Abstract
Twenty percent of patients with Sjögren’s syndrome experience associated neurological disease. Transverse myelitis (TM) frequently forms part of a neuromyelitis optica spectrum disorder associated with the presence of anti-aquaporin 4 (AQP4) antibodies. We report the first described case of a patient who developed TM and the presence of a newly recognized antibody, anti-myelin oligodendrocyte protein (MOG), who went on to develop Sjögren’s syndrome. AQP4 and MOG antibodies should be tested to guide prognostically the chances of further relapse as well as the type and duration of immunotherapy in patients with coexisting Sjögren’s syndrome and TM.
Keywords: Transvere myelitis, primary Sjögren’s syndrome, neuromyelitis optica spectrum disorder, neurological manifestations, myelin-oligodendrocyte glycoprotein, antibodies
Introduction
Twenty percent of patients with Sjögren’s syndrome experience associated neurological disease, and occasionally, this is the presenting feature (1). Although peripheral nervous system involvement is more common, central nervous system (CNS) involvement is well described. Transverse myelitis (TM) is thought to occur in 1% to 5% of patients with Sjögren’s syndrome (2). It frequently forms part of a neuromyelitis optica spectrum disorder (NMOSD) associated with the presence of the autoantibody anti-aquaporin 4 (AQP4). The coexistence of Sjögren’s syndrome in patients with NMOSD is estimated to be 2% (1). Of patients with NMO, 16% have anti-SSA and/or anti-SSB antibodies, and 43% have antinuclear antibodies (3). The relationship between primary Sjögren’s syndrome (pSS) and demyelinating disease of the CNS is well described with the majority associated with optic neuritis (54%) and AQP4 seropositivity (89%) (1). In those with spinal cord lesions, longitudinally extensive TM (LETM) is the most common pattern found in patients with coexisting pSS (1). We describe a single case of a patient with TM associated with the presence of the autoantibody anti-myelin oligodendrocyte glycoprotein (anti-myelin oligodendrocyte protein (MOG)) who was also found to have pSS. To our knowledge, this is the first documented case of anti-MOG antibody-associated TM occurring in a patient with Sjögren’s syndrome.
Case Presentation
A 13-year-old boy with no significant medical history presented with a 1-day history of progressive bilateral leg weakness, evolving from normal power to complete lower limb paralysis over the course of 24 h. Weakness was accompanied by lumbar back pain, numbness, and painless urinary retention requiring catheterization. Two weeks prior to symptom onset, he had suffered a severe upper respiratory tract infection. He had recovered well prior to his presentation.
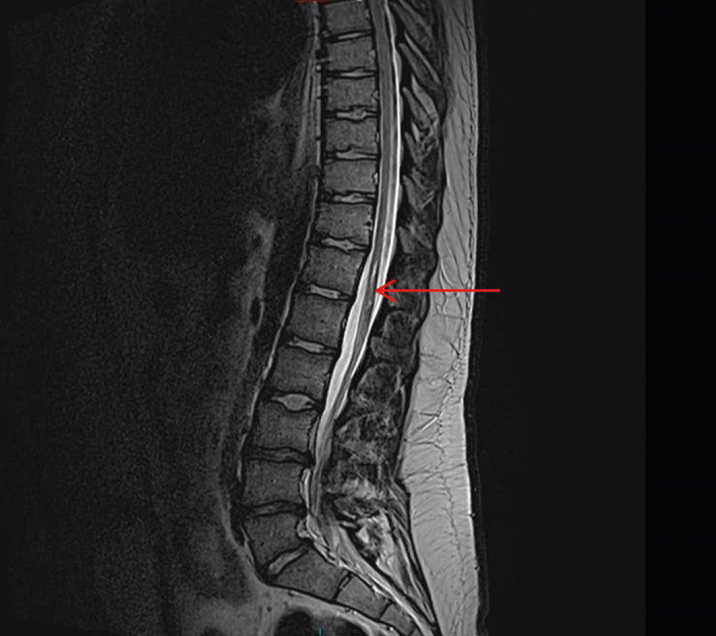
First-line investigations revealed normal routine blood tests. Anti-Ro and anti-La were highly positive. A magnetic resonance imaging (MRI) of the spine demonstrated a subtle TM of the conus medullaris, accounting for his current symptoms (Figure 1, 2). He was treated with a high dose of intravenous methylprednisolone, followed by a rapid oral taper of prednisolone, and started to significantly improve over the course of 72 h, reaching to the point where the catheter could be removed at 6 weeks, and he could walk without the aid of crutches at 4 months. He had only an increased left lower limb tone, a right foot drop, and a fixed contracture at the knee.
Figure 1.
MRI T2-weighted images showing axial views of the spinal cord
Figure 2.
