Abstract
Intravenous fluid therapy is the most common intervention received by acutely ill patients. Historically, saline (0.9% sodium chloride) has been the most frequently administered intravenous fluid, especially in North America. Balanced crystalloid solutions (e.g., lactated Ringer’s, Plasma-Lyte) are an increasingly used alternative to saline. Balanced crystalloids have a sodium, potassium, and chloride content closer to that of extracellular fluid and, when given intravenously, have fewer adverse effects on acid–base balance. Preclinical research has demonstrated that saline may cause hyperchloremic metabolic acidosis, inflammation, hypotension, acute kidney injury, and death. Studies of patients and healthy human volunteers suggest that even relatively small volumes of saline may exert physiological effects. Randomized trials in the operating room have demonstrated that using balanced crystalloids rather than saline prevents the development of hyperchloremic metabolic acidosis and may reduce the need for vasopressors. Observational studies among critically ill adults have associated receipt of balanced crystalloids with lower rates of complications, including acute kidney injury and death. Most recently, large randomized trials among critically ill adults have examined whether balanced crystalloids result in less death or severe renal dysfunction than saline. Although some of these trials are still ongoing, a growing body of evidence raises fundamental concerns regarding saline as the primary intravenous crystalloid for critically ill adults and highlights fundamental unanswered questions for future research about fluid therapy in critical illness.
Keywords: intravenous fluid, saline, balanced crystalloids, acute kidney injury, critical illness
Intravenous fluid therapy has been fundamental to acute care for more than a century (1). Each year, more than 30 million patients receive intravenous fluid for resuscitation or maintenance of intravascular volume or as a medication carrier (2). Crystalloid solutions are the most commonly administered intravenous fluid and are composed of electrolytes in water that cross easily from the vascular space into the interstitium (3). Two basic classes of crystalloid are available to clinicians: saline (0.9% sodium chloride) and balanced crystalloids (e.g., lactated Ringer’s, Plasma-Lyte A [Baxter Healthcare]). Although saline has been the predominantly used crystalloid in North America and in many other parts of the world (4), recent evidence from basic science research, observational research, and clinical trials suggests that using balanced crystalloids rather than saline may have beneficial effects on acid–base balance, renal physiology, and patient outcomes.
Crystalloid Solutions
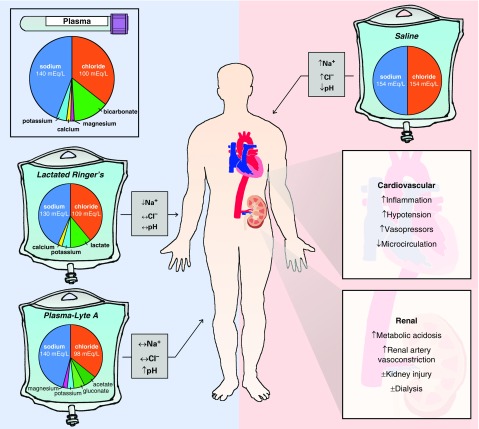
Saline (a/k/a 0.9% sodium chloride or “normal saline”) contains 154 mmol/L of both sodium and chloride (Table 1). Balanced crystalloids (a/k/a “buffered crystalloids”) are solutions in which chloride anions are replaced with bicarbonate or buffers to reduce the perturbations in acid–base balance resulting from fluid administration (Figure 1) (5). The original balanced crystalloids achieved this by replacing chloride anions with bicarbonate anions (1). Because of the instability of bicarbonate-containing solutions in standard gas-permeable bags, modern balanced crystalloids contain alternative anions, such as lactate, acetate, and gluconate, that are rapidly metabolized or excreted (6).
Table 1.
Composition of Crystalloid Solutions
| Fluid | Sodium | Potassium | Calcium | Magnesium | Chloride | Acetate | Gluconate | Malate | Lactate | Osmolarity |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | 135–145 | 4.5–5.0 | 2.2–2.6 | 0.8–1.0 | 94–111 | 0.02–0.2 | 1–2 | 275–295 | ||
| Plasma-Lyte A | 140 | 5.0 | 3.0 | 98 | 27 | 23 | 294 | |||
| Normosol-R | 140 | 5.0 | 3.0 | 98 | 27 | 23 | 295 | |||
| Isolyte S | 141 | 5.0 | 3.0 | 98 | 27 | 23 | 295 | |||
| Ringer’s acetate | 145 | 4.0 | 2.5 | 1.0 | 127 | 24 | 5 | 309 | ||
| Lactated Ringer’s | 130 | 4.0 | 2.7 | 109 | 28 | 273 | ||||
| Hartmann’s solution | 131 | 5.4 | 1.8 | 112 | 28 | 280 | ||||
| 0.9% sodium chloride | 154 | 154 | 308 |
All values are in mEq/L, except calculated osmolarity, which is in mOsm/L. Plasma-Lyte A is “Multiple Electrolyte Injection, Type 1, USP,” from Baxter Healthcare Corporation; Normosol-R is “Multiple Electrolyte Injection, Type 1, USP,” from Hospira, Inc.; Isolyte S is from B. Braun Medical Inc.; Ringer’s acetate is “Sterofundin ISO, Isotonic Electrolyte Solution,” from B. Braun; lactated Ringer’s is “lactated Ringer’s Injection, USP,” from Baxter Healthcare Corporation; Hartmann’s solution is “Compound Sodium Lactate” from Baxter Healthcare Corporation; and 0.9% saline is “Sodium Chloride Injection, USP,” from Baxter Healthcare Corporation.
