Abstract
Alkaptonuria (AKU) is a rare autosomal recessive disorder that results from the deficient activity of homogentisate 1,2-dioxygenase and leads to increased levels of homogentisic acid (HGA) and its oxidized product benzoquinone acetic acid (BQA). Both HGA and BQA form polymerized deposits that lead to a bluish-black discoloration of the cartilage as well as degeneration, inflammation, and calcification of the tendons, ligaments, intervertebral discs, and large joints and increased bone resorption. A brittle and fragmented cartilage forms and leads to aberrant loading of the subchondral bone. These fragments then adhere to the synovial membrane and cause fibrosis or chondromatosis, leading to ochronotic arthropathy. Ochronotic tendinopathy most commonly affects the patellar or Achilles tendon and can lead to enthesopathy or spontaneous tendon ruptures. Ochronotic pigments deposited in the bone impair the bone mineralization process and lead to osteopenia or osteoporosis. Here, we report a case of a patient with several musculoskeletal manifestations of AKU and reviewed the literature to summarize the pathophysiology, clinical characteristics, and radiologic findings of the rheumatic features of AKU. Though medical treatment options are limited, early identification of AKU can facilitate prompt surgical intervention.
Keywords: Alkaptonuria, ochronosis, homogentisic acid oxidase deficiency, ochronotic arthropathy, ochronotic tendinopathy
Introduction
Alkaptonuria (AKU) is a rare autosomal recessive disorder that results from a deficiency of the homogentisic acid dioxygenase (HGD) activity, which is the third enzyme in tyrosine degradation. HGD deficiency leads to the accumulation of homogentisic acid (HGA) that can be excreted in the urine (homogentisic aciduria) or can oxidize and polymerize to form an ochronotic pigment that is deposited in the connective tissues (ochronosis) or within the joints (ochronotic arthropathy) (1). Patients are typically asymptomatic in childhood. However, during the second to third decade of life, ochronosis may begin to manifest as a blue or brown pigmentation within the ear cartilage or the sclera; stones (renal, prostatic, gall bladder, and salivary glands); back or peripheral joint pain; rupture of the tendons, muscles, or ligaments; renal failure; osteoporosis; or fractures (2).
Alkaptonuria is caused by missense mutations in the HGD gene, which maps the human chromosome 3q21–q23, and the prevalence of the mutations is approximately 1:1, 000, 000–250, 000 in most ethnic groups (3). Loss of approximately 99% of the HGD activity is required to cause symptoms of ochronosis.
We report a case of a patient with AKU involving the cervical and lumbar spines, shoulders, and knees who underwent numerous orthopedic surgical procedures prior to his diagnosis of AKU at the age of 61 years. We also review the literature on the common musculoskeletal manifestations of AKU, including arthropathy, tendinopathy, and osteoporosis.
Methods
We searched PubMed for articles published between 1974 and 2017 using the search terms “alkaptonuria”, “ochronosis”, “ochronotic arthropathy”, “ochronotic tendinopathy”, and “alkaptonuria and osteoporosis.” We also checked the references in the retrieved articles.
Case Presentation
A 65-year-old African-American man with a history of hypertension, benign prostatic hypertrophy, nephrolithiasis, aortic stenosis, and osteoarthritis (OA) was initially referred to the rheumatology clinic because of a history of severe degenerative arthritis of the spine and peripheral joints for 20 years and a recent Achilles tendon rupture. He underwent multiple orthopedic surgeries through the years. These included open reduction and internal fixation of a right humerus fracture in 1984, cervical fusion of C4–C6 with fibular strut graft in 2000, L2-S1 laminectomy for lumbar spinal stenosis in 2001, discectomy with C3–6 fusion in 2005, fusion of the first metatarsal head with the proximal phalanx in 2009, open reduction and internal fixation of the left distal clavicle fracture in 2010, right knee arthroplasty in 2011, L4–5 microdiscectomy in 2011, repair of the right dorsal distal radial intra-articular fracture in 2014, reconstruction of the left Achilles tendon avulsion in 2014, right total hip replacement in 2015, left total knee arthroplasty in 2016, L4-pelvis posterior internal fixation and fusion for sacral fracture in 2017, and L4–L5 microdecompression in 2017. His medications include calcium and vitamin D supplements, prazosin, and hydrocodone/acetaminophen, as needed. Family history includes a brother who underwent bilateral knee replacement for knee OA at the age of 57 years.
