42.Qu D, Chuang PJ, Prateepchinda S, et al. 2016. Micro- and ultrastructural characterization of age-related changes at the anterior cruciate ligament-to-bone insertion. ACS Biomater Sci Eng 3:2806–2814 [DOI] [PubMed] [Google Scholar]
- 43.Spalazzi JP, Dagher E, Doty SB, et al. 2008. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res Part A 86A:1–12. [DOI] [PubMed] [Google Scholar]
- 44.Subramony SD, Qu D, Ma R, et al. 2014. In vitro optimization and in vivo evaluation of a multi-phased nanofiberbased synthetic ACL scaffold In: Transactions of the 60th Orthopaedic Research Society. [Google Scholar]
- 45.Samavedi S, Guelcher SA, Goldstein AS, et al. 2012. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials 33:7727–7735. [DOI] [PubMed] [Google Scholar]
- 46.Samavedi S, Olsen Horton C, Guelcher SA, et al. 2011. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament–bone interface. Acta Biomater 7:4131–4138. [DOI] [PubMed] [Google Scholar]
- 47.Phillips JE, Burns KL, Le Doux JM, et al. 2008. Engineering graded tissue interfaces. Proc Natl Acad Sci 105:12170–12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang CY, Wang VM, Pawluk RJ, et al. 2005. Inhomogeneous mechanical behavior of the human supraspinatus tendon under uniaxial loading. J Orthop Res 23:924–930. [DOI] [PubMed] [Google Scholar]
- 49.Liu YX, Thomopoulos S, Birman V, et al. 2012. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech Mater 44 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3839427/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano H, Saijo Y, Kokubun S. 2006. Non-mineralized fibro-cartilage shows the lowest elastic modulus in the rabbit supraspinatus tendon insertion: measurement with scanning acoustic microscopy. J Shoulder Elbow Surg 15:743–749. [DOI] [PubMed] [Google Scholar]
- 51.Rumian AP, Wallace AL, Birch HL. 2007. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features—a comparative study in an ovine model. J Orthop Res 25:458–464. [DOI] [PubMed] [Google Scholar]
- 52.Chang CH, Chen CH, Su CY, et al. 2009. Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc 17:1447–1453. [DOI] [PubMed] [Google Scholar]
- 53.Sundar S, Pendegrass CJ, Blunn GW. 2009. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res Part B Appl Biomater 88:115–122. [DOI] [PubMed] [Google Scholar]
- 54.Moffat KL, Cassilly RT, Subramony SD, et al. 2010. In vivo evaluation of a bi-phasic nanofiber-based scaffold for integrative rotator cuff repair. Trans 56th Orthop Res Soc 2010 https://www.ors.org/Transactions/56/0184.pdf.
- 55.Genin GM, Kent A, Birman V, et al. 2009. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J 97:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Xie J, Lipner J, et al. 2009. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett 9:2763–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Lipner J, Xie J, et al. 2014. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl Mater Interfaces 6:2842–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickerson DA, Misk TN, Van Sickle DC, et al. 2013. In vitro and in vivo evaluation of orthopedic interface repair using a tissue scaffold with a continuous hard tissue-soft tissue transition. J Orthop Surg Res 8:18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui W, Li X, Chen J, et al. 2008. In situ growth kinetics of hydroxyapatite on electrospun poly(dl-lactide) fibers with gelatin grafted. Cryst Growth Des 8:4576–4582. [Google Scholar]
- 60.Cui W, Li X, Xie C, et al. 2010. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials 31:4620–4629. [DOI] [PubMed] [Google Scholar]
- 61.Yang PJ, Temenoff JS. 2009. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev 15:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wren TA, Yerby SA, Beaupre GS, et al. 2001. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon) 16:245–251. [DOI] [PubMed] [Google Scholar]
- 63.Trotter JA. 2002. Structure–function considerations of muscle–tendon junctions. Comp Biochem Physiol Part A Mol Integr Physiol 133:1127–1133. [DOI] [PubMed] [Google Scholar]
- 64.Larkin LM, Calve S, Kostrominova TY, et al. 2006. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng 12: 3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladd MR, Lee SJ, Stitzel JD, et al. 2011. Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering. Biomaterials 32:1549–1559. [DOI] [PubMed] [Google Scholar]
- 66.Patel S, Gualtieri AP, Lu HH, et al. 2016. Advances in biologic augmentation for rotator cuff repair. Ann New York Acad Sci 1383:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomopoulos S 2011. The role of mechanobiology in the attachment of tendon to bone. IBMS BoneKEy 8:271–285. [Google Scholar]
- 68.Wang Z, Zhang Y, Zhu J, et al. 2015. In vitro investigation of a tissue-engineered cell-tendon complex mimicking the transitional architecture at the ligament-bone interface. J Biomater Appl 29:1180–1192. [DOI] [PubMed] [Google Scholar]
- 69.Kimura Y, Hokugo A, Takamoto T, et al. 2008. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue engineering Part C. Methods 14:47–57. [DOI] [PubMed] [Google Scholar]
- 70.Santoni BG, McGilvray KC, Lyons AS, et al. 2010. Bio-mechanical analysis of an ovine rotator cuff repair via porous patch augmentation in a chronic rupture model. Am J Sports Med 38:679–686. [DOI] [PubMed] [Google Scholar]
- 71.Moffat KL, Kwei AS, Spalazzi JP, et al. 2009. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A 15:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]