Abstract
Purpose
This study aimed to investigate whether changes in psychosocial factors and pain severity were associated with reduction in disability due to pain among patients with chronic pain. We hypothesized that increased self-efficacy would reduce disability.
Patients and methods
This longitudinal observational study included 72 patients. Patients’ psychological and physical variables were assessed before and after 3 months of treatment. Demographic and clinical information were collected, including the Pain Disability Assessment Scale (PDAS), the Pain Self-Efficacy Questionnaire (PSEQ), the Hospital Depression and Anxiety Scale, and the Numeric Rating Scale (NRS) to assess pain intensity. First, univariate regression analyses were conducted to clarify associations between change in PDAS and sex, age, pain duration, changes in psychosocial factors (self-efficacy, anxiety, and depression) and change in pain intensity. Second, multivariate regression was conducted using the variables identified in the univariate analyses (PSEQ and NRS) to detect the most relevant factor for reducing disability.
Results
Univariate regression analyses clarified that changes in PSEQ (β = −0.31; 95% CI: −0.54–−0.08, p = 0.008) and NRS (β = 0.24; 95% confidence interval [CI]: 0.01–0.47, p = 0.04) were associated with reduction in PDAS. Multivariate regression analysis demonstrated that change in PSEQ (β = 0.26; 95% CI: −0.50–−0.02; p = 0.01) was associated with a reduction in disability, independent of change in NRS.
Conclusion
These findings suggest improved self-efficacy is associated with reduced disability in patients with chronic pain, independent of reduction in pain intensity. Focusing on improvement in self-efficacy may be an effective strategy in chronic pain treatment in addition to pain relief.
Introduction
Chronic pain carries a serious social burden, and is an important global health issue [1] [2] [3]. For many individuals, chronic pain interferes with social life, especially because it negatively impacts activities of daily living (ADL) and health-related quality of life (QOL) [4] [5]. Moreover, chronic pain bears a high economic cost [6].
Chronic pain accompanied by negative psychosocial factors often hinders healthcare providers in achieving treatment goals. For example, the fear-avoidance model explains that negative psychosocial factors (e.g., catastrophizing, fear, and depression) have a possible role in mediating disability in patients with chronic pain [7]. Although medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are often used to eliminate pain, it is difficult to improve the psychosocial factors associated with chronic pain by medication only. In addition, NSAIDs often cause adverse gastrointestinal and cardiovascular events [8] [9]. Opioids also cause various adverse events, and over-use of opioids for non-cancer pain may result in narcotics addiction and induce an opioid crisis [10]. Completely eliminating pain is not a feasible strategy in chronic pain treatment; for example, Thomas and Lee noted that “zero pain is not the goal” [11]. Improvement in ADL should be a target in chronic pain treatment. Improving both ADL and QOL using a multimodal approach that includes rehabilitation, exercise therapy, cognitive behavioral therapy, and mindfulness is recommended in several chronic pain treatment guidelines [12] [13].
Several physical and psychological factors are thought to contribute to enhancing disability in patients with chronic pain. Pain intensity is related to physical activity [14] and is also reported to have a negative association with return to work in patients with chronic low back pain [15]. Depression is a common comorbid psychological condition in those with chronic pain, and is known to be a significant determinant of pain-related disability [14]. Anxiety often leads patients with chronic pain to avoid activities that can exacerbate pain and worsen disability [16]. Pain-related self-efficacy has been found to affect pain severity, negative psychological factors, and disability related to low back and shoulder pain [17] [18] [19]. Self-efficacy is also reported to mediate the relationship between pain intensity and disability [20] [21]. Moreover, increased self-efficacy was a significant predictor of positive change in health status through self-management programs among patients with arthritis [22]. To our knowledge, few cross-sectional studies have investigated the direct association between self-efficacy and disability in patients with chronic pain [23], and no longitudinal studies have been conducted in Asia, including Japan. A meta-analysis integrating the results of 83 studies that were conducted outside Japan showed that self-efficacy was associated with pain-related outcomes such as impairment, affective distress, and pain severity in various types of painful diseases [24]. The meta-analysis reported that several studies had longitudinally investigated the relationship between self-efficacy and disability among patients with specific site pain, such as low back or arthritis pain [17] [18] [19]. However, no studies targeted chronic pain, including neuropathic pain, headache, orofacial pain, and visceral pain. However, there were no studies that targeted chronic pain, including neuropathic pain, headache, orofacial pain, and visceral pain. For research purposes, chronic pain is usually categorized by pain site or cause of pain. However, widespread pain and duplicate causes of pain are likely to be misclassified in such categorization. Regardless of pain site or cause of pain, subjective persistent pain itself has a pathological meaning. Therefore, evaluation of all-inclusive chronic pain is required for comprehensive research. The present study aimed to examine the association between change in psychosocial factors and pain severity with reduction in disability among patients with chronic pain in a Japanese clinical pain care setting using a longitudinal approach.