The patient presented to the clinic with complaints of pain in his bilateral shoulders, hips and lower back associated with 10–15 min of morning stiffness with gradual worsening of pain throughout the day with physical activity. On general physical examination, a slight blue-black hyperpigmentation of the bilateral pinna of the ear and a 2/6 systolic murmur best heard along the left sternal border were noted. On musculoskeletal examination, tenderness over the left acromioclavicular joint, right glenohumeral joint, and bilateral knees with no apparent joint effusions was observed. The patient also had limited range of motion in his bilateral shoulders, right wrist, and right hip.
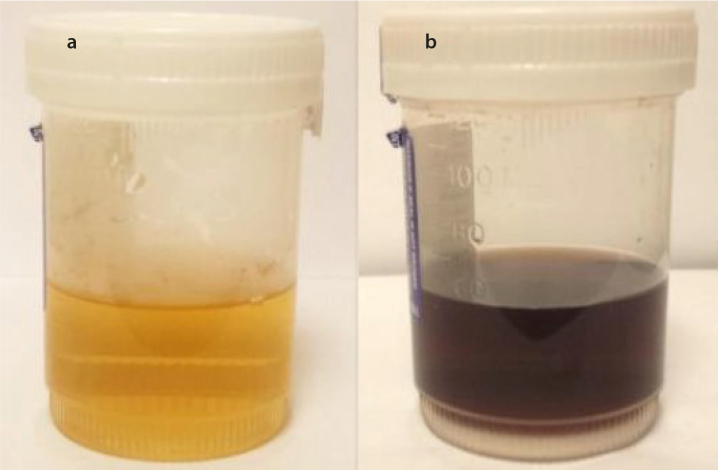
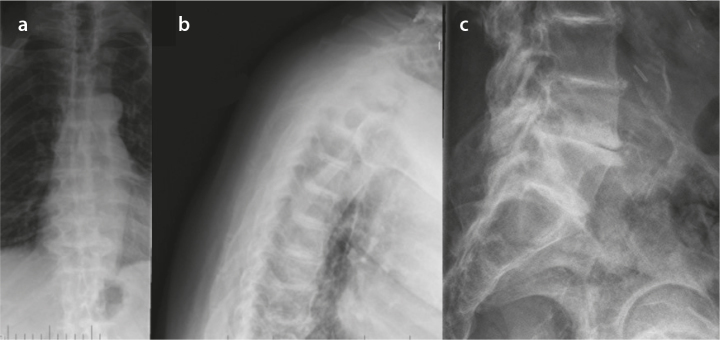
Results of comprehensive metabolic panel, ferritin, and transferrin were normal. He had mild anemia with a hemoglobin level of 12.4 g/dL (normal range: 13.3–17.7 g/dL). Urine qualitative measurement of organic acids was positive for increased level of homogentisic acid. His urine sample turned brown following alkalinization (Figure 1).Thoracic spine radiograph showed calcification of the intervertebral disc spaces at all levels of the thoracic vertebrae with disc space narrowing and anterior and posterior osteophytes (Figure 3a, b), whereas lumbar spine radiograph revealed diffuse severe osteopenia with interbody disc space calcification in the lumbar region (Figure 3c). Dual-energy X-ray absorptiometry was notable for the mean femoral neck and mean total hip bone mineral densities in the osteopenic range, with a T-score of −1.6 SD and −1.4 SD, respectively. The operative report from his Achilles tendon surgery noted black ends in the area where the Achilles tendon was ruptured and a tendinopathy of the flexor hallucis longus with blackening of the flexor tendon. Histological section of the cartilage and tendon sheath/fibrous tissue was notable for deposition of ochronotic pigment with associated homogenization and rigid appearance, transverse fracture with jagged or pointed ends, and fragmentations (Figure 4).
Figure 1. a, b.
Photographs of the patient’s urine sample before (a) and after alkalinization (b)
Figure 3. a–c.
Plain X-rays of the patient’s thoracic spine revealing calcification of the intervertebral disc spaces and osteophytes in AP (a) and lateral (b) views; radiograph of the patient’s lumbar spine showing severe osteopenia with interbody disc space calcification (c)
Figure 4.
