Abstract
Advanced (Ad) systemic mastocytoses (SM) include aggressive SM (ASM) and mast cell leukemia (MCL) with or without an associated clonal hematological non-mast cell lineage disease (AHNMD). They are rare (<15%) but are associated with a poor prognosis due to rapid organ dysfunction. To date, responses to high-dose chemotherapy, cladribine, and imatinib were revealed to be suboptimal with a median survival time of 24 months. Midostaurin is a potent multikinase inhibitor including the most frequent KIT D816V mutation (>80%). We herein present a review of the most recent data of the use of midostaurin in AdSM. First, a multicenter Phase II study (CPKC412D2213) revealed an unprecedented overall response rate (ORR) of 69% regardless of KIT mutational status, with 38% of major response (MR) among 26 AdSM patients treated with midostaurin alone 200 mg daily. Second, a sponsor-initiated, multicenter, single-arm open Phase II study (CPKC412D2201) confirmed a high and durable ORR of 60% including 45% of MR among 89 AdSM patients. Finally, a French compassionate use program managed by the French Reference Centre for Mastocytosis allowed the treatment of almost a hundred AdSM patients to date in France since the CPKC412D2201 study closure. The outcome of the first 28 treated patients under cover of this on-going procedure revealed an ORR of 71% including 57% of MR. Most importantly, survival analysis revealed in comparison to a historical control cohort of AdSM patients who did not receive midostaurin a twofold lower risk of death (p=0.02) in midostaurin-treated patients. Side effects revealed were acceptable and manageable (mostly digestive). Midostaurin appears to be an effective and safe treatment of AdSM. However, its effect on the course of the AHNMD is less clear. For the future, combined therapy (hypomethylating agents, cladribine, mammalian target of rapamycin inhibitors, chemotherapy, and allogeneic bone marrow transplantation) may further improve long-term survival, particularly that of MCL and AdSM patients with AHNMD.
Keywords: aggressive systemic mastocytosis, mast cell leukemia, tyrosine kinase inhibitor, midostaurin
Introduction
Mastocytosis is considered as a myeloproliferative neoplasm characterized by the unregulated activation, proliferation, and accumulation of clonal mast cells in various organs including bone marrow, liver, spleen, and skin.1 The aggressive forms, commonly denominated advanced systemic mastocytosis (AdSM), are rare accounting for <15% of all cases of mastocytosis.2,3 They are characterized by rapid organ damage caused by infiltrating mast cells with consequent dysfunction (C-findings), including cytopenias, enlargement of the spleen and liver with associated hypersplenism, liver function abnormalities, and portal hypertension notably ascitis, malabsorption with weight loss and hypoalbuminemia, and osteolysis with or without pathological fractures.4,5 According to the World Health Organization (WHO), aggressive systemic mastocytosis (ASM) with or without associated clonal hematological non-mast cell lineage disease (AHNMD) is the most frequent aggressive sub-type followed by mast cell leukemia (MCL), which constitutes the less frequent form of AdSM.2,3 Mast cell sarcoma (MCS), which is even more rare, exhibits extremely aggressive features with a very bad prognosis.6,7 In contrast, smoldering systemic mastocytosis (SSM), characterized by high mast cell burden with organ enlargement and signs of dysplastic features of bone marrow cells (B-findings) but without organ dysfunction (no C-findings), may also reduce the overall survival (OS) and impair quality of life.8 No efficient standard of care is currently available to induce prolonged responses and cure of ASM, MCL, MCS, and even SSM,5 except allogeneous bone marrow transplantation.9 However, cytoreductive therapies are needed to induce response prior to bone marrow transplantation. Patients treated by cladribine (2-chloro-deoxy-adenosine [2-CdA]) may exhibit responses with control of C-findings and mast cell burden decrease.10–14 In the most recent data on 2-CdA in mastocytosis, the multicenter retrospective French study including 32 AdSM patients reported an overall response rate (ORR) of 50% with 37.5% of major response (MR).14 However, responses are usually of short duration requiring repeated cycles of treatment, while the number of cycles is limited by cumulative hematological toxicities.11,14 Moreover, highly aggressive AdSM with rapid progression is refractory to 2-CdA in first line with the risk to increase cytopenia. Interferon may induce MR in only 21% in AdSM with early relapses after discontinuation.13,15,16 However, interferon induced slow responses, with a marginal effect on cell proliferation and is therefore not a good option for rapidly progressive diseases but rather for indolent forms of mastocytosis with the preferential effect on mast cell activation.17 Concerning alternative therapeutic options such as thalidomide and mammalian target of rapamycin (mTOR) inhibitors, indications are limited to refractory situations because of unpredictable responses and absence of large prospective cohort to date.2,3,18–21 Thus, prognosis of AdSM is still very poor with a median survival time estimated at 24 months, respectively 3.5 years in ASM, 2 years in SM-AHNMD, and <6 months in MCL.2,3