Figure 1.
Effects of crystalloid composition on plasma electrolytes and organ function. The figure displays the electrolyte content of human plasma and of each intravenous crystalloid solution. Gray boxes indicate the expected effect of administration of each crystalloid on plasma electrolytes. Panels on the right summarize detrimental effects of saline-induced hyperchloremic metabolic acidosis on the cardiovascular and renal systems reported in preclinical and clinical research.
Preclinical Research
Both balanced crystalloids and saline have been available for clinical use and scientific examination for more than 100 years. Concerns about the effects of saline’s composition were first raised at the turn of the 20th century, when researchers noted that saline did not maintain electrical or mechanical activity of isolated muscle preparations. In 1901, Harvey Cushing declared saline to be “poisonous” (7). Despite the concerns, in the first three decades of the 20th century, saline went from a fluid developed for laboratory use to the most common fluid administered to patients globally. By the 1940s, saline was so ubiquitous in clinical practice that when acidosis resulting from fluid resuscitation was first observed, it was mistakenly attributed to dilution of bicarbonate (8) rather than to saline. After the introduction of standard base excess in the 1970s (9) and strong ion difference in the 1980s (10), quantitative analyses made it apparent that chloride concentration was an important contributor to acid–base derangements in critical illness.
Acid–Base Balance
In 1998, Kellum and colleagues first quantified the effect of saline on acid–base balance (11). Among endotoxemic dogs resuscitated with saline, changes in strong ion difference demonstrated that saline alone accounted for more than one-third of the overall acidosis. Subsequent work in the same laboratory identified the supraphysiologic chloride content of saline as a potential regulator of acid–base physiology and clinical outcomes (12).
Despite the consistently observed relationship between intravenous saline resuscitation and hyperchloremic metabolic acidosis, uncertainty remained about whether, and how, saline-induced acidosis might affect organ function and survival. Observations of the clinical effects of acidosis from the field of exercise physiology suggested even profound metabolic acidosis (arterial blood pH <7.0) generated by vigorous exercise was well tolerated and without lasting effects on organ function (13, 14). Similarly, permissive hypercapnia for patients with acute respiratory distress syndrome produced moderate to severe acidosis without negative effects on organ function or survival (15). Indeed, healthy humans and animals appear to be able to tolerate rather significant acidosis, at least for short periods. In the context of critical illness, however, acidosis appears to have meaningful detrimental effects on the vasculature (16). Acidosis induces vasodilation (17), which may be beneficial in exercise but may worsen shock. In rats, after cecal ligation and puncture, even modest acidosis (standard base excess of −5 to −10 mEq/L) induced by infusion of dilute hydrochloric acid, decreases blood pressure (18) in a dose–response fashion. These hemodynamic effects take up to 8 hours to fully develop and are not completely explained by changes in plasma nitrate/nitrite concentrations. The concept that resuscitation with saline-containing fluids induces acidosis that can contribute to cardiovascular collapse provides a potential mechanism for the findings of several recent randomized trials examining fluid resuscitation for acutely ill patients (19–21).
Inflammation
Saline-induced hyperchloremic metabolic acidosis also appears to induce inflammation in cell culture and animal models. Macrophage-like RAW 264.7 cells incubated with LPS and a pH of 7.0 exhibit increased nuclear factor-κB DNA binding when acidosis is induced with hydrochloric acid, but not when acidosis is induced with lactic acid (22). Studies demonstrating antiinflammatory effects of CO2 (23) suggest that acidosis may have variable effects on the inflammatory response, depending on the acid involved, and highlight important differences between endogenous acids such as lactate and CO2 and hyperchloremic acidosis, which is often iatrogenic.
Among rats made septic by cecal ligation and puncture, saline-treated animals experienced higher plasma IL-6 concentrations (24). These results are consistent with prior animal studies demonstrating increased cytokine expression with hydrochloric acid–induced hyperchloremic acidosis, even when blood pressure is maintained (25).
Renal Function
The effects of crystalloid solutions with high chloride content on the kidney have been equally controversial. A 1983 study of isolated, perfused greyhound kidneys found that increasing chloride concentration in the perfusate produced a progressive renal vasoconstriction and fall in glomerular filtration rate (26). These effects were independent of the renal nerves and appeared to be related to tubular chloride reabsorption (26). Subsequent studies among healthy human volunteers, discussed below, found similar decreases in renal blood flow velocity and perfusion with high-chloride solutions (27, 28).
Healthy rats treated with intravenous saline did not develop kidney damage, but animals made septic with cecal ligation and puncture were a different story. Compared with Plasma-Lyte treatment, resuscitation with saline resulted in significantly worse kidney injury severity, as measured by RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) criteria (29). These results were consistent with kidney histology and biomarkers of acute kidney injury (24). Twenty-four-hour survival also favored Plasma-Lyte resuscitation (76.6% vs. 53.3%; P = 0.03).
Clinical Research
Building on a growing body of preclinical research, studies over the last three decades have compared balanced crystalloids with saline in controlled experiments among healthy volunteers, observational studies in acute illness, randomized trials in the operating room, and large randomized trials among critically ill adults.