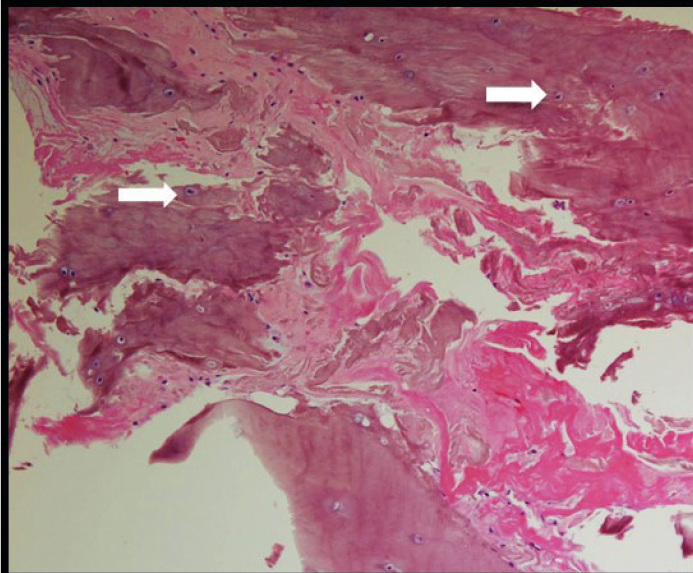
H&E stained section (10× magnification) of the cartilage and fibrous tissue showing deposition of ochronotic pigment (white arrows) with associated homogenization, rigid appearance, transverse fracture with jagged ends, and fragmentation
A diagnosis of AKU was established based on the increased level of urinary HGA, operative and pathology reports from his Achilles tendon surgery, and radiological findings. On further questioning, the patient reported that his urine turns dark brown when left standing.
Discussion
Arthropathy, tendinopathy, and osteopenia/osteoporosis are musculoskeletal manifestations of AKU. Though AKU is present at birth, the renal tubular excretion system efficiently secretes HGA up to several grams per day. Thus, the musculoskeletal symptoms of AKU are typically delayed until the fourth decade of life due to reduced renal clearance of HGA with age (4). Musculoskeletal symptoms tend to begin with spinal arthropathy, followed by peripheral arthropathy, tendinopathy, and then eventually, osteopenia and osteoporosis, though they may not always occur in this sequence.
Ochronotic arthropathy
Ochronotic arthropathy is characterized by severe early onset spine or peripheral joint pain associated with limited range of motion, swelling, and stiffness. Though the mechanism by which HGA deposition causes arthropathy is not clearly elucidated, it is hypothesized that oxidized HGA deposits in the deeper layers of the articular cartilage, accumulating more frequently in the senescent cartilage with poor metabolism. As a result, the cartilage becomes brittle and loses its elasticity, leading to fragmentation and formation of loose bodies. These fragments may then adhere to the synovial membrane and cause fibrosis or chondromatosis, eventually forming osteophytes or subchondral cysts (5). It is also proposed that the process of HGA oxidation itself produces free radicals that may be associated with the induction of both inflammatory and degenerative processes, leading to tissue damage. In particular, lysyl hydroxylase, a cartilaginous enzyme required for the formation of hydroxylysine residues and crosslinking, is inhibited by the HGA oxidation process (5). Conditions that allow for pigment deposition have not been clearly identified but are likely related to tissue damage or alteration in the gene expression. One study showed that the matrix becomes more susceptible to pigmentation in response to tissue injury, such as biochemical or mechanical damage, including microtrauma (6).
It has also been shown that tissue turnover in the cartilage may be affected by ochronosis, and that this may play a role in disease initiation and progression. A recent study analyzing hip and knee cartilages obtained at surgery from patients with arthropathy due to AKU found that compared with OA, AKU cartilage demonstrates a very low turnover state and has low levels of extractable matrix proteins. In particular, Taylor et al. (7) found that there are few extractable glycosaminoglycan and cartilage oligomeric matrix protein in AKU samples as compared with OA comparators.
The spine is commonly involved earlier than the peripheral joints, and patients typically present with back stiffness, with eventual loss of lordosis and exaggeration of thoracic kyphosis. Ochronotic arthropathy of the spine has been associated with myelopathy and disc prolapse or herniation. Degenerative changes in the spine with osteophytes from the posterior part of the vertebral body or uncinate process may intrude into the neural canal, causing myelopathy. Though disc herniation is less common, several case reports illustrate lumbar disc herniation in patients with ochronosis (8). Typical radiologic findings include intervertebral calcification at multiple levels and vacuum phenomena with radiolucent collections of gas, representing areas of severe degeneration. Kyphosis, apophyseal joint abnormalities, disc space obliteration, and bony bridging may occur with long-standing disease, simulating the spinal changes of ankylosing spondylitis (5). However, in contrast to ankylosing spondylitis, there is relative sparing of the sacroiliac joints and the absence of bamboo spine, annular ossification, syndesmophyte, or erosion in ochronotic spine disease.