The discovery that survival and proliferation of mast cells are driven by somatic mutations leading to constitutive activation of the tyrosine kinase receptor c-KIT led to the development of therapeutic strategies aiming at inhibiting its kinase activity. First-generation tyrosine kinase inhibitors (TKIs), notably imatinib mesylate, are inefficient due to the high frequency of naturally resistant c-KIT D816V mutation, which drives >80% of AdSM.22–25 Preclinical studies demonstrated that midostaurin is a potent TKI of all common mutant forms of c-KIT, including D816V and D816Y inhibiting the stem cell factor-induced phosphorylation of c-KIT in Ba/F3 cells expressing c-KIT D816V and D816Y and inducing apoptosis of MCL cell line.26–28 Clinical response reported in 2005 in a patient with MCL treated by midostaurin26 led to an investigator-initiated, multicenter Phase II study (CPKC412D2213), which included 26 patients with AdSM (ASM and MCL). Results of this study were reported in 2010 at the 52nd Annual Meeting of the American Society of Hematology (ASH) and consisted in an unprecedented ORR of 69% regardless of KIT mutational status, with 38% of MR. On the basis of these encouraging results, a sponsor-initiated, global, multicenter, single-arm open Phase II study (CPKC412D2201)29 was aimed to evaluate the efficacy and safety of oral midostaurin 100 mg twice daily in adult patients with ASM or MCL with or without AHNMD according to WHO criteria.4,30 Marked and durable clinical benefits were again demonstrated in the 89 AdSM patients included from 2009 to 2012 with an ORR of 60% including 45% of MR.29 After the CPKC412D2201 study closure, a compassionate use program was approved in France in August 2012 to use PKC412 (a novel staurosporine-derived TKI) in AdSM under cover of a transitory use authorization (TUA). The French National Reference Centre for Mastocytosis (CEREMAST) conducted a prospective survey of patients treated in this program. To date, almost a hundred patients received midostaurin under cover of this on-going procedure in France for an AdSM. Among them, the outcome of the first 28 midostaurin-treated French patients was compared to that of 44 matched patients for age at diagnosis and WHO-defined subtypes,4,30 who did not receive midostaurin. The ORR was 71% including MRs in 57% and survival analysis revealed in comparison to the historical control cohort a twofold lower risk of death (p=0.02).31 We herein present a review on those most recent resulting data to illustrate in details clinical potential of midostaurin in AdSM.
Analysis of clinical potential of midostaurin in term of response, survival, and quality of life
All patients received a continuous regimen of midostaurin alone at the dose of 100 mg twice daily, although the reduction of dose could occur in case of adverse events. Based on C-findings, organ enlargement, tryptase level, and bone marrow histological analysis (refer to response criteria used in the CPKC412D2201 study presented in Table S11,29,32), to date all studies are concordant (Table 1) in revealing an unprecedented high ORR of 69%, 60%, and 71%, respectively, in the CPKC412D2213 study, the CPKC412D2201 study, and the French compassionate use program with a majority of MRs in 38%, 45%, and 57%, respectively, including mainly incomplete MRs (23%, 21%, and 39%) and pure clinical responses (15%, 17%, and 18%) but no complete response.29,31 Partial responses were noted in 30%, 15%, and 14%, respectively; stable disease in 15%, 12%, and 11%; and progressive disease in 15%, 11%, and 18%, respectively. Reversal of organ damage was observed across all types of C-findings with notable correction of cytopenia with red blood cell and platelet transfusion independency, regain of the weight loss, restoration of the liver functions as well as decrease in liver and spleen volume, bone marrow mast cell burden, serum tryptase level, eosinophilia, and monocytosis. Moreover, we observed an improvement of responses with time, sometimes until 2 years after midostaurin initiation in the French compassionate cohort. As such, the time to best response remains to be determined. Response rates according to AdSM subtypes were documented in the CPKC412D2201 study and the French compassionate cohort as follows: 75% in ASM in both studies, 58% and 72%, respectively, in SM-AHNMD with no therapeutic impact on the AHNMD course, and 50% and 66% in MCL.29,31 No response was observed in the only patient from the French compassionate cohort with MCS who experienced a progressive disease.31
Table 1.
Demographic and disease characteristics of patients treated by midostaurin in the CPKC412D2213, CPKC412D2201, and French compassionate use program and of a historical cohort of patients treated before the era of midostaurin
| Characteristics (clinical/biological) | CPKC412D221351 | CPKC412D220129 | French TUA compassionate cohort31,a | French historical control groupa |
|---|---|---|---|---|
| Number of patients | 26 | 89 | 28 | 44 |
| Male sex, n (%) | 15 (57) | 57 (64) | 24 (85) | 27 (61) |
| Age, median (range) | 62 (24–79) | 64 (25–82) | 67 (29–85) | – |
| Age at diagnosis, median (range) | na | na | 65 (12–84) | 66 (14–87) |
| SM subtype according to the WHO, n (%) | ||||
| ASM | 4 (15) | 16 (18) | 4 (14) | 5 (11) |