Healthy Volunteers
Multiple randomized crossover trials have compared intravenous administration of high-chloride versus low-chloride solutions in healthy human volunteers (27, 28, 30). When healthy volunteers received 2 L of either saline or Plasma-Lyte over 1 hour, saline significantly decreased renal artery blood velocity, decreased renal cortical tissue perfusion, decreased urine output, and increased extravascular fluid accumulation compared with Plasma-Lyte (27). Similarly, when healthy volunteers received 1 L of intravenous 6% hydroxyethyl starch (HES) over 30 minutes, HES in saline decreased renal cortical perfusion compared with HES in balanced crystalloid (28). These findings provide modest support for the idea that in humans, like in animal models (26), saline-induced hyperchloremia may cause increased tubuloglomerular feedback and decreased renal cortical perfusion.
Observational Studies
Much of the early interest in the potential effects of crystalloid composition on clinical outcomes for acutely ill adults arose from large observational studies in the operating room and ICU. A retrospective study of more than 30,000 major abdominal surgery patients drawn from the Premier Perspective Comparative Database found that after propensity score adjustment and matching, patients treated with balanced crystalloids experienced fewer complications and less renal failure requiring dialysis (31). A prospective observational study of 542 patients undergoing major surgery found that after adjusting for fluid balance and amount of fluid received, low-chloride solutions were independently associated with a lower risk of acute kidney injury than high-chloride solutions (32).
Three observational studies compared balanced crystalloids with saline among patients receiving fluid resuscitation for sepsis or septic shock (33–35). A retrospective analysis of more than 100,000 adults from a Cerner database meeting systemic inflammatory response criteria found that, after adjusting for the total volume of resuscitation fluid received, receipt of a larger chloride load was associated with increased odds of death (odds ratio, 1.09; 95% confidence interval [CI], 1.06–1.13) (33). A propensity-matched analysis of over 6,000 adults in a Premier database with a diagnosis of sepsis who received at least 2 L of intravenous fluid found that receipt of balanced crystalloids was associated with a 3.2% (95% CI, 1.5–5.0%) lower absolute risk of in-hospital mortality (relative risk, 0.86; 95% CI, 0.78–0.94) (34). Another analysis of a Premier database examining more than 60,000 patients with sepsis and receiving vasopressors also found that after propensity score matching, balanced crystalloids were associated with a lower risk of in-hospital mortality (risk ratio, 0.84; 95% CI, 0.76–0.92) (35). Yet another study found that among patients receiving large-volume resuscitation (>60 ml/kg in 24 h), chloride load was associated with decreased 1-year survival, even after controlling for total fluid volume, age, and baseline severity of illness (36).
Finally, a before-and-after study compared 760 patients admitted to a single ICU during a period in which high-chloride solutions (saline, 4% gelatin, 4% albumin) were used with 773 patients admitted to the same ICU during a period in which low-chloride solutions (Hartmann solution, Plasma-Lyte 148, 20% albumin) were used (37). Acute kidney injury (odds ratio, 0.52; 95% CI, 0.37–0.75) and receipt of renal replacement therapy (odds ratio, 0.52; 95% CI, 0.33–0.81) were less common during the low-chloride period. The odds ratio for mortality during the low-chloride period compared with the high-chloride period was 0.88 (95% CI, 0.66–1.18). A subsequent analysis, however, suggested that unidentified confounders (e.g., decreasing use of gelatins over time, Hawthorne effect) may have contributed to the observed differences in acute kidney injury and renal replacement therapy between the low-chloride and high-chloride periods (38).
Clinical Trials in the Operating Room
Numerous small clinical trials have compared balanced crystalloids with saline among patients undergoing renal and nonrenal surgery. Early trials comparing lactated Ringer’s to saline among patients undergoing gynecologic surgery (39) or abdominal aortic aneurysm repair (40) found that saline resuscitation produced hyperchloremia, metabolic acidosis, and decreased strong ion difference. These findings were confirmed across multiple operative populations, including women undergoing cesarean section (41), adults undergoing neurosurgery (42–44), and adults undergoing major abdominal surgery (45). Balanced crystalloids also appeared to result in lower concentrations of biomarkers of early acute kidney injury (e.g., neutrophil gelatinase-associated lipocalin) among adults undergoing major abdominal surgery (45).
A recent, patient-level, double-blind, randomized trial compared an acetate-buffered balanced crystalloid with saline among patients undergoing major abdominal surgery (21). The trial was terminated after the enrollment of 60 of 240 planned patients, when, at the interim analysis, 97% of patients in the saline group met the primary outcome of requiring catecholamine infusion to maintain mean arterial pressure, compared with 67% in the balanced crystalloid group (P = 0.03). Patients randomized to the saline group experienced more frequent hyperchloremic metabolic acidosis, earlier and more frequent receipt of vasopressors, and higher overall doses of vasopressors, although early stopping of the trial for safety may overestimate the magnitude of these effects.
The LICRA (Limiting I.V. Chloride to Reduce AKI) study is the largest trial to date comparing balanced crystalloids with saline in the operating room. LICRA was a single-cluster, double-crossover trial comparing low-chloride solutions (balanced crystalloids or 20% albumin) with high-chloride solutions (saline or 4% albumin) among 1,136 adults undergoing cardiac surgery at a single academic center (46). Patients received nearly 5 L of study fluid, with a mean difference between groups in highest plasma chloride concentration of 4 mmol/L. The incidence of acute kidney injury was not significantly different between the balanced crystalloid (29.1%) and saline (33.3%) groups (odds ratio, 0.82; 95% CI, 0.64–1.05). Temporal changes over the course of the crossover study, however, resulted in imbalances between the study groups in baseline factors expected to modify the risk of acute kidney injury. Specifically, the balanced crystalloid and saline groups differed with regard to stage of chronic kidney disease at baseline, receipt of extracorporeal membrane oxygenation or a ventricular assist device, and intraoperative receipt of vancomycin, leaving residual uncertainty regarding the relative effects of balanced crystalloids and saline during cardiac surgery.