Although peripheral articular symptoms of ochronotic arthritis may resemble those of rheumatoid arthritis (RA) with chronic painful swollen joints, in contrast to RA, the smaller joints of the hands and feet are often spared. The knee is the most commonly affected peripheral joint and is found in up to 64% of cases; however, degeneration of the knee often occurs many years after the onset of spinal symptoms (5). Knee radiographs reveal joint space narrowing with loose osteochondral bodies that are characteristic of ochronotic arthropathy. Aspiration of the synovial effusion often reveals floating black particles, also called as the “ground pepper sign” (9).
Owing to the rarity of the disease and symptoms mimicking other types of arthritis, the diagnosis of AKU is frequently not made until it is discovered intraoperatively during orthopedic surgery, and the affected joint bears the characteristic bluish-black discoloration. Histopathological findings include macroscopic brown to black coloring of the articular cartilages and synovial tissues, as well as microscopic fibrillation and eburnation (10). Darkening of the urine on oxidation or increased levels of urine HGA confirm the diagnosis.
Currently, no available medical treatment options have been shown to prevent the complications of AKU. A low tyrosine and phenylalanine diet, vitamin C or antioxidants, and nitisinone are few of the suggested treatments. A low tyrosine and phenylalanine diet has been proposed because the HGD enzyme is located in the phenylalanine and tyrosine degradation pathway. Therefore, a low-protein diet would theoretically decrease HGA production. However, the long-term effect of such a diet on the symptoms and outcomes of AKU has not been studied (11). Vitamin C and other antioxidants are also proposed as they could theoretically prevent the oxidation of HGA (12). Nitisinone (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione) is a herbicide and potent competitive inhibitor of 4-hydroxyphenylpyruvate dioxygenase, which is the enzyme that produces HGA. In alkaptonuric mice, nitisinone treatment from birth can prevent ochronosis in adult mice, whereas treatment from mid-life stops disease progression (13). A recent international trial demonstrated that nitisinone decreases urinary HGA excretion in a dose-dependent manner and increases tyrosine levels (14). Once significant ochronotic arthropathy develops, surgical intervention through total joint replacement is the best treatment option. Unfortunately, reports of successful surgical intervention for spinal involvement are uncommon. Physical therapy, along with nonsteroidal anti-inflammatory drugs, has been successful in limiting the progression of symptoms but has not been shown to affect the progression of underlying disease (15).
Ochronotic tendinopathy
Tendons are also sites of ochronotic pigment deposition due to their high collagen content, leading to tendinopathy. Among those patients with ochronotic tendinopathy, the prevalence of spontaneous tendon or ligament ruptures is estimated to be approximately 20%–30% (16). The patellar and Achilles tendons are the most commonly affected, often involving the insertion sites of traction tendons, leading to enthesopathy. The deposition of pigmented HGA in collagenous tissues affects the structural integrity of collagen, increasing the risk of tendon rupture due to their high collagen content (16). An ultrasound examination of the Achilles tendon shows loss of fibrillary pattern, increased thickness, small focus of calcification, and increase in size of the retrocalcaneal bursa (5). Although the patellar and Achilles tendons are the most commonly affected, other tendons may not always be spared. There is one report of a man who presented with a nontraumatic calcaneal avulsion in the setting of ochronosis (17). Another case report describes bilateral spontaneous quadriceps tendon rupture in a previously healthy patient as the initial presentation of AKU (18).
Ochronotic pigments may also deposit in the ligaments, causing them to become stiff and weakened. A case of patellar ligament rupture during total knee replacement surgery has been reported. Intraoperatively, it was noted that the distal half of the patellar ligament was heavily pigmented and fragile, resulting in rupture during retraction with mild force (19).
Medical treatment to prevent ochronotic involvement of the tendons and ligaments is limited as in the case of the above-mentioned arthropathy. However, improved preoperative preparation and greater care in handling of the tendons and ligaments during operative procedures may help avoid intraoperative complications, including tendon or ligament ruptures (19).
