Abstract
Objective
Silent myocardial involvement is associated with poor prognosis in patients with systemic sclerosis (SSc). Here we aimed to evaluate the subclinical left ventricular (LV) and right ventricular (RV) systolic dysfunction in patients with SSc without any cardiovascular diseases, by using both strain imaging methods, speckle tracking echocardiography (STE) and real-time 3D echocardiography (RT3DE).
Methods
A total of 47 patients with SSc and 20 age- and gender-matched healthy controls (HC) were studied. Conventional echocardiography, STE-based strain imaging, and real-time 3D echocardiography (Bothell, WA, USA) were performed to assess the biventricular deformation. Clinical and serological findings were sought.
Results
Conventional echocardiographic LV measurements were similar between SSc and HC. Both the LV and RV longitudinal peak systolic strain/strain rates were significantly impaired in SSc, demonstrating subclinical LV and RV systolic dysfunction (p≤0.001). Systolic pulmonary artery pressure (SPAP) was negatively correlated with both the LV and RV longitudinal peak systolic strain/strain rates (LV, r=−0.554 and r=−0.642, respectively, p<0.001; and RV, r=−0.554 and r=−0.642, respectively, p=0.001). There was a trend for decreasing LV strain and increasing LEVSV in a 1-year analysis of patients with SSc.
Conclusion
SSc is associated with myocardial systolic dysfunction. A deformation scrutiny conducted by both the STE-based strain imaging and end-systolic LV volume analysis by real-time 3D echocardiography are promising modalities that allow us for non-invasive, comprehensive investigation of subtle deterioration in the biventricular systolic function of patients with SSc.
Keywords: Systemic sclerosis, echocardiography, systolic dysfunction
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by inflammation, vasculopathy, and fibrosis of the skin and internal organs, including the heart, lung, and kidneys. The heart involvement is usually occult, but it occurs in a significant percentage of patients. All cardiac structures can be affected in SSc and clinically manifested as myocardial disease, conduction system abnormalities, arrhythmias, pericardial disease, or heart failure (1, 2). Myocardial involvement is associated with poor prognosis, and when clinically evident, the mortality rate rises to 70% at 5 years (3, 4). The detection of myocardial involvement is important to increase the survival rates.
Myocardial involvement is very common in patients with SSc and is related to abnormal vasoreactivity. A recurrent ischemic injury, myocardial Raynaud’s phenomenon, is responsible for irreversible myocardial fibrosis. Depressed myocardial contractility is the hallmark of primary myocardial involvement (2). The EULAR registry reported the prevalence of depressed left ventricular ejection fraction (LVEF) as 5.4% in patients with SSc (5). On the other hand, the systolic and diastolic dysfunction can occur in the early stages of the disease before clinical manifestations become relevant. Some studies reported that reduced myocardial contractility can be underestimated by using the conventional echocardiography technique (6–7). This conventional method lacks sensitivity to detect the preclinical stage, and more sensitive techniques are needed to determine the subclinic myocardial involvement.
In the present study, we aimed to evaluate the subclinic myocardial involvement in patients with SSc who had no other cardiovascular diseases or overt heart failure. For this purpose, we used two novel echocardiographic techniques: speckle tracking echocardiography (STE) and real-time 3D echocardiography (RT3DE). The left ventricular (LV) and right ventricular (RV) systolic function were evaluated using the strain imaging method, and LV end-diastolic and end-systolic volumes were measured by RT3DE.
Methods
Fifty-four (F/M:50/4) consecutive patients who were regularly seen at our rheumatology outpatient clinic were enrolled into this cross-sectional and prospective study. All patients fulfilled the ACR/EULAR SSc classification criteria (8). Twenty age- and gender-matched volunteers, without any cardiac disease and with a preserved LVEF, were included in the study as a healthy control (HC) group. We excluded patients younger than 18 years of age, having pulmonary hypertension (PH) (systolic pulmonary pressure ≥40 mmHg on echocardiography), coronary artery disease (CAD), left ventricular hypertrophy (LVH), valvular heart disease, and uncontrolled hypertension.