Patients undergoing renal transplant represent a unique population in which the potential effects of balanced crystalloids versus saline on acid–base status, plasma electrolyte concentrations, and renal function may be magnified. Seven randomized trials have compared balanced crystalloids with saline during renal transplant (47–53). All found that use of balanced crystalloids resulted in lower rates of hyperchloremic metabolic acidosis. Four found lower rates of hyperkalemia with balanced crystalloids than with saline (47–50), perhaps owing to saline-induced hyperchloremic acidosis shifting potassium out of cells into the extracellular fluid (54). The effect of crystalloid composition on renal allograft function after transplant and the effect of balanced crystalloids versus saline on plasma potassium concentration among other patient populations remain unclear (55).
Clinical Trials in Acute Care
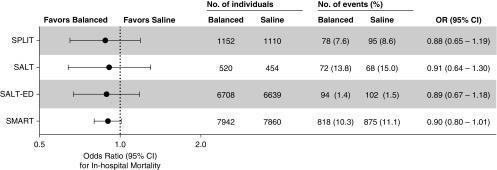
Two cluster-randomized cluster-crossover pilot trials compared balanced crystalloids with saline among adult ICU patients (56, 57). The SPLIT (0.9% Saline versus Plasma-Lyte 148 for ICU fluid Therapy) trial compared Plasma-Lyte 148 with 0.9% sodium chloride among 2,278 patients admitted to four ICUs in New Zealand (56). Patients were predominantly admitted after cardiovascular surgery, were at low risk of death by baseline Acute Physiologic Assessment and Chronic Health Evaluation score, received primarily balanced crystalloid before ICU admission, and received a median of 2.0 L of isotonic crystalloid after enrollment. The relative risk of in-hospital mortality with balanced crystalloids compared with saline was 0.87 (95% CI, 0.64–1.18) (Figure 2).
Figure 2.
Effect of balanced crystalloids versus saline on mortality among critically ill adults. The odds ratios (ORs) and 95% confidence intervals (CIs) for in-hospital mortality with balanced crystalloids compared with saline are displayed for the four large randomized trials among critically ill adults (56–59).
The SALT (Isotonic Solution Administration Logistical Testing) trial compared balanced crystalloids (primarily lactated Ringer’s) with 0.9% sodium chloride among 974 adults admitted to a single medical ICU (57). Patients were predominantly admitted from the emergency department (ED) (with sepsis as the most common diagnosis), received primarily saline before ICU admission, and received a median of 1.5 L (IQR, 0.5–3.5 L) of isotonic crystalloid after enrollment. The odds ratio of 30-day in-hospital mortality with balanced crystalloids compared with saline was 0.91 (95% CI, 0.64–1.30). The incidence of death, new renal replacement therapy, or persistent renal dysfunction was lower with balanced crystalloids than with saline among patients who received larger volumes of isotonic crystalloid and among patients with sepsis.
These pilot studies laid the foundation for two recently completed large trials comparing balanced crystalloids with saline among nearly 30,000 acutely ill adults (58, 59). SMART (isotonic Solutions and Major Adverse Renal Events Trial) and SALT-ED (Saline Against Lactated Ringer’s or Plasma-Lyte in the Emergency Department) were cluster-randomized, cluster-crossover trials comparing balanced crystalloid (lactated Ringer’s or Plasma-Lyte A) with 0.9% sodium chloride for patients in the ICUs and ED at a single academic medical center.
The SMART trial enrolled 15,802 adult patients from five ICUs, 50% of whom were admitted from the ED and approximately 20% of whom were admitted from the operating room (58). Approximately one-fourth of patients were receiving vasopressors, one-third were receiving mechanical ventilation, and 15% had an admitting diagnosis of sepsis or septic shock. Fluid therapy in the ED, operating room, and ICUs was coordinated, so that most patients received the assigned crystalloid during initial resuscitation before ICU admission. Patients received a median of 2.5 L (IQR, 0.8–5.2 L) of intravenous crystalloid between 24 hours before ICU admission and the first of 30 days after ICU admission or hospital discharge. The primary outcome was major adverse kidney events within 30 days (MAKE30): the composite of death, new receipt of renal replacement therapy, or persistent renal dysfunction (60, 61). The National Institute of Diabetes and Digestive and Kidney Diseases work group on clinical trials in acute kidney injury recommends the use of the major adverse kidney events composite outcome to capture effects of acute kidney injury that are more patient centered than acute changes in creatinine, in a manner that appropriately accounts for competing risks (60, 62). Of patients in the balanced crystalloid group, 14.3% experienced MAKE30, compared with 15.4% in the saline group (P = 0.04). The 1.1% absolute risk difference in MAKE30 between groups (odds ratio, 0.90; 95% CI, 0.82–0.99) was driven primarily by death (odds ratio, 0.90; 95% CI, 0.80–1.01) and renal replacement therapy (odds ratio, 0.84; 95% CI, 0.68–1.02), not by changes in creatinine (odds ratio, 0.96; 95% CI, 0.84–1.11). In the prespecified subgroup of patients with sepsis or septic shock, 30-day in-hospital mortality was 25.2% with balanced crystalloids and 29.4% with saline (odds ratio, 0.80; 95% CI, 0.67–0.97; P = 0.02). Although the relative risk reduction in MAKE30 and in-hospital mortality with balanced crystalloids compared with saline was consistent across the spectrum of baseline risk, for patients at the highest risk of MAKE30 or death, use of balanced crystalloids rather than saline resulted in an absolute risk reduction in MAKE30 of 3.7% (0.6–6.9%) and in-hospital mortality of 4.2% (−7.9% to 16.4%) (63).