Osteopenia and osteoporosis
Alkaptonuria is also associated with decreased bone mineral density (BMD) and subsequent increased risk for fragility fractures. It is hypothesized that the newly formed and uncalcified osteoid matrix exposed to the effects of ochronotic pigment could be impaired in the mineralization process. Aliberti et al. characterized the biochemical markers of bone turnover and showed that HGA deposition in the bone matrix results in increased bone resorption, which coupled with normal bone formation, and leads to overall increased bone loss. In addition, matrix microdamage and collagen crosslink impairment due to HGA deposits may further weaken the bone (20). Another study of the lumbar spine and femoral bone mineral densities in patients found that all patients had lower than normal BMD at the femoral neck but had overestimated lumbar spine BMD likely due to intervertebral disc calcification and osteophyte formation (21). Patients with ochronosis are more prone to fractures due to decreased BMD. One case report highlights a patient who suffered a fracture of the left femur neck following a trivial fall (22).
Currently, no treatments have been shown to reverse the bone loss process in ochronotic osteoporosis. Aliberti et al. compared the BMD measurements of those treated with alendronate versus those without and found that there is no clinically significant difference in BMD after 2 years of treatment. The effects of HGA deposition render the bone matrix to be unaffected by bisphosphonate therapy (21).
Conclusion
Musculoskeletal manifestations of AKU may be divided into three main categories: early onset arthropathy, tendinopathy, and osteopenia/osteoporosis. Though medical treatment options are limited, early identification can facilitate prompt surgical intervention. A low tyrosine and phenylalanine diet, vitamin C or antioxidants, and nitisinone may also slow the progression of AKU, though their long-term effects have not been studied.
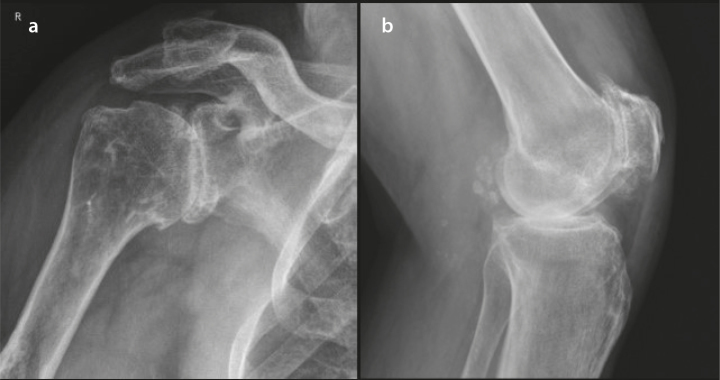
Figure 2. a, b.
Radiographs of the patient’s right shoulder (a) and left knee (b) showing severe osteoarthritis; chondrocalcinosis is present in the popliteal bursa
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - K.W., E.B., M.A.F.; Design - K.W., E.B., M.A.F.; Supervision - M.A.F.; Data Collection and/or Processing - K.W., E.B., G.M., M.A.F.; Analysis and/or Interpretation - K.W., E.B., G.M., M.A.F.; Literature Search - K.W., M.A.F.; Writing Manuscript - K.W., G.M., M.A.F.; Critical Reviews - K.W., E.B., G.M., M.A.F.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Mistry JB, Bukhari M, Taylor AM. Alkaptonuria. Rare Dis. 2013;1:e27475. doi: 10.4161/rdis.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phornphutkul C, Introne WJ, Perry MB, Bernardini I, Murphey MD, Fitzpatrick DL, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347:2111–21. doi: 10.1056/NEJMoa021736. [DOI] [PubMed] [Google Scholar]
- 3.Nemethova M, Radvanszky J, Kadasi L, Ascher DB, Pires DE, Blundell TL, et al. Twelve novel HGD gene variants identified in 99 alkaptonuria patients: focus on ‘black bone disease’ in Italy. Eur J Hum Genet. 2016;24:66–72. doi: 10.1038/ejhg.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdi N, Cooke TD, Hassan B. Ochronotic arthropathy: case report and review of the literature. International Orthopaedics. 1999;23:122–5. doi: 10.1007/s002640050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventura-Ríos L, Hernández-Díaz C, Gutiérrez-Pérez L, Bernal-González A, Pichardo-Bahena R, Cedeño-Garcidueñas AL, et al. Ochronotic arthropathy as a paradigm of metabolically induced degenerative joint disease. A case-based review. Clin Rheumatol. 2016;35:1389–95. doi: 10.1007/s10067-014-2557-7. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AM, Boyde A, Wilson PJ, Jarvis JC, Davidson JS, Hunt JA, et al. The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum. 2011;63:3887–96. doi: 10.1002/art.30606. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AM, Hsueh MF, Ranganath LR, Gallagher JA, Dillon JP, Huebner JL, et al. Cartilage biomarkers in the osteoarthropathy of alkaptonuria reveal low turnover and accelerated ageing. Rheumatology. 2017;56:156–64. doi: 10.1093/rheumatology/kew355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurkanlar D, Daneyemez M, Solmaz I, Temiz C. Ochronosis and lumbar disc herniation. Acta Neurochir. 2006;148:891–94. doi: 10.1007/s00701-006-0774-9. [DOI] [PubMed] [Google Scholar]
- 9.Hunter T, Gordon DA, Ogryzlo MA. The ground pepper sign of synovial fluid: a new diagnostic feature of ochronosis. J Rheumatol. 1974;1:45–53. [PubMed] [Google Scholar]
- 10.Doganavsargil B, Pehlivanoglu B, Bicer EK, Argin M, Bingul KB, Sezak M, et al. Black joint and synovia: histopathological evaluation of degenerative joint disease due to ochronosis. Pathol Res Pract. 2015;211:470–7. doi: 10.1016/j.prp.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Arnoux JB, Le Quan Sang KH, Brassier A, Grisel C, Servais A, Wippf J, et al. Old treatments for new insights and strategies: proposed management in adults and children with alkaptonuria. J Inherit Metab Dis. 2015;38:791–6. doi: 10.1007/s10545-015-9844-6. [DOI] [PubMed] [Google Scholar]
- 12.Morava E, Kosztolanyi G, Engelke UF, Wevers RA. Reversal of clinical symptoms and radiographic abnormalities with protein restriction and ascorbic acid in alkaptonuria. Ann Clin Biochem. 2003;40:108–11. doi: 10.1258/000456303321016268. [DOI] [PubMed] [Google Scholar]
- 13.Keenan CM, Preston AJ, Sutherland H, Wilson PJ, Psarelli EE, Cox TF, et al. Nitisinone arrests but does not reverse ochronosis in alkaptonuric mice. JIMD Rep. 2015;24:45–50. doi: 10.1007/8904_2015_437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganath LR, Milan AM, Hughes AT. Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis. 2016;75:362–7. doi: 10.1136/annrheumdis-2014-206033. [DOI] [PubMed] [Google Scholar]
- 15.Gil JA, Wawrzynski J, Wayasz GR. Orthopedic manifestations of ochronosis: pathophysiology, presentation, diagnosis, and management. Am J Med. 2016;129:536e1–6. doi: 10.1016/j.amjmed.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Manoj Kumar R, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85:883–6. doi: 10.1302/0301-620X.85B6.13662. [DOI] [PubMed] [Google Scholar]
- 17.Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2017;57:179–83. doi: 10.1053/j.jfas.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Chua SY, Chang H. Bilateral spontaneous rupture of the quadriceps tendon as an initial presentation of alkaptonuria-a case report. Knee. 2006;13:408–10. doi: 10.1016/j.knee.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Sahoo MM, Mahapatra SK, Sethi GC, Dash SK. Patellar ligament rupture during total knee arthroplasty in an ochronotic patient. Acta Orthop Traumatol Turc. 2014;49:367–70. doi: 10.3944/AOTT.2014.3245. [DOI] [PubMed] [Google Scholar]
- 20.Aliberti G, Pulignano I, Schiappoli A, Minisola S, Romagnoli E, Proietta M. Bone metabolism in ochronotic patients. J Intern Med. 2003;254:296–300. doi: 10.1046/j.1365-2796.2003.01145.x. [DOI] [PubMed] [Google Scholar]
- 21.Aliberti G, Pulignano I, Pisani D, March M, Porto F, Proietta M. Bisphosphonate treatment in ochronotic osteoporotic patients. Clin Rheumatol. 2007;26:729–35. doi: 10.1007/s10067-006-0390-3. [DOI] [PubMed] [Google Scholar]
- 22.Zacharia B, Chundarathil J, Ramakrishnan V, Krishnankutty RM, Veluthedath R, Puthezhath K, et al. Black hip, fracture neck of femur and scoliosis: a case of ochronosis. J Inherit Metab Dis. 2009;32(Suppl 1):S215–20. doi: 10.1007/s10545-009-1172-2. [DOI] [PubMed] [Google Scholar]