The demographic data, including age, gender, disease duration from the time of formal diagnosis, disease subset (diffuse, limited, or sine scleroderma), organ involvement (interstitial lung disease, etc.), and cardiovascular risk factors including the smoking status, diabetes, hypertension, hyperlipidemia, and obesity were recorded. The lung involvement was evaluated by pulmonary function tests (PFT), the lung carbon monoxide transfer factor, and high-resolution computerized tomography (HRCT). Interstitial lung disease was defined as the presence of typical features, such as bibasilar interstitial fibrosis on a HRCT scan of the chest, along with the restrictive pattern on PFT. Restrictive ventilator defect was defined as the FEV1/FVC ≥70% and the FVC <80% according to the American Thoracic Society criteria (9).
The biventricular deformation was evaluated using STE in all patients with SSc and healthy individuals at the baseline and 1-year follow-up. LV volumes were measured in 44 patients with SSc at the baseline and 30 of them at a 1-year follow-up by RT3DE. The study was approved by the Ethical Committee of our İstanbul Bilim University, and all patients and controls gave written informed consent.
Echocardiographic measurements
Patients underwent a transthoracic echocardiography (Siemens, Sequoia, C256; Mountainview, CA, USA; and matrix iE33, Philips, The Netherlands) using a 2.3–3.5 MHz transducer. Interventricular septum (IVS) and posterior wall thicknesses and left ventricular end-diastolic (LVEDD) and end-systolic diameters (LVESD) were measured from the parasternal long-axis view using the M-mode. LVEF was calculated using a modified Simpson’s method from the apical four-chamber view (10). The RV systolic function was evaluated by tricuspidal annular plane systolic excursion (TAPSE), and the RV end-diastolic and end-systolic diameters were measured from the apical four-chamber view.
Speckle tracking echocardiography
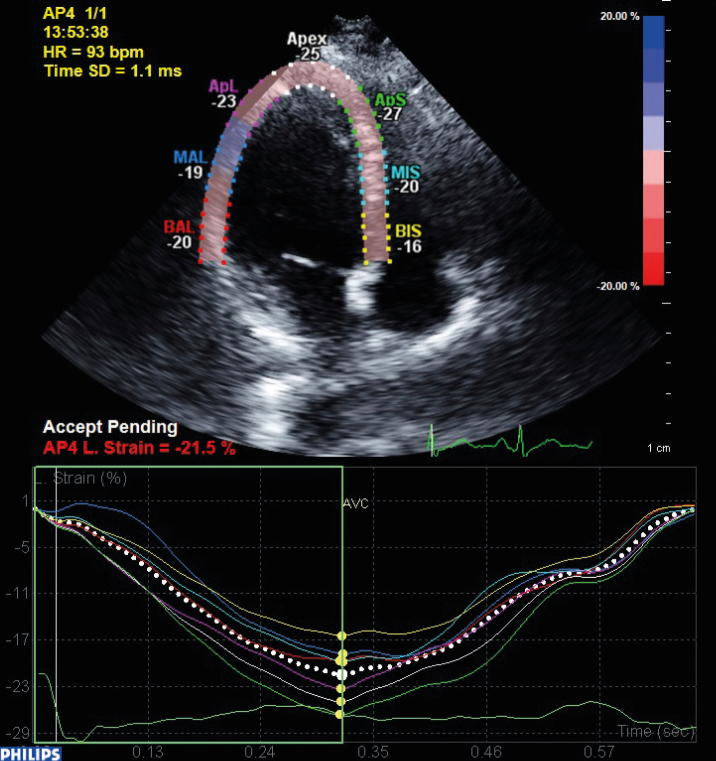
We obtained the LV apical four-chamber views using a frame rate of 50–100 frames/s, and at least three cardiac cycles were acquired at the end-expiration breath holding and stored digitally on a hard disk for an offline analysis. The analysis was performed offline on a PC workstation using a custom analysis software (QLab advanced quantification software version 8.1, Philips, The Netherlands). The LV and RV endocardial border of the end-systolic frame was traced. From this line, a region of interest, including the entire transmural wall in all patients, was created automatically, and the software selected natural acoustic markers moving with the tissue. Frame-by-frame tracking of these markers during the cardiac cycle provided a measure of strain and the strain rate at any point of myocardium. In the present study, the LV and RV longitudinal global strain and SRs were measured on the apical four-chamber view (11) (Figure 1).