Methods
Participants
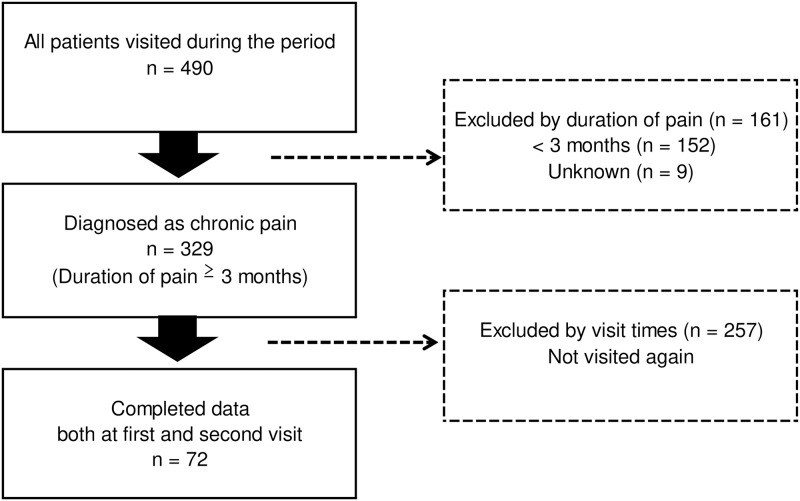
We identified 490 outpatients from March 2016 to December 2016 in a pain clinic (Fig 1).
Fig 1. Diagram of the participant selection process.
Individuals were eligible for participation if they: 1) were aged 20 years or older; 2) were able to read, write, and understand questionnaires written in Japanese; and 3) had persistent pain for at least 3 months. Participants completed four measures: the Pain Disability Assessment Scale (PDAS) [25], the Pain Self-Efficacy Questionnaire (PSEQ) [26], the Hospital Depression and Anxiety Scale (HADS) [27], and a Numeric Rating Scale (NRS) [28]. These measures were completed at pre- and post-treatment (3 months after the initial visit) evaluations. We did not control therapeutic interventions in the present study. In addition, we did not use a standardized treatment protocol, and physicians selected the most suitable therapy for each patient. Physicians conducted flexible treatment, which was based on standard clinical guidelines for chronic pain in Japan [13]. Treatment options included pharmacotherapy, interventional management, psychological approach, and rehabilitation [13]. The questionnaires were completed in the pain clinic waiting room. Demographic data, medical history, duration of pain, and diagnosis were collected from participants’ medical records. Physicians specialized in pain management categorized each participant using pain classifications of the International Classification of Diseases, 11th revision [29]. Of 490 identified patients, 161 with pain duration less than 3 months or unknown duration were excluded. A further 257 patients who did not visit the clinic again were also excluded. This left 72 participants (33 men and 39 women) with complete data on all variables for inclusion in the study. In this study, we did not identify reasons why more than half of the patients had not visited our clinic again at 3 months after their first visit. We assume that there may be various reasons for this, including: 1) patients improved and did not need any further medical care at 3 months; 2) patients improved and their physician referred them to a primary care doctor during the 3-month study period; and 3) patients discontinued therapy without notice.
Ethical considerations
This study was approved by the Institutional Review Board for Clinical Research of Juntendo University Hospital (IRB No.17-234). Informed consent was obtained from all participants before they completed the measures.
Measures
Pain-related disability
The PDAS is a 20-item tool that assesses the degree of impact of pain-related disability on a person’s lifestyle during the past week [25]. Respondents are asked to rate each activity on a Likert scale from 0 (“Pain did not interfere with this activity”) to 3 (“Pain completely interfered with this activity”) [25]. Total scores range from 0 to 60, with higher scores indicating greater levels of pain interference [25].
Pain-related self-efficacy
The PSEQ is a measure of generalized pain-related self-efficacy and assesses the degree of an individual’s confidence in performing a number of activities despite pain [26]. Each item is rated on a 7-point Likert scale from 0 (“not at all confident”) to 6 (“completely confident”). Total scores can range from 0 to 60, with higher scores indicating greater self-efficacy for performing despite pain. A previous study reported that a score over 40 points was associated with work restoration and functional improvement in patients with chronic upper limb pain, although a clear cut off value representing recovery from chronic pain has not yet been defined [30].
Anxiety and depression
The HADS is a self-report measure that was originally developed to assess anxiety and depression in clinical populations [27]. It comprises 14 items on two 7-item subscales (depression and anxiety), with each item rated on a scale from 0 to 3 according to how respondents’ felt recently. Respondents are scored from 0 and 21 for each subscale. A systematic review that included a large number of studies identified a cut-off point of 8 (of 21) as indicating possible anxiety or depression [31].
Pain intensity
The NRS is a common scale for quantifying pain, and is anchored by 0 (“no pain”) and 10 (“worst pain”) [28]. The scale is used for recording average pain intensity. The scores participants reported for pain experienced in the past 24 hours were used in this study [28].