The SALT-ED trial enrolled 13,347 patients who received intravenous crystalloids in the ED and were hospitalized outside an ICU (59). Patients received a median of 1.0 L (IQR, 1.0–2.0 L) of crystalloid in the ED. Despite the study only controlling choice of crystalloid in the ED, plasma chloride concentrations were lower and serum bicarbonate concentrations were higher in the balanced crystalloid group for at least 72 hours after enrollment. Although the primary outcome of hospital-free days was similar between groups (25 d vs. 25 d; odds ratio, 0.98; 95% CI, 0.92–1.04; P = 0.41), the secondary outcome of MAKE30 occurred in 4.7% of patients in the balanced crystalloid group compared with 5.7% of patients in the saline group (odds ratio, 0.82; 95% CI, 0.70–0.95; P = 0.01). The difference in acute kidney injury and MAKE30 between balanced crystalloids and saline appeared to be greatest among the prespecified subgroups of patients with hyperchloremia or an elevated plasma creatinine value at ED presentation.
The SMART and SALT-ED trials have important limitations. They were conducted at a single center; fluid was open label; the average volume of crystalloid received was relatively small; and initiation of renal replacement therapy was determined by treating clinicians. Importantly, the trials were not powered to detect differences in each individual component of the MAKE30 outcome, and the MAKE30 composite itself is imperfect because it equally weights three endpoints that are likely valued differently by patients and relies on short-term measures of kidney injury and recovery as surrogates for longer-term kidney function and health status. In both trials, the absolute difference in the MAKE30 outcome between groups was only 1%, suggesting that in an unselected population of adults receiving intravenous fluid, the effects of crystalloid composition on death and severe renal dysfunction are relatively small. Moreover, the trials were not designed to evaluate the mechanism by which crystalloid composition may have influenced the composite MAKE30 outcome. In the SMART trial, differences between the balanced crystalloid group and the saline group in the incidence of hyperchloremia (24.5% vs. 35.6%; P < 0.001) and metabolic acidosis (35.2% vs. 42.1%; P < 0.001) were modest, suggesting that crystalloid assignment was responsible for around one-third of the observed hyperchloremia and one-sixth of the observed metabolic acidosis. Whether differences between groups in clinical outcomes were mediated by the proposed mechanism of chloride-induced acute kidney injury or by an alternative mechanism is unclear. Potential alternative mechanisms include effects of fluid composition on recovery from established acute kidney injury or the effects of acidosis and chloride-induced inflammation on vasodilation, receipt of vasopressors, and receipt of renal replacement therapy.
Two ongoing trials may provide additional insights into the effect of isotonic crystalloid composition on outcomes among critically ill adults. The PLUS (Plasma-Lyte 148 v Saline) study is a multicenter, parallel-group, blinded, randomized trial comparing Plasma-Lyte 148 with 0.9% sodium chloride with regard to 90-day mortality among 8,800 ICU patients with central access receiving an intravenous fluid bolus for evidence of hypovolemia (64). The BaSICS study (Balanced Solution versus Saline in Intensive Care Study) is a multicenter, parallel-group, blinded, randomized trial comparing Plasma-Lyte 148 with 0.9% sodium chloride with regard to 90-day mortality among 11,000 ICU patients at risk for acute kidney injury with evidence of hypoperfusion and fluid responsiveness (65). Upon completion of these trials, data from randomized trials enrolling more than 50,000 patients will be available to inform the choice between balanced crystalloids and saline for critically ill adults.
Areas of Uncertainty
Specific patient populations exist for whom saline might be expected to produce better outcomes than balanced crystalloids. Despite several preliminary studies of balanced crystalloids for patients with traumatic brain injury (66–69), the hypotonicity of some balanced crystalloids relative to the extracellular fluid makes their safety for patients with elevated intracranial pressure unknown. Administration of isotonic or hypertonic saline to maintain or increase serum osmolarity is an appropriate therapy for patients with elevated intracranial pressure. Saline is an intuitive choice for patients with hypovolemic hyponatremia or hypochloremic metabolic alkalosis, although preliminary data suggest that plasma chloride concentrations may not accurately predict which patients will experience better clinical outcomes with balanced crystalloid versus saline (70). Because most trials comparing balanced crystalloids with saline have occurred in a limited number of academic centers, how the findings generalize to centers with different patient and provider populations in different geographical regions remains uncertain. Although balanced crystalloids and saline are similar in availability and cost in many resource-intensive settings (71), the clinical effects and cost-effectiveness of balanced crystalloids versus saline for patients in low- and middle-income countries may require additional considerations (41).