Figure 1.
Left ventricular global longitudinal strain measurement by speckle tracking echocardiography. After tracing the LV endocardial border by echocardiographer, a region of interest included the entire wall, and natural acoustic markers were created by software automatically. From this, the markers strain and strain rate can be measured
Real-time 3D echocardiography data analysis
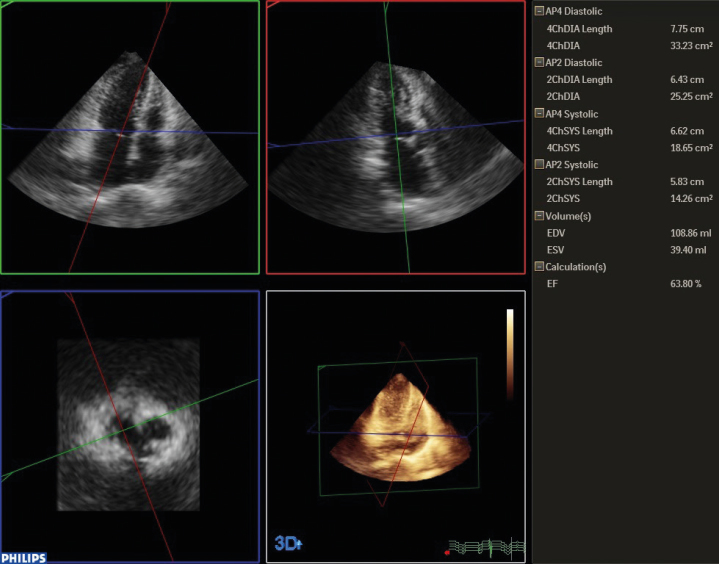
Analysis was performed using the QLab advanced quantification software version 8.1 (Philips, Bothell, WA, USA). The observer determined the end-diastolic and end-systolic frame visually. Contour tracing was executed semi-automatically by defining the mitral valve annulus in the LV four-chamber and two-chamber views and the apex in four-chamber view in both the end-systolic and end-diastolic frames (Figure 2).
Figure 2.
Ejection fraction measurement by real-time 3D echocardiography. After defining the mitral anulus and apex manually, the software produces a 3D shell automatically. Ejection fraction can be measured by this method without geometric assumptions
Statistical analysis
Continuous variables were expressed as the mean±standard deviation, unless stated otherwise. Comparisons between the groups were made using the Mann–Whitney U test and chi-squared fit test for binary variables. Comparisons were made between the baseline and 1-year follow-up parameters with the Wilcoxon test. Correlations were expressed in terms of the Pearson correlation coefficients. P-values ≤0.05 were considered as significant. All statistical analyses were performed using the Statistical Package for the Social Sciences version 11.5 (SPSS Inc.; Chicago, IL, USA)
Results
We screened a total of 54 patients, and seven of them were excluded because of cardiovascular problems (four PH, 1 LVH, one CAD, and one mitral valve disease). We studied 47 patients with SSc (diffuse, 12; limited, 35). Patients characteristics were shown in Table 1. The mean age was 48.2±12.4 and 47.5±9.4 years in SSc patients and HC, respectively.
Table 1.