Statistical analysis
Statistical analyses were performed using EZR version 1.27 [32]. Participants’ demographic data were reported as means and standard deviations (SD), with the proportion of each classification as a percentage. Changes in each score at the first and second visits were analyzed using paired t-tests.
Univariate regression analyses were conducted to investigate which variables showed single associations with disability. Change in PDAS was considered an objective variable, with changes in PSEQ, HADS-Anxiety, HADS-Depression, and NRS considered as explanatory variables. Age, sex, and pain duration were variables used for adjustment. Subsequently, multivariate regression analysis was conducted to detect the most relevant factor for improving physical disability, using the identified explanatory variables. All statistical inferences were based on a significance level of P<0.05 (two-tailed tests).
Results
Participants’ characteristics
Demographic variables are shown in Table 1.
Table 1. Participants’ characteristics.
| Variables | |
| Number of patients (Men/Women) | 72 (33/39) |
| Age, years [mean (SD)] | 65.2 (14.8) |
| Duration of disease, months [mean (SD)] | 52.6 (86.4) |
| Classification of chronic pain for ICD-11 candidate | Number (%) |
| 1.Primary Pain | 7 (9.7) |
| 2.Cancer Pain | 2 (2.8) |
| 3.Postsurgical and Posttraumatic Pain | 9 (12.5) |
| 4.Neuropathic Pain | 45 (62.5) |
| 5.Headache and Orofacial Pain | 1 (1.4) |
| 6.Viscaral Pain | 1 (1.4) |
| 7.Muscloskeletal Pain | 7 (9.7) |
SD, standard deviation.
Changes in measures
All measures were significantly improved at the post-treatment evaluation (3 months after the initial visit) (Table 2).
Table 2. Changes in pain-related assessments at pre- and post-treatment.
| Pre treatment | Post treatment | P-value | |
|---|---|---|---|
| PDAS | 23.68 (13.71) | 19.81 (13.99) | <0.01 |
| PSEQ | 32.00 (17.59) | 36.60 (15.03) | <0.01 |
| HADS Anxiety | 6.36 (4.32) | 4.78 (3.66) | <0.01 |
| HADS Depression | 6.74 (4.88) | 5.54 (3.95) | 0.02 |
| NRS | 5.56 (2.26) | 4.26 (2.35) | <0.01 |
Data presented as mean (standard deviation), PDAS; Pain Disability Assessment Scale, PSEQ; Pain Self-efficacy Questionnaire, HADS; Hospital Anxiety and Depression Scale, NRS; Numeric Rating Scale,
Univariate regression analyses
Changes in both the PSEQ (β = −0.31; 95% CI: −0.54–−0.08) and NRS (β = 0.24; 95% confidence interval [CI]: 0.01–0.47) were significantly associated with change in PDAS. Changes in HADS depression and HADS anxiety scores were not significantly associated with PDAS (Table 3).
Table 3. Univariate regression analysis examining predictors of change in disability.
| β (95% CI) | R2 | Fscore | p value | |
|---|---|---|---|---|
| Dependent = ΔPDAS | ||||
| Sex | 0.11 (-0.13–0.35) | 0.01 | 0.9 | 0.35 |
| Age | -0.02 (-0.26–0.22) | <0.001 | 0.02 | 0.89 |
| Duration | 0.03 (-0.20–0.27) | 0.001 | 0.1 | 0.78 |
| ΔPSEQ | -0.31 (-0.54–0.08)** | 0.10 | 7.5 | 0.008 |
| ΔHADS Anxiety | 0.20 (-0.03–0.43) | 0.04 | 2.9 | 0.09 |
| ΔHADS Depression | 0.18 (-0.06–0.41) | 0.03 | 2.3 | 0.14 |
| ΔNRS | 0.24 (0.01–0.47)* | 0.24 | 4.2 | 0.04 |
n = 72;
*p<0.05,
**p<0.01
β; standardized regression coefficient, CI; confidence interval.
Multivariate regression analyses
We used changes in PSEQ and NRS as explanatory variables in the multivariate regression analysis as an additional step to identify the most relevant factor. PSEQ (β = 0.26; 95%CI: −0.50–0.02) was associated with reduction in PDAS (R2change = 0.12, Fchange = 4.5). There was no association between NRS and PDAS (Table 4).
Table 4. Multivariate regression analysis examining predictors of change in disability.
| β (95% CI) | R2change | Fchange | p | |
|---|---|---|---|---|
| Dependent = ΔPDAS | 0.12 | 4.5 (2,69) | 0.01 | |
| ΔPSEQ | -0.26 (-0.50–0.02)* | |||
| ΔNRS | 0.15 (-0.09–0.39) |
n = 72;
*p<0.05
β; standardized regression coefficient from the each step equation, CI; confidence interval.