Balanced crystalloids may be either lactate buffered (e.g., lactated Ringer’s) or acetate/gluconate buffered (e.g., Plasma-Lyte, Ringer’s acetate). Sodium lactate may cause small increases in serum lactate concentration (72), especially among patients with impaired hepatic function, but it does not cause lactic acidosis and may provide bioenergetic fuel because it is metabolized into carbon dioxide and water, which equilibrate with bicarbonate (73). Similarly, acetate is metabolized through the citric acid cycle into carbon dioxide and water. When used as a dialysate buffer, large doses of sodium acetate may be funneled into alternative metabolic pathways, producing nitric oxide and hemodynamic instability (74), but such effects have not been described at the low rate of acetate administration occurring during intravenous fluid infusion. In contrast, little is known about gluconate metabolism, and some studies suggest that gluconate is predominantly excreted unchanged in the urine, which may limit its alkalinizing effects (75, 76). Whether clinical outcomes or adverse effects differ between balanced crystalloids containing different buffers (77, 78) is the subject of ongoing research (www.clinicaltrials.gov [NCT03537898]).
Whether fluids with greater alkalinizing effects than balanced crystalloids (e.g., isotonic or hypertonic sodium bicarbonate solutions) can improve outcomes for select populations of critically ill adults remains uncertain. Specifically, administration of sodium bicarbonate to critically ill adults with severe acidosis or severe acute kidney injury has a physiologic rationale, but whether such an approach can prevent renal replacement therapy and death merits further rigorous evaluation (79). Whether other aspects of fluid composition and administration, including osmolarity, temperature, and infusion speed, modify the effect of crystalloid composition on clinical outcomes requires additional research (65).
Conclusions
A growing body of evidence suggests that using balanced crystalloids rather than saline may have the potential to reduce morbidity and mortality for critically ill patients. Preclinical research has demonstrated that administration of saline causes hyperchloremic metabolic acidosis, inflammation, hypotension, acute kidney injury, and death. For patients undergoing major surgery, randomized trials have found that balanced crystalloids cause less hyperchloremic metabolic acidosis and reduce the need for vasopressors. Among acutely ill adults in the ED or ICU, data from several recent large randomized trials suggest that using balanced crystalloids decreases the risk of death or severe kidney dysfunction.
Important questions remain regarding the mechanism by which balanced crystalloids may influence clinical outcomes, which patients are most likely to benefit from balanced crystalloids versus saline, and whether clinical outcomes differ between the available balanced crystalloids. While awaiting the results of further research, the data already available provide clinicians the opportunity to improve care for tens of millions of acutely ill adults treated with intravenous crystalloid solutions each year.
Supplementary Material
Footnotes
Supported in part by NHLBI grant K23HL143053 (M.W.S.).
Author Contributions: Drafting of the manuscript: M.W.S. and J.A.K.; critical revision of the manuscript for important intellectual content: M.W.S. and J.A.K.
CME will be available for this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201809-1677CI on November 8, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Glassford NJ, Bellomo R. The complexities of intravenous fluid research: questions of scale, volume, and accumulation. Korean J Crit Care Med. 2016;31:276–299. [Google Scholar]
- 3.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 4.Hammond NE, Taylor C, Saxena M, Liu B, Finfer S, Glass P, et al. Resuscitation fluid use in Australian and New Zealand intensive care units between 2007 and 2013. Intensive Care Med. 2015;41:1611–1619. doi: 10.1007/s00134-015-3878-y. [DOI] [PubMed] [Google Scholar]
- 5.Morgan TJ. The ideal crystalloid – what is ‘balanced’? Curr Opin Crit Care. 2013;19:299–307. doi: 10.1097/MCC.0b013e3283632d46. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg L, Collins N, Van Mourik K, Tan C, Bellomo R. Plasma-Lyte 148: a clinical review. World J Crit Care Med. 2016;5:235–250. doi: 10.5492/wjccm.v5.i4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushing H. Concerning the poisonous effect of pure sodium chloride solutions upon the nerve-muscle preparation. Am J Physiol. 1901;6:77–90. [Google Scholar]
- 8.Shires GT, Holman J. Dilution acidosis. Ann Intern Med. 1948;28:557–559. doi: 10.7326/0003-4819-28-3-557. [DOI] [PubMed] [Google Scholar]
- 9.Siggaard-Andersen O. The van Slyke equation. Scand J Clin Lab Invest Suppl. 1977;146:15–20. doi: 10.3109/00365517709098927. [DOI] [PubMed] [Google Scholar]
- 10.Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–368. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- 14.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568–1578. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 16.Schieve JF, Wilson WP. The changes in cerebral vascular resistance of man in experimental alkalosis and acidosis. J Clin Invest. 1953;32:33–38. doi: 10.1172/JCI102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You JP, Wang Q, Zhang W, Jansen-Olesen I, Paulson OB, Lassen NA, et al. Hypercapnic vasodilatation in isolated rat basilar arteries is exerted via low pH and does not involve nitric oxide synthase stimulation or cyclic GMP production. Acta Physiol Scand. 1994;152:391–397. doi: 10.1111/j.1748-1716.1994.tb09821.x. [DOI] [PubMed] [Google Scholar]