| SM-AHNMD | 20 (77) | 57 (64) | 18 (64) | 33 (75) |
| MCL | 2 (8) | 16 (18) | 3 (11) | 2 (5) |
| MCS | – | – | 1 (4) | 2 (5) |
| Progressive SSM | – | – | 2 (7) | 2 (5) |
| C-findings, median (range) | All patients had 1 or | 2 (1 to ≥3) | 2.5 (0–4) | 2 (0–4) |
| C-findings excluding cytopenia | more C-findings | na | 2 (0–3) | 1 (0–3) |
| Hematopoietic organ enlargement,b n (%) | na | 82 (92) | 27 (96) | 38 (86) |
| Urticaria pigmentosa, n (%) | na | na | 13 (46) | na |
| Mast cell mediator symptoms,c (%) | na | na | 13 (46) | 26 (59) |
| Bone marrow mast cell burden, % (range) | na | 50 (8–98) | 35 (10–80) | na |
| Tryptase, median (range) | na | 236 (27–12,069) | 200 (85–2000) | 103 (4–900) |
| Gene mutations, % | ||||
| WT c-KIT | na | 11 | 3.5 | 16 |
| D816V c-KIT | 69 | 87 | 96.5 | 84 |
| ASXL1 | na | na | 30 (75% of SM-AHNMD) | 19 (85% of SM-AHNMD) |
| TET2 | na | na | 43 (83% of SM-AHNMD) | 29 (70% of SM-AHNMD) |
| Number of previous therapies – median (range) | 1.5 (0–4) | 0 (0 to ≥3) | 1.5 (1–3) | 2 (1–4) |
| Steroids, % | na | 6 | 21 | 41 |
| 2-CdA, % | na | 13 | 21 | 49 |
| Interferon, % | na | 8 | 11 | 8 |
| TKI other than midostaurin, % | na | 17 | 0 | 13 |
| Thalidomid, % | na | na | 0 | 18 |
| mTOR inhibitor, % | na | na | 11 | 5 |
| Other, % | na | na | 0 | 5 |
| ORRd, % | 69 | 60 | 71 | NA |
| Major response | 38 | 45 | 57 | |
| Partial response | 30 | 15 | 14 | |
| Stable disease | 15 | 12 | 11 | |
| Progressive disease | 15 | 11 | 18 | |
| RR%d according to WHO-SM subtype | ||||
| ASM | na | 75 | 75 | NA |
| SM-AHNMD | na | 58 | 72 | |
| MCL | na | 50 | 66 | |
| MCS | – | – | 0 | |
| Progressive SSM | – | – | 100 | |
| Median treatment duration, months (range) | na | 11.4 (0.3–51.5) | 10.5 (2–32) | NA |
| Median follow-up, months (range) | na | 26 (12–54) | 18.5 (3–36) | NA |
| Median response duration, months (range) | na | 24.1 (18.1-not estimated) | 17 (5–32) | NA |
| Safety/adverse events Any grade % is available (grade 3 and/or 4, % or number if percentage not available) | Decreasing frequency: nausea/vomiting (G3, n=2) Diarrhea Fatigue (G3, n=2) Anemia (G3, n=1) Thrombocytopenia (G3, n=1) Hyperlipasemia (G3, n=1) |
Nausea 79% (6%) Vomiting 66% (6%) Diarrhea 54% (8%) Peripheral edema 34% (4%) Abdominal pain 28% (3%) Fatigue 28% (9%) Constipation 24% (1%) Headache 23% (2%) Arthralgia 20% (2%) Cough 19% (1%) Dizziness 13% QT interval prolongation (12%) Neutropenia 48% (24%) Anemia 63% (41%) Thrombocytopenia 52% (29%) |
Nausea 89% (39%) Vomiting 25% (3.5%) Photosensitivity 25% Fatigue 14% (3.5%) Diarrhea 10.5% Drug-induced toxidermia 3.5% (3.5%) Peripheral edema 3.5% Lymphopenia 67% Cytolytic hepatitis 7% |
NA |
| Discontinuation due to adverse events | na | 22% | 10% | NA |
Notes: Response rates to midostaurin and safety data.
No statistical difference was observed between groups in age at diagnosis, sex, WHO-defined SM subtype distribution,4,5 C-findings, organ enlargement, MCAS, gene mutations distribution, tryptase level, hematological parameters, and follow-up time from diagnosis except for the number of previous treatment lines.
Hematopoietic organ enlargement refers to hepatomegaly and/or splenomegaly and/or adenopathy.
MCAS was defined by the presence of two symptoms among flush, pruritus, diarrhea, and anaphylactic shock.
Response criteria were those of the CPKC412D2201 Phase II trial.
Abbreviations: AHNMD, associated clonal hematological non-mast cell lineage disease; ASM, aggressive systemic mastocytosis; C, clinical; G, grade; MCL, mast cell leukemia; MCS, mast cell sarcoma; mTOR, mammalian target of rapamycin; na, not available; NA, not applicable; ORR, overall response rate; RR, response rate; SM, systemic mastocytosis; SSM, smoldering systemic mastocytosis; TKI, tyrosine kinase inhibitor; TUA, transitory use authorization; WHO, World Health Organization; WT, wild type; 2-CdA, 2-chloro-deoxy-adenosine; MCAS, mast cell activation syndrome.
At the histological level, no complete response was observed under midostaurin alone.29,31 Two patterns were observed, one in which mast cell infiltration was significantly reduced and the other with no or minor changes in bone marrow mast cell infiltration and in accordance tryptase levels remained unchanged. Gotlib et al29 reported a concomitant decrease in both bone marrow mast cell burden and serum tryptase level in 78% of the patients with a correlation between the duration of the decrease in mast cell burden and OS. However, we amazingly observed in the French compassionate use program that in patients without mast cell burden decrease, the survival could also be increased and quality of life improved (CEREMAST, unpublished data). This apparent discrepancy might be explained by the combined inhibitory effect of midostaurin on mast cells proliferation and tissue invasion as well as on mast cell mediator release. Indeed, in some cases, organ dysfunctions may also be due to mast cell mediators release, such as some cytopenia, bleeding disorders,33 and cachexia. This observation raised the following question: is complete response really a goal to achieve? One may postulate that control of C-findings and disease control, including partial and MR and even stable disease, should be considered as a therapeutic success that eventually will translate in a significant improvement of OS.