Demographic Characteristics of Patients With Scleroderma (n=47)
| Age (year) (mean±SD) | 48.2±12.4 |
| Gender (F/M) (n) | 43/4 |
| Disease duration (years) (mean±SD) | 8.8±8.2 |
| Disease subset Limited n(%) | 35 (74.5) |
| Diffuse n(%) | 12 (25.5) |
| Digital ulcer n(%) | 20 (42.5) |
| Interstitial lung disease n(%) | 28 (59.5) |
| Gastrointestinal involvement n(%) | 12 (25.5) |
| Rodnan skin score (mean±SD) | 11.1±5.5 |
| Anti-Scl-70 n(%) | 20 (42.5) |
| Concomitant diseases n(%) | |
| Diabetes mellitus | 3 (6.3) |
| Hypertension | 18 (41.2) |
| Hyperlipidemia | 13 (27.6) |
| Obesity | 10 (21.2) |
| Smoking history n(%) | 7 (14.8) |
Conventional echocardiographic measurements
Conventional echocardiographic findings are presented in Table 2. LV conventional echocardiographic measurements were similar between SSc and HC. Regarding the right heart conventional parameters, right atrium and RV were significantly enlarged, while the TAPSE was decreased, and the systolic pulmonary artery pressure was increased in SSc compared to HC (p=0.001) (Table 2).
Table 2.
Conventional Echocardiographic Findings
| SSc (n=47) | HC (n=20) | p | |
|---|---|---|---|
| LVEDD (cm) | 4.95±0.22 | 4.91±0.17 | 0.45 |
| LVESD (cm) | 3.09±0.2 | 3.05±0.2 | 0.51 |
| EF (%) | 60.1±2.18 | 60.7±1.83 | 0.30 |
| RAD (cm) | 3.75±0.27 | 3.49±0.34 | 0.001 |
| RVD (cm) | 2.34±0.18 | 2.19±0.15 | 0.002 |
| TAPSE (cm) | 2.02±0.33 | 2.96±0.51 | 0.0001 |
| SPAP (mmHg) | 30.78±4.83 | 23.35±3.23 | 0.001 |
| TRV (m/sn) | 2.62±0.31 | 2.02±0.19 | 0.001 |
LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; EF: ejection fraction; RAD: right atrial diameter; RVD: right ventricular diameter; TAPSE: tricuspidal annular plane systolic excursion; SPAP: systolic pulmonary artery pressure
Speckle tracking echocardiography analysis
Both the LV and RV longitudinal global peak systolic strain/strain rates were significantly impaired in SSc, demonstrating a subclinical LV and RV systolic dysfunction (Table 3). We obtained a significant positive correlation between the TAPSE and RV longitudinal global peak systolic strain/strain rate (r=0.753 and r=0.71, respectively, p=0.0001). Systolic PAP was negatively correlated with both the LV and RV longitudinal global peak systolic strain/strain rate (LV, r=−0.554 and r=−0.642, respectively, p=0.001; RV, r=−0.554 and r=−0.642, respectively, p=0.001). The anti-Scl-70-positive patients had impaired LV longitudinal peak systolic strain rate values, compared to others; however, the difference did not reach a statistical significance (13.01±1.26% to 13.04±1.90%, p=0.96, for strain; 0.30±0.06 1/s to 0.31±0.15 1/s, p=0.79, for strain rate). There weren’t any statistically difference for the strain measurements at the first year follow up (Table 4).
Table 3.
Speckle Tracking Echocardiographic Findings
| SSc (n=47) | HC (n=20) | p | |
|---|---|---|---|
| LV LGS (%) | 12.81±1.12 | 20.35±3.05 | 0.0001 |
| LVSr (1/s) | 0.50±0.03 | 1.70±0.47 | 0.0001 |
| RV LGS (%) | 11.68±1.61 | 14.63±2.55 | 0.0001 |
| RVSr (1/s) | 0.31±0.01 | 1.73±0.50 | 0.0001 |
LV LGS; left ventricular longitudinal global strain; LVSr: left ventricular strain rate; RV LGS: right ventricular longitudinal global strain; RVSr: right ventricular strain rate
Table 4.