- 18.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 19.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 20.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, et al. FEAST trial group. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfortmueller CA, Funk GC, Reiterer C, Schrott A, Zotti O, Kabon B, et al. Normal saline versus a balanced crystalloid for goal-directed perioperative fluid therapy in major abdominal surgery: a double-blind randomised controlled study. Br J Anaesth. 2018;120:274–283. doi: 10.1016/j.bja.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 22.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 23.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill – too little of a good thing? Lancet. 1999;354:1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42:e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 1-L infusions of 6% hydroxyethyl starch suspended in 0.9% saline (voluven) and a balanced solution (Plasma Volume Redibag) on blood volume, renal blood flow velocity, and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2014;259:881–887. doi: 10.1097/SLA.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 29.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg L, Li M, Churilov L, Armellini A, Gibney M, Hewitt T, et al. Associations of fluid amount, type, and balance and acute kidney injury in patients undergoing major surgery. Anaesth Intensive Care. 2018;46:79–87. doi: 10.1177/0310057X1804600112. [DOI] [PubMed] [Google Scholar]
- 33.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–1905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 35.Raghunathan K, Bonavia A, Nathanson BH, Beadles CA, Shaw AD, Brookhart MA, et al. Association between initial fluid choice and subsequent in-hospital mortality during the resuscitation of adults with septic shock. Anesthesiology. 2015;123:1385–1393. doi: 10.1097/ALN.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 36.Sen A, Keener CM, Sileanu FE, Foldes E, Clermont G, Murugan R, et al. Chloride content of fluids used for large-volume resuscitation is associated with reduced survival. Crit Care Med. 2017;45:e146–e153. doi: 10.1097/CCM.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 38.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–264. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 39.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Ayebale ET, Kwizera A, Mijumbi C, Kizito S, Roche AM. Ringer’s lactate versus normal saline in urgent cesarean delivery in a resource-limited setting: a pragmatic clinical trial. Anesth Analg. 2017;125:533–539. doi: 10.1213/ANE.0000000000002229. [DOI] [PubMed] [Google Scholar]
- 42.Song JW, Shim JK, Kim NY, Jang J, Kwak YL. The effect of 0.9% saline versus Plasmalyte on coagulation in patients undergoing lumbar spinal surgery; a randomized controlled trial. Int J Surg. 2015;20:128–134. doi: 10.1016/j.ijsu.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 43.Takil A, Eti Z, Irmak P, Yilmaz Göğüş F. Early postoperative respiratory acidosis after large intravascular volume infusion of lactated Ringer’s solution during major spine surgery. Anesth Analg. 2002;95:294–298. doi: 10.1097/00000539-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Hafizah M, Liu CY, Ooi JS. Normal saline versus balanced-salt solution as intravenous fluid therapy during neurosurgery: effects on acid-base balance and electrolytes. J Neurosurg Sci. 2017;61:263–270. doi: 10.23736/S0390-5616.16.03221-5. [DOI] [PubMed] [Google Scholar]
- 45.Volta CA, Trentini A, Farabegoli L, Manfrinato MC, Alvisi V, Dallocchio F, et al. Effects of two different strategies of fluid administration on inflammatory mediators, plasma electrolytes and acid/base disorders in patients undergoing major abdominal surgery: a randomized double blind study. J Inflamm (Lond) 2013;10:29. doi: 10.1186/1476-9255-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIlroy D, Murphy D, Kasza J, Bhatia D, Wutzlhofer L, Marasco S. Effects of restricting perioperative use of intravenous chloride on kidney injury in patients undergoing cardiac surgery: the LICRA pragmatic controlled clinical trial. Intensive Care Med. 2017;43:795–806. doi: 10.1007/s00134-017-4772-6. [DOI] [PubMed] [Google Scholar]
- 47.Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysamie AP, Khashayar P, et al. Effects of normal saline vs. lactated Ringer’s during renal transplantation. Ren Fail. 2008;30:535–539. doi: 10.1080/08860220802064770. [DOI] [PubMed] [Google Scholar]
- 48.Modi MP, Vora KS, Parikh GP, Shah VR. A comparative study of impact of infusion of Ringer’s lactate solution versus normal saline on acid-base balance and serum electrolytes during live related renal transplantation. Saudi J Kidney Dis Transpl. 2012;23:135–137. [PubMed] [Google Scholar]
- 49.O’Malley CMN, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double-blind comparison of lactated Ringer’s solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–1524. doi: 10.1213/01.ANE.0000150939.28904.81. [DOI] [PubMed] [Google Scholar]
- 50.Weinberg L, Harris L, Bellomo R, Ierino FL, Story D, Eastwood G, et al. Effects of intraoperative and early postoperative normal saline or Plasma-Lyte 148 on hyperkalaemia in deceased donor renal transplantation: a double-blind randomized trial. Br J Anaesth. 2017;119:606–615. doi: 10.1093/bja/aex163. [DOI] [PubMed] [Google Scholar]
- 51.Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264–269. doi: 10.1213/ane.0b013e3181732d64. [DOI] [PubMed] [Google Scholar]
- 52.Kim SY, Huh KH, Lee JR, Kim SH, Jeong SH, Choi YS. Comparison of the effects of normal saline versus Plasmalyte on acid-base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplant Proc. 2013;45:2191–2196. doi: 10.1016/j.transproceed.2013.02.124. [DOI] [PubMed] [Google Scholar]