A significant response on the skin with regression of the cutaneous lesions of mastocytosis (urticaria pigmentosa) when initially present was also registered notably in the French compassionate cohort associated with a concomitant significant control on mast cell mediator release symptoms such as pruritus, flush, anaphylactic shock, and diarrhea in responder patients, illustrating the impact of midostaurin also on mast cell activation.34,35 This was also the case in the CPKC412D2201 study with a significant benefit on patient-reported symptoms and quality of life29,36 although the latter may also be due to mast cell burden decrease. However, further emphasizing the hypothesis that midostaurin may act by blocking mast cell activation, some patients experienced decrease of their symptoms while mast cell infiltration and tryptase levels were not significantly modified. Whatever, the opportunity and benefits to use midostaurin in patients with indolent SM with refractory disabling symptoms as recently reported with masitinib,37 another TKI with a more selective spectrum of activity, as well as in symptomatic and progressive smoldering SM patients, should be weighed against adverse events, notably the still unknown long-term adverse events in case of continuous exposure.
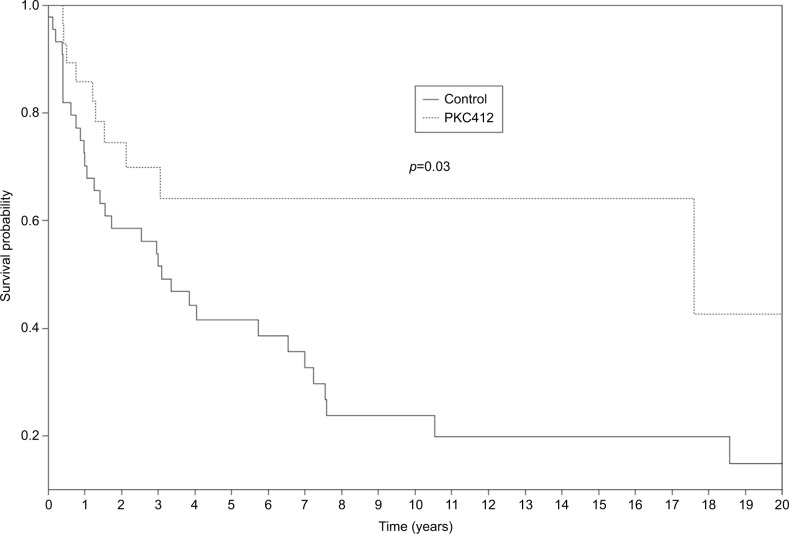
The survival benefit to use midostaurin in AdSM could be documented via a comparison of the outcome of 28 midostaurin-treated French patients across the compassionate use program to that of an historical cohort of AdSM patients extracted from the CEREMAST French national database, who did not receive midostaurin made up of 44 matched patients for age at diagnosis and WHO-defined SM subtypes,4,30 treated before the era of midostaurin.31 Although the comparator group (midostaurin-untreated group) had received more lines of treatment, notably more cladribine exposure (49% in the control group versus 21% in the midostaurin group), mean follow-up time from diagnosis was similar in both groups, and the OS rate was revealed to be significantly superior in the midostaurin-treated group (42.7% [95% CI: 0.181–1] versus 14.9% [95% CI: 0.0613–0.36]) in the control group (p=0.03)31 (Figure 1). In the multivariate analysis by pooling both groups, age at diagnosis, C-findings, WHO mastocytosis classification, and midostaurin exposure were independent prognosis factors, with a threefold higher risk of death in the control group than the midostaurin-treated cohort.31
Figure 1.
Survival curves in midostaurin (PKC412)-treated patients under cover of the French compassionate use program (n=28)31 and historical control (n=44) groups at the last follow-up in April 2015.
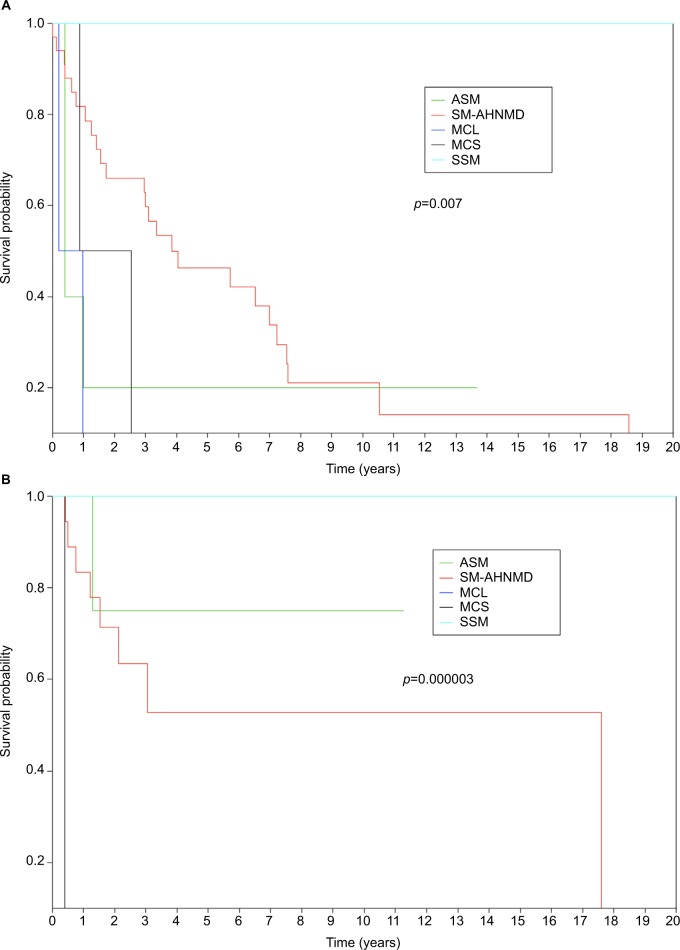
Moreover, in this French compassionate cohort, OS according to the SM subtypes was revealed to be significantly (p<0.01) higher in ASM and MCL than in SM-AHNMD and MCS patients (Figure 2A, B) with 80% of deaths being related to AHNMD progression.31 Thus, the poor prognosis of MCL and ASM appears reversed by midostaurin (Figure 2A, B). In contrast, in SM-AHMND and MCS, the prognosis remains related to independent AHNMD progression and probably to c-KIT independent oncogenic events that are not inhibited by midostaurin.38–40 This is in accordance with the results of the CPKC412D2201 study in which median OS was 28.7 months and, as in the French analysis, median OS was not reached in ASM whereas it was estimated to be 20.7 months in patients with SM-AHNMD and 9.4 months in patients with MCL but this was not reached among the subgroup of patients with MCL who had a response contrary to those who had not (median OS: 7.6 months).29 In the multivariate analysis, a better survival was detected in the case of response rather than non-response (p=0.03) and AdSM other than MCL (p=0.04), whereas exposure to previous therapy was associated with shorter survival (p=0.02).29
Figure 2.