Follow-Up STE Analysis
| Basal (n=47) | First Year (n=30) | p | |
|---|---|---|---|
| LV LGS (%) | 12.81±1.12 | 12.37±1.10 | 0.346 |
| LVSr (1/s) | 0.50±0.03 | 0.48±0.02 | 0.19 |
| RV LGS (%) | 11.68±1.61 | 11.66±1.55 | 0.98 |
| RVSr (1/s) | 0.31±0.01 | 0.30±0.03 | 0.82 |
STE: speckle tracking echocardiography; LV LGS: left ventricular longitudinal global strain; LVSr: left ventricular strain rate; RV LGS: right ventricular longitudinal global strain; RVSr: right ventricular strain rate
We observed no association between the age, disease duration, or Rodnan skin score and STE measurements. Both LV and RV longitudinal peak systolic strain and strain rate values were similar among patients according to disease subset, anti-Scl-70, the presence of digital ulcer, or interstitial lung disease.
Real-time 3D echocardiography analysis
Left ventricular end-diastolic and end-systolic volumes were similar between SSc and HC (Table 5). According to the first year follow-up, diastolic volumes were similar, whereas systolic volumes had a tendency to increase, although they did not reach statistical significance (Table 6).
Table 5.
RT3DE Analysis
| SSc (n=47) | HC (n=20) | p | |
|---|---|---|---|
| LVEDV (mL) | 104.97±16.27 | 106.70±17.52 | 0.63 |
| LVESV (mL) | 44.42±7.26 | 42.35±7.27 | 0.32 |
RT3DE: real-time 3D echocardiography; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume
Table 6.
Follow-Up RT3DE Analysis
| Basal (n=30) | First Year (n=30) | p | |
|---|---|---|---|
| LVEDV (mL) | 104.97±16.27 | 105.65±13.61 | 0.45 |
| LVESV (mL) | 44.42±7.26 | 44.48±7.27 | 0.16 |
RT3DE: real-time 3D echocardiography; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume
Discussion
In the present study, we observed that the LV conventional echocardiographic measurements were similar between the patients with SSc and HC. However, both LV and RV longitudinal peak systolic strain/strain rates were significantly impaired in patients with SSc, suggesting a subclinical biventricular dysfunction. A year after the first results, LV strain measurements tended to be lower in the patient group. Compatible to these findings, based on the RT3DE measurements, LV end-systolic volumes of the patients with SSc were found to be significantly increased compared to HC, suggesting a lower cardiac output and EF. First year RT3DE measurements in patients revealed a tendency of increasing LVES volumes without statistical significance.
Systemic sclerosis can cause a wide variety of cardiac abnormalities, including microvascular CAD, myocardial fibrosis, LV systolic and diastolic dysfunction, pericardial disease, and conduction abnormalities. Clinically evident cardiac involvement in SSc is associated with an increased risk of death (1, 2). Cardiac involvement is usually clinically asymptomatic and begins in the early stages of the disease. Nevertheless, several clinical and histological studies revealed that it is more common than expected and associated with poor prognosis (12–14). Significant improvements have been recently achieved regarding organ-specific therapy, and therefore, a detection of cardiac involvement in the subtle status is important for risk stratification and selecting an appropriate therapy.
In general practice, cardiac evaluation is usually performed by the LV global systolic function assessment based on the conventional LVEF. Still, 2DE LVEF is insufficient because of a complex myocardium motion. 2DE LVEF is considered to reflect the circumferential and radial function but not the longitudinal movement, which is influenced by the motion of subendocardial fibers, which is most susceptible to evident myocardial damage (15). Due to its ability to provide a quantitative endocardial deformation analysis, strain imaging is considered to be superior to conventional echocardiographic parameters (16, 17). The LV global longitudinal strain reflects the long-axis mechanics. Subclinical impairment of the long-axis mechanics has been demonstrated to be associated with mortality in the general population (18, 19). Strain imaging can be achieved by different methods. STE is performed by using a semi-automated algorithm, which minimizes the user interference. It is based on the real-time tracking of acoustic markers, and it allows a calculation of deformation parameters. In previous studies, it has been validated, showing high levels of reproducibility and accuracy in detecting subtle LV systolic function abnormalities (depressed longitudinal deformation) in patients without an altered EF (20–22).