MRI T2-weighted images showing sagittal views of the spinal cord
Over the following years, anti-Ro and anti-La tests remained highly positive and are up to the present. At follow-up, he denied the presence of significant sicca symptoms, Raynaud’s phenomenon, and previous genital or oral ulcers. On examination, there was no significant lymph node or salivary gland swelling or tenderness. Schirmer’s test remained negative. Unstimulated salivary flow rate was positive at 0.5 ml/15 min. An ultrasound of the salivary glands demonstrated hypoechoic changes suggestive of pSS. A follow-up salivary gland biopsy confirmed the diagnosis of pSS with a focus score of two. Anticardiolipin antibodies, Coombs test, rheumatoid factor, and anti-AQP4 antibodies were negative. ANA titer was 1/40. He was negative for dsDNA antibodies.
Six years after the onset of symptoms, based on new knowledge of the relevance of distal cord inflammatory disease, MOG autoantibody was tested and found to be positive. A repeat MRI at this time was unchanged.
Written informed consent was obtained from the patient.
Discussion
TM has a number of different causes including multiple sclerosis (MS), neuromyelitis optica, and in association with connective tissue diseases, such as systemic lupus erythematosus and Sjögren’s syndrome. Neuromyelitis optica is considered a more aggressive inflammatory disease than MS and is typically characterized by an LETM i.e., a myelitis that extends over the distance of three vertebral bodies along with severe (and not uncommonly) bilateral optic neuritis (4). Unlike in MS, the inflammatory process is believed to be driven by a pathogenic antibody, anti-AQP4 antibody, resulting in an autoimmune astrocytopathy (5). Serological identification of this antibody allows early recognition of NMO at a time where there may be only a single demyelinating lesion and prompts clinicians to initiate early and prolonged immunosuppressive therapy as it predicts a high rate of relapse and, compared with MS, a much higher rate of relapse-associated disability with a much poorer chance of recovery with corticosteroids.
A subset of patients who meet the criteria for NMOSD are seronegative for AQP4. In recent years, a proportion of these are recognized to be seropositive for anti-MOG antibody (one-third of patients) (5). As yet, it remains unclear as to whether anti-MOG antibodies are directly pathogenic or a simple bystander to an alternative inflammatory process. However, they are being increasingly tested for in the context of AQP4-negative NMOSD. Experience of patients with anti-MOG positive antibodies is limited, but case series suggest that patients are more frequently male, tend to have a more benign course compared with AQP4-positive patients, the condition is more likely to be monophasic with relapses acutely steroid responsive, and typically leave relatively minimal residual disability (6). TM tends to be more distal cord, and anti-MOG antibody may be positive several years following the incident demyelinating event or only positive during an acute attack and disappear (author’s own experience). The implications of this on the underlying inflammatory process are unclear. More recently, in a review by Weber et al., a case is made for anti-MOG-associated CNS demyelination to be considered as an entirely discrete disease entity based on a homogenous group of characteristics including predominant optic neuritis, supratentorial lesions on MRI, and on a histopathological basis, a relative sparing of astrocytes (5). The occurrence of anti-MOG antibodies in NMO with other coexisting autoimmune disease is thought to be rare (5).
Several case reports are found in the literature of NMOSD occurring in the pediatric population with coexisting pSS (7). The vast majority of these are seropositive for AQP4. Nevertheless, the reports do act to remind us of the misconceived frequency of coexisting pediatric systemic autoimmunity and NMOSD. This reinforces the message that the finding of anti-Ro/La antibodies in a patient with NMO, adult or pediatric, should prompt further investigation for pSS. Similarly, we suggest that patients with Sjögren’s syndrome presenting with TM should have AQP4 and MOG antibodies tested to guide prognostically the chances of further relapse and hence the type and duration of immunotherapy.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed
Author Contributions: Concept - K.J., D.L., W.F.N., J.G.; Design - K.J., D.L., W.F.N., J.G.; Supervision - K.J., D.L., W.F.N., J.G.; Data Collection and/or Processing - K.J., D.L., W.F.N., J.G.; Analysis and/or Interpretation - K.J., D.L., W.F.N., J.G.; Literature Search - K.J., D.L., W.F.N., J.G.; Writing Manuscript - K.J., D.L., W.F.N., J.G.; Critical Review - K.J., D.L., W.F.N., J.G.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Berkowitz A, Samuels M. The neurology of Sjögren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol. 2014;14:14–22. doi: 10.1136/practneurol-2013-000651. [DOI] [PubMed] [Google Scholar]
- 2.Sa MJ. Acute transverse myelitis: a practical reappraisal. Autoimmun Rev. 2009;9:128–31. doi: 10.1016/j.autrev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homberger HA, Wingerchuck D, et al. Neuromyelitis optica and non-organ specific autoimmunity. Arch Neurol. 2008;65:78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk D, Banwell B, Bennet J, Cabre P, Carroll W, Chitnes T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber MS, Derfuss T, Metz I, Brück W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord. 2018;11:1–15. doi: 10.1177/1756286418762083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato D, Callegaro D, Lana-Peixoto M, Waters P, de Haidar Jorge F, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–81. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gmuca S, Lieberman SM, Mehta J. Paediatric neuromyelitis optica spectrum disorder and sjogren’s syndrome: more common than previously thought? J Rheumatol. 2017;44:959–60. doi: 10.3899/jrheum.160978. [DOI] [PMC free article] [PubMed] [Google Scholar]