(A) Survival curves in midostaurin-untreated patients (French historical control group, n=44) according to WHO-defined systemic mastocytosis subtypes. (B) Survival curves in midostaurin-treated patients under cover of the French compassionate use program (n=28) according to WHO-defined systemic mastocytosis subtypes. Notes: (A) SM-AHNMD n=33; ASM n=5; MCL n=2; SSM n=2; MCS n=2. (B) SM-AHNMD n=18; ASM n=4; MCL n=3; SSM n=2; MCS n=1. The MCL and SSM curves have the same color because no death was registered, so curves are superimposed.
Abbreviations: AHNMD, associated clonal hematological non-mast cell lineage disease; ASM, aggressive systemic mastocytosis; MCL, mast cell leukemia; MCS, mast cell sarcoma; n, number; SM, systemic mastocytosis; SSM, smoldering systemic mastocytosis; WHO, World Health Organization.
MCL is still considered as the subtype of AdMS with the worst prognosis with a median survival of <6 months.2,3,9,26,41 From those recent studies testing midostaurin alone in AdSM, although we did observe an unprecedented improvement of OS, it is difficult to definitely conclude that midostaurin is sufficient to induce cure of the disease. The clinical and biological heterogeneity of MCL does not allow drawing firm interpretation on the real long-term impact of midostaurin. As such, the exceptional MCL survival curve in the French compassionate use program with no death to date31 (Figure 2B) is probably biased by the excess of chronic MCL patients (2/3), assessed by the low Ki67 expression level (CEREMAST, unpublished data). Patients with chronic MCL appeared better responders to midostaurin than patients with acute MCL because the only acute MCL patient in the French cohort did not respond to midostaurin but benefited of a sequential combined therapy using cytarabine–temsirolimus bridged to haploidentical allogeneic hematopoietic stem cell transplantation (alloHCT), with an unexpected persisting survival of >36 months to date (CEREMAST, unpublished data).
The 11% rate of leukemia transformation documented in the CPKC412D2201 study is identical to that observed in patients with AdSM who did not receive midostaurin as reported in the Mayo Clinic study.2,29 Facing the highest rate of AHNMD-related mortality and the subsequent absence of prognosis impact of midostaurin exposure as a single agent on the survival of the SM-AHNMD subgroup31 as illustrated by (Figure 2A, B), combined therapies should be considered. The combination of midostaurin with standard high-dose induction chemotherapy regimen (3+7) has already proven feasible and safe in Phase I/II trials42 and efficient in Phase III in AML with FLT3 ITD mutations.43 Depending on AHNMD type and staging, age, and comorbidities, the combinations of midostaurin with hypomethylating agents,44,45 or traditional induction (3+7)42 or other chemotherapy-based regimens46 should be evaluated in prospective trials, either in a concomitant or in a sequential manner more likely as a bridge to alloHCT.9
In the French compassionate cohort, corticosteroids therapy could be briefly used (always <2 months to prevent bias of interpretation in midostaurin response) only in selected patients in the case of immediate life-threatening events incompatible with the initiation of a cytoreductive therapy (CEREMAST, unpublished data). In fact, corticosteroids appear highly efficient in emergency situations to control acute phases of mast cell activation and proliferation as illustrated, for example, by the rapid resolution of bleeding episodes,33 cytopenia, heart failure (CEREMAST, unpublished data), ascitis, pleural and pericardial effusions, and massive cachexia in some patients. However, the duration of response to corticosteroids alone is short and therefore should only be considered as an adjuvant therapy allowing to start midostaurin in better conditions (better performance status and hematopoiesis). Yet, it is not ruled out whether the combination of midostaurin and high-dose steroids may have a synergistic effect and improve response rate and survival. Whatever, either with or without steroids, responses to midostaurin appeared to be detectable within the first 3 months of treatment in both studies (CPKC412D2201 and French compassionate cohort).29,31 This allows an early identification of refractory patients and thus a switch to another therapy without delay.