The LV volume is the key measurement for accurate LVEF. A volume analysis by 2D echocardiography is limited due to geometric assumptions and foreshortening of the LV, and therefore, volume underestimation by 2D techniques has been revealed by several studies (23–26). 3D echocardiography was found to result in slightly larger LV volumes than 2D echocardiography, whereas EF was similar between both methods. 3D echocardiography has been reported to improve accuracy in the assessment of LV volumes (27, 28).
In the EUSTAR database, the male gender, age, diffuse cutaneous disease, digital ulceration, myositis, and no use of calcium channel blockers were found as independent risk factors for LV dysfunction (5). However, we did not observe any relationship between clinical findings and echocardiographic measurements.
In our study, we were able to assess the myocardial function of patients with SSc, both by STE and RT3DE methods, and we observed the subclinical cardiac dysfunction compared to normal subjects. We excluded patients with PH, which can be related with RV dysfunction as a result of pulmonary complications and valvular and known coronary heart diseases to avoid evident myocardial dysfunction and investigate subtle changes, which can be in connection to SSc. In the first year follow-up, conventional parameters remained unchanged, while strain measurements were impaired, compared to the basal evaluation. The deterioration of the LV systolic function in the follow-up examination can be the result of progressive myocardial function impairment due to SSc.
Limitations and strengths
To the best of our knowledge, this study is the first that evaluated the myocardial function using two novel techniques with a 1-year follow-up data in patients with SSc. A detailed assessment of biventricular myocardial functions, based on novel tools, in patients with preserved EF is the strength of this study. The major limitation of our study is that we were not able to demonstrate the exact mechanism of subtle changes in myocardial contractility, based on other methods, such as cardiac magnetic resonance imaging. Myocardial fibrosis, which is the potential mechanism of myocardial dysfunction in these patients, was not verified. In addition, the right heart catheterization (RHC) was not part of the study protocol for diagnosing PH and coronary angiography to exclude coronary heart disease. Therefore, PH can be assessed only by echocardiographic measurements, which can underestimate exact values. However, according to the 2015 ESC/ERS recommendations for the diagnosis and treatment of pulmonary hypertension, RHC should be performed for the high-risk echocardiographic probability of patients with PH, who were excluded from our study (29).
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of İstanbul Bilim University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Ş.T.Ş., Ş.Y., S.A.; Design - Ş.T.Ş., N.Y.; Supervision - Ş.Y., S.A.; Resources - S.Y. Y.C., N.Y.; Materials - S.Y., Ş.T.Ş., B.C.; Data Collection and/or Processing - Ş.T.Ş., B.C.; Analysis and/or Interpretation - Ş.T.Ş., N.Y.; Literature Search - B.C., E.K.; Writing Manuscript - Ş.T.Ş., N.Y.; Critical Review - Ş.Y., S.A.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):45–8. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 2.Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Di Cesare E, Battisti S, Di Sibio A, Cipriani P, Giacomelli R, Liakouli V, et al. Early assessment of sub-clinical cardiac involvement in systemic sclerosis (SSc) using delayed enhancement cardiac magnetic resonance (CE-MRI) Eur J Radiol. 2013;82:268–73. doi: 10.1016/j.ejrad.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA, Jr, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of systemic sclerosis patients. Ann Rheum Dis. 2010;69:218–21. doi: 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 6.Allanore Y, Meune C, Vignaux O, Weber S, Legmann P, Kahan A. Bosentan increases myocardial perfusion and function in systemic sclerosis: a magnetic resonance imaging and Tissue-Doppler echography study. J Rheumatol. 2006;33:2464–67. [PubMed] [Google Scholar]