- 53.Potura E, Lindner G, Biesenbach P, Funk GC, Reiterer C, Kabon B, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg. 2015;120:123–129. doi: 10.1213/ANE.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 54.Lee Hamm L, Hering-Smith KS, Nakhoul NL. Acid-base and potassium homeostasis. Semin Nephrol. 2013;33:257–264. doi: 10.1016/j.semnephrol.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Wan S, Roberts MA, Mount P. Normal saline versus lower-chloride solutions for kidney transplantation. Cochrane Database Syst Rev. 2016;(8):CD010741. doi: 10.1002/14651858.CD010741.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 57.Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, et al. SALT Investigators; Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–1372. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. SALT-ED Investigators. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844–850. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 61.Billings FT, IV, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127:89–93. doi: 10.1159/000363725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molitoris BA, Okusa MD, Palevsky PM, Chawla LS, Kaufman JS, Devarajan P, et al. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol. 2012;7:856–860. doi: 10.2215/CJN.12821211. [DOI] [PubMed] [Google Scholar]
- 63.McKown AC, Huerta LE, Rice TW, Semler MW Pragmatic Critical Care Research Group. Heterogeneity of treatment effect by baseline risk in a trial of balanced crystalloids versus saline. Am J Respir Crit Care Med. 2018;198:810–813. doi: 10.1164/rccm.201804-0680LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammond NE, Bellomo R, Gallagher M, Gattas D, Glass P, Mackle D, et al. The Plasma-Lyte 148 v Saline (PLUS) study protocol: a multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc. 2017;19:239–246. [PubMed] [Google Scholar]
- 65.Zampieri FG, Azevedo LCP, Corrêa TD, Falavigna M, Machado FR, Assunção MSC, et al. BaSICS Investigators and the BRICNet. Study protocol for the Balanced Solution versus Saline in Intensive Care Study (BaSICS): a factorial randomised trial. Crit Care Resusc. 2017;19:175–182. [PubMed] [Google Scholar]
- 66.Rowell SE, Fair KA, Barbosa RR, Watters JM, Bulger EM, Holcomb JB, et al. The impact of pre-hospital administration of lactated Ringer’s solution versus normal saline in patients with traumatic brain injury. J Neurotrauma. 2016;33:1054–1059. doi: 10.1089/neu.2014.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roquilly A, Loutrel O, Cinotti R, Rosenczweig E, Flet L, Mahe PJ, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care. 2013;17:R77. doi: 10.1186/cc12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young JB, Utter GH, Schermer CR, Galante JM, Phan HH, Yang Y, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg. 2014;259:255–262. doi: 10.1097/SLA.0b013e318295feba. [DOI] [PubMed] [Google Scholar]
- 69.Hassan MH, Hassan WMNW, Zaini RHM, Shukeri WFWM, Abidin HZ, Eu CS. Balanced fluid versus saline-based fluid in post-operative severe traumatic brain injury patients: acid-base and electrolytes assessment. Malays J Med Sci. 2017;24:83–93. doi: 10.21315/mjms2017.24.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown RM, Wanderer JP, Ehrenfeld JM, Stollings JL, McKown AC, Wang L, et al. SALT Investigators; Pragmatic Critical Care Research Group. Balanced crystalloids versus saline for adults with sepsis or septic shock [abstract] Am J Respir Crit Care Med. 2018;197:A6188. [Google Scholar]
- 71.National Clinical Guideline Centre. Appendix N, Cost sensitivity analysis: intravenous fluids for routine maintenance. In: Intravenous fluid therapy in adults in hospital (NICE Clinical Guidelines, No. 174). London, UK: Royal College of Physicians; 2013 Dec [accessed 2018 Sep 12]Available from: https://www.ncbi.nlm.nih.gov/books/NBK333109/
- 72.Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558–564. doi: 10.1111/j.1399-6576.2011.02398.x. [DOI] [PubMed] [Google Scholar]
- 73.Nalos M, Leverve X, Huang S, Weisbrodt L, Parkin R, Seppelt I, et al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care. 2014;18:R48. doi: 10.1186/cc13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veech RL, Gitomer WL. The medical and metabolic consequences of administration of sodium acetate. Adv Enzyme Regul. 1988;27:313–343. doi: 10.1016/0065-2571(88)90024-6. [DOI] [PubMed] [Google Scholar]
- 75.Stetten MR, Stetten D., Jr The metabolism of gluconic acid. J Biol Chem. 1950;187:241–252. [PubMed] [Google Scholar]
- 76.Naylor JM, Forsyth GW. The alkalinizing effects of metabolizable bases in the healthy calf. Can J Vet Res. 1986;50:509–516. [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar L, Seetharaman M, Rajmohan N, Ramamurthi P, Rajan S, Varghese R. Metabolic profile in right lobe living donor hepatectomy: comparison of lactated Ringer’s solution and normal saline versus acetate based balanced salt solution - a pilot study. Indian J Anaesth. 2016;60:719–725. doi: 10.4103/0019-5049.191669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weinberg L, Chiam E, Hooper J, Liskaser F, Hawkins AK, Massie D, et al. Plasma-Lyte 148 vs. Hartmann’s solution for cardiopulmonary bypass pump prime: a prospective double-blind randomized trial. Perfusion. 2018;33:310–319. doi: 10.1177/0267659117742479. [DOI] [PubMed] [Google Scholar]
- 79.Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, et al. BICAR-ICU Study Group. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392:31–40. doi: 10.1016/S0140-6736(18)31080-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.