Long-term prognosis of AdSM remains uncertain with no curative option to date as illustrated by the absence of complete response, the usual occurrence of relapses in the case of treatment discontinuation (e.g., for adverse events; CERE-MAST, unpublished data), and updated results of median OS and median progression free survival (PFS) of only 28.7 and 14.1 months, respectively, in the CPKC412D2201 study.29,31 Midostaurin-combined regimens using chemotherapy42–45 and other TKI to prevent clonal selection by targeting other kinases than c-KIT and to encompass and overtake KIT-independent signaling in neoplastic mast cells,28,38,47 innovative drugs such as mTOR inhibitors,20 alone or bridged to alloHCT,9 for young and high-risk patients like those with MCL and with additional mutations such as ASXL1, RUNX1, or SRSF248,49 should also be prospectively evaluated.
Safety data associated with the use of midostaurin
Tolerance was acceptable and manageable in most cases in all three studies29,31 (Table 1). Most frequent adverse events were nausea/vomiting in 85% and 82% (grades 3–4 in 12% and 40%), respectively, in the CPKC412D2201 study and the French compassionate cohort.29,31 Although this could lead to treatment compliance failure or even discontinuation, prophylactic measures using anti-emetics combined with a strict administration with meals are usually effective. Diarrhea and fatigue were also frequently reported in 54% (grade 3 or 4 in 3%) and 28% (grade 3 or 4 in 9%), respectively, in the CPKC412D2201 study.29 Although grades 3–4 neutropenia, anemia, and thrombocytopenia were reported in 24%, 41%, and 29%, respectively, of the patients of the CPK412D2201 study, no permanent limiting bone marrow suppression was registered. This occurred mostly in patients with pre-existing cytopenia, notably patients with AHNMD.29 Transitory or permanent dose reduction is usually sufficient to reverse toxic cytopenia unless they are also related to mastocytosis and/or AHNMD progression. In the French cohort, we also registered two other adverse events suspected to be related to midostaurin but that were not reported elsewhere. We observed a frequent global lymphopenia (median 500/mm3 [300–1100]) but without opportunistic infection under cover of valacyclovir more or less cotrimoxazole prophylaxis. We also detected 3 cases of carcinoma among 28 patients (1 gastric and 2 extensive basocellular) while on treatment.31 However, the early appearance with regard to midostaurin initiation, the age of the patients and, considering all studies, the very low frequency of report of such tumors do not allow drawing final conclusions on the putative role of midostaurin in promoting carcinogenesis or reducing immune surveillance against tumors. However, carcinogenesis analyses are worth to be performed particularly if midostaurin may also be an alternative treatment option in refractory indolent SM patients, in whom long-term life expectancy is preserved contrary to AdSM in whom to date expected survival was <5 years.2,3
The incidence of treatment discontinuation for adverse events was 10% and 22% of patients in the French compassionate cohort and the CPKC412D2201 study, respectively,29,31 and they revealed mostly digestive adverse events. Midostaurin monitoring may improve safety and response, taking into account not only compliance and absorption, but also organ dysfunction and drugs interactions.50 It is important to note that no dose adaptation is required in case of renal insufficiency, whereas safety data are lacking in case of liver function abnormalities waiting for results of sequential midostaurin monitoring in this setting.50 However, treatment usually improves liver functions in responders patients via mast cell burden decrease with reduction in liver volume as well as correction of portal hypertension and normalization of albumin level.29,31 Finally, in comparison to other drugs, midostaurin appears to have lower cytotoxic side effects, notably no limiting myelosuppression as observed with 2-CdA.11,14 Taken together, results of all studies suggest that midostaurin, alone or in combination, can be used in first-line therapy in AdSM without any major risk of cumulative toxicity notably for the bone marrow. However, facing a multi-target drug and the need to treat for life/until progression, we do not know yet exactly about potential long-term side effects, notably the induction of secondary neoplasia and the role in progression of the AHNMD.
Conclusion and perspectives
Midostaurin as a single agent appears highly effective in AdSM demonstrating a high rate of response with improved survival, particularly in ASM and MCL, despite no complete response. Although it has few or no impact on the course of the AHNMD (without impairing the response on ASM), it may facilitate its treatment by appropriate therapies. With the limitation of the very low number of cases, midostaurin does not appear very effective in MCS. To improve the response rate, quality of the response, and long-term survival in all subgroups of AdSM, combined therapies (cladribine, 5-azacitidine, decitabine, and chemotherapy)42–46 should be evaluated in clinical trials as well as the role of alloHCT.9
Supplementary material
Table S1.
Response criteria the CPKC412D2201 study
| Response | C-Finding(s) (CF)a | Subcategory | Mast cell infiltrate in organ | Tryptase level | Organo-megaly |
|---|---|---|---|---|---|
| Major responseb | ≥1 CF resolved; and no CF ↑ | Complete remission | Disappeared [and] ↓ <20 ng/ml [and] disappeared | ||
| ≥1 CF resolved; and no CF ↑ | Incomplete remission | Decrease [and/or] ↓ >50% [and/or] ↓ >50% | |||
| ≥1 CF resolved; and no CF ↑ | Pure clinical response | No significant change | ↓ ≤50%–0% | No significant change | |
| Partial responseb | ≥1 CF ↓ by >50%; no CF ↑ | Good partial responsed | N/A | N/A | No significant change |
| ≥1 CF ↓ by >20%–≤50% no CF ↑ | Minor partial response | N/A | N/A | No significant change | |
| No response | CFs ↓ ↑ by +/–0–20% | Stable disease | N/A | N/A | N/A |
| ≥1 CF ↑ by >20% | Progressive disease | N/A | N/A | N/A | |
Notes: Only measurable C-findings were eligible for this study: transfusion-independent and -dependent anemia and thrombocytopenia; neutropenia; liver function abnormalities (increased alanine aminotransferase, aspartate aminotransferase, and/or total bilirubin); hypoalbuminemia; and medically documented weight loss ≥10% in the 6 months prior to the study. Ascites and bone lesions were not permitted as sole C-findings because they were not considered quantifiable.