- 7.Meune C, Allanore Y, Pascal O, Devaux JY, Dessault O, Duboc D, et al. Myocardial contractility is early affected in systemic sclerosis: a tissue Doppler echocardiography study. Eur J Echocardiogr. 2005;6:351–7. doi: 10.1016/j.euje.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal AN, Agarwal W. The new ATS/ERS guidelines for assessing the spirometric severity of restrictive lung disease differ from previous standards. Respirology. 2007;12:759–62. doi: 10.1111/j.1440-1843.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 10.Feigenbaum H, Armstrong WF, Ayan T. Feigenbaum’s Echocardiography. 6th Ed. Lippincotts: Williams&Wilkins; 2005. [Google Scholar]
- 11.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricu- lar contraction and relaxation in patients with cardiovascular risk factors: A study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008;21:1138–44. doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Follansbee WP, Miller TR, Curtiss EI, Orie JE, Bernstein RL, Kiernan JM, et al. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma) J Rheumatol. 1990;17:656–62. [PubMed] [Google Scholar]
- 13.Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34:181–90. doi: 10.1016/j.rdc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candell-Riera J, Armadans-Gil L, Simeón CP, Castell-Conesa J, Fonollosa-Pla V, García-del-Castillo H, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum. 1996;39:1138–45. doi: 10.1002/art.1780390710. [DOI] [PubMed] [Google Scholar]
- 15.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64. doi: 10.1161/01.CIR.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 17.Gilman G, Khandheria BK, Hagen ME, Abraham TP, Seward JB, Belohlavek M. Strain rate and strain: Step-by-step approach to image and data acquasition. J Am Soc Echocardiogr. 2004;17:1011–20. doi: 10.1016/j.echo.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Andersen NH, Poulsen SH. Evaluation of the longitudinal contraction of the left ventricle in normal subject by Doppler tissue tracking and strain rate. J Am Soc Echocardiogr. 2003;16:716–23. doi: 10.1016/S0894-7317(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 19.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–85. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 20.Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, et al. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414–19. doi: 10.1093/ejechocard/jen299. [DOI] [PubMed] [Google Scholar]
- 21.Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, et al. Global and regional myocardial function quantification by two-dimensional strain: Application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–81. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson JE. Heart failure with a normal ejection fraction. Heart. 2007;93:155–8. doi: 10.1136/hrt.2005.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewey M, Müller M, Eddicks S, Schnapauff D, Teige F, Rutsch W, et al. Evaluation of global and regional left ventricular function with 16-slice computed tomography, biplane cineventriculography, and two-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol. 2006;48:2034–44. doi: 10.1016/j.jacc.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 24.Greupner J, Zimmermann E, Grohmann A, Dübel HP, Althoff TF, Borges AC, et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3- dimensional transthoracic echocardiography: comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol. 2012;59:1897–907. doi: 10.1016/j.jacc.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Møgelvang J, Stokholm KH, Saunamäki K, Reimer A, Stubgaard M, Thomsen C, et al. Assessment of left ventricular volumes by magnetic resonance in comparison with radionuclide angiography, contrast angiography and echocardiography. Eur Heart J. 1992;13:1677–83. doi: 10.1093/oxfordjournals.eurheartj.a060124. [DOI] [PubMed] [Google Scholar]
- 26.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur Radiol. 2004;14:1813–22. doi: 10.1007/s00330-004-2387-0. [DOI] [PubMed] [Google Scholar]
- 27.Kühl HP, Schreckenberg M, Rulands D, Katoh M, Schäfer W, Schummers G, et al. High-resolution transthoracic real-time three-dimensional echocardiography: quantitation of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;43:2083–90. doi: 10.1016/j.jacc.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 29.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]