For major response, partial response, or stable disease, no C-findings can show progression.
Details on how response to transfusion dependence was determined are shown.
Subtype could not be determined due to lack of bone marrow involvement and/or liver/spleen measurement and/or serum tryptase levels.
Minimum increases required for hematologic response: hemoglobin, 1.5 g/dL; absolute neutrophil count, 0.2×109/L; platelets, 20×109/L.
Abbreviations: C, clinical; N/A, not applicable.
Footnotes
Disclosure
Olivier Hermine declares research grants from Novartis, Celgene, Hybrigenics, and Inatherys and is a founder, stockholder, and consultant and received research grant from AB science. The other authors report no conflicts of interest in this work.
References
- 1.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27(7):635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 2.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SS, Skovbo S, Vestergaard H, et al. Epidemiology of systemic mastocytosis in Denmark. Br J Haematol. 2014;166(4):521–528. doi: 10.1111/bjh.12916. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250–262. doi: 10.1002/ajh.23931. [DOI] [PubMed] [Google Scholar]
- 6.Georgin-Lavialle S, Aguilar C, Guieze R, et al. Mast cell sarcoma: a rare and aggressive entity–report of two cases and review of the literature. J Clin Oncol. 2013;31(6):e90–e97. doi: 10.1200/JCO.2012.41.9549. [DOI] [PubMed] [Google Scholar]
- 7.Weiler CR, Butterfield J. Mast cell sarcoma: clinical management. Immunol Allergy Clin North Am. 2014;34(2):423–432. doi: 10.1016/j.iac.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116(26):5812–5817. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 9.Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32(29):3264–3274. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Li CY, Butterfield JH, Hoagland HC. Treatment of systemic mast-cell disease with cladribine. N Engl J Med. 2001;344(4):307–309. doi: 10.1056/NEJM200101253440415. [DOI] [PubMed] [Google Scholar]
- 11.Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102(13):4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- 12.Pardanani A, Hoffbrand AV, Butterfield JH, Tefferi A. Treatment of systemic mast cell disease with 2-chlorodeoxyadenosine. Leuk Res. 2004;28(2):127–131. doi: 10.1016/s0145-2126(03)00185-1. [DOI] [PubMed] [Google Scholar]
- 13.Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84(12):790–794. doi: 10.1002/ajh.21561. [DOI] [PubMed] [Google Scholar]
- 14.Barete S, Lortholary O, Damaj G, et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood. 2015;126(8):1009–1016. doi: 10.1182/blood-2014-12-614743. [DOI] [PubMed] [Google Scholar]
- 15.Delaporte E, Pierard E, Wolthers BG, et al. Interferon-alpha in combination with corticosteroids improves systemic mast cell disease. Br J Dermatol. 1995;132(3):479–482. doi: 10.1111/j.1365-2133.1995.tb08689.x. [DOI] [PubMed] [Google Scholar]
- 16.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28(3):249–257. doi: 10.1016/s0145-2126(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 17.Casassus P, Caillat-Vigneron N, Martin A, et al. Treatment of adult systemic mastocytosis with interferon-alpha: results of a multicentre phase II trial on 20 patients. Br J Haematol. 2002;119(4):1090–1097. doi: 10.1046/j.1365-2141.2002.03944.x. [DOI] [PubMed] [Google Scholar]
- 18.Damaj G, Bernit E, Ghez D, et al. Thalidomide in advanced mastocytosis. Br J Haematol. 2008;141(2):249–253. doi: 10.1111/j.1365-2141.2008.07038.x. [DOI] [PubMed] [Google Scholar]
- 19.Gruson B, Lortholary O, Canioni D, et al. Thalidomide in systemic mastocytosis: results from an open-label, multicentre, phase II study. Br J Haematol. 2013;161(3):434–442. doi: 10.1111/bjh.12265. [DOI] [PubMed] [Google Scholar]
- 20.Gabillot-Carre M, Lepelletier Y, Humbert M, et al. Rapamycin inhibits growth and survival of D816V-mutated c-kit mast cells. Blood. 2006;108(3):1065–1072. doi: 10.1182/blood-2005-06-2433. [DOI] [PubMed] [Google Scholar]
- 21.Parikh SA, Kantarjian HM, Richie MA, Cortes JE, Verstovsek S. Experience with everolimus (RAD001), an oral mammalian target of rapamycin inhibitor, in patients with systemic mastocytosis. Leuk Lymphoma. 2010;51(2):269–274. doi: 10.3109/10428190903486220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Zeng S, Metcalfe DD, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99(5):1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 23.Growney JD, Clark JJ, Adelsperger J, et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106(2):721–724. doi: 10.1182/blood-2004-12-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish network on mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13(2):180–188. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotlib J, Berube C, Growney JD, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106(8):2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleixner KV, Mayerhofer M, Aichberger KJ, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107(2):752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 28.Gleixner KV, Mayerhofer M, Sonneck K, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92(11):1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 29.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 30.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 31.Chandesris MO, Damaj G, Canioni D, et al. Midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2605–2607. doi: 10.1056/NEJMc1515403. [DOI] [PubMed] [Google Scholar]
- 32.Gotlib J, Pardanani A, Akin C, et al. International Working Group-Myelo-proliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121(13):2393–2401. doi: 10.1182/blood-2012-09-458521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalhosa AB, Aouba A, Damaj G, et al. A French national survey on clotting disorders in mastocytosis. Medicine (Baltimore) 2015;94(40):e1414. doi: 10.1097/MD.0000000000001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009;39(11):1711–1720. doi: 10.1111/j.1365-2222.2009.03353.x. [DOI] [PubMed] [Google Scholar]
- 35.Peter B, Winter GE, Blatt K, et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia. 2016;30(2):464–472. doi: 10.1038/leu.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 37.Lortholary O, Chandesris MO, Livideanu CB, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389(10069):612–620. doi: 10.1016/S0140-6736(16)31403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gleixner KV, Mayerhofer M, Cerny-Reiterer S, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and Btk activation and disruption by dasatinib and bosutinib. Blood. 2011;118(7):1885–1898. doi: 10.1182/blood-2010-06-289959. [DOI] [PubMed] [Google Scholar]
- 39.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 40.Jawhar M, Schwaab J, Schnittger S, et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29(5):1115–1122. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
- 41.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 42.Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26(9):2061–2068. doi: 10.1038/leu.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlenk R, Döhner K, Salih H, et al. Midostaurin in combination with intensive induction and as single agent maintenance therapy after consolidation therapy with allogeneic hematopoietic stem cell transplantation or high-dose cytarabine. Blood. 2015;126:322. [Google Scholar]
- 44.Williams CB, Kambhampati S, Fiskus W, et al. Preclinical and phase I results of decitabine in combination with midostaurin (PKC412) for newly diagnosed elderly or relapsed/refractory adult patients with acute myeloid leukemia. Pharmacotherapy. 2013;33(12):1341–1352. doi: 10.1002/phar.1316. [DOI] [PubMed] [Google Scholar]
- 45.Strati P, Kantarjian H, Ravandi F, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276–281. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsingh G, Westervelt P, McBride A, et al. Phase I study of cladribine, cytarabine, granulocyte colony stimulating factor (CLAG regimen) and midostaurin and all-trans retinoic acid in relapsed/refractory AML. Int J Hematol. 2014;99(3):272–278. doi: 10.1007/s12185-014-1503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molinos-Quintana A, Aquino V, Montero I, et al. Emerging BCR/ABL1 mutations under treatment with tyrosine kinase inhibitors in paediatric acute lymphoblastic leukaemia. Acta Haematol. 2015;134(2):71–75. doi: 10.1159/000371831. [DOI] [PubMed] [Google Scholar]
- 48.Damaj G, Joris M, Chandesris O, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9(1):e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30(1):136–143. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 50.Bourget P, Amin A, Chandesris MO, et al. Liquid chromatography-tandem mass spectrometry assay for therapeutic drug monitoring of the tyrosine kinase inhibitor, midostaurin, in plasma from patients with advanced systemic mastocytosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;944:175–181. doi: 10.1016/j.jchromb.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Gotlib JDD, George T, Corless CL, et al. KIT inhibitor midostaurin exhibits a high rate of clinically meaningful and durable responses in advanced systemic mastocytosis: report of a fully accrued phase II trial. Blood. 2010;116:316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Response criteria the CPKC412D2201 study
| Response | C-Finding(s) (CF)a | Subcategory | Mast cell infiltrate in organ | Tryptase level | Organo-megaly |
|---|---|---|---|---|---|
| Major responseb | ≥1 CF resolved; and no CF ↑ | Complete remission | Disappeared [and] ↓ <20 ng/ml [and] disappeared | ||
| ≥1 CF resolved; and no CF ↑ | Incomplete remission | Decrease [and/or] ↓ >50% [and/or] ↓ >50% | |||
| ≥1 CF resolved; and no CF ↑ | Pure clinical response | No significant change | ↓ ≤50%–0% | No significant change | |
| Partial responseb | ≥1 CF ↓ by >50%; no CF ↑ | Good partial responsed | N/A | N/A | No significant change |
| ≥1 CF ↓ by >20%–≤50% no CF ↑ | Minor partial response | N/A | N/A | No significant change | |
| No response | CFs ↓ ↑ by +/–0–20% | Stable disease | N/A | N/A | N/A |
| ≥1 CF ↑ by >20% | Progressive disease | N/A | N/A | N/A | |
Notes: Only measurable C-findings were eligible for this study: transfusion-independent and -dependent anemia and thrombocytopenia; neutropenia; liver function abnormalities (increased alanine aminotransferase, aspartate aminotransferase, and/or total bilirubin); hypoalbuminemia; and medically documented weight loss ≥10% in the 6 months prior to the study. Ascites and bone lesions were not permitted as sole C-findings because they were not considered quantifiable.
For major response, partial response, or stable disease, no C-findings can show progression.
Details on how response to transfusion dependence was determined are shown.
Subtype could not be determined due to lack of bone marrow involvement and/or liver/spleen measurement and/or serum tryptase levels.
Minimum increases required for hematologic response: hemoglobin, 1.5 g/dL; absolute neutrophil count, 0.2×109/L; platelets, 20×109/L.
Abbreviations: C, clinical; N/A, not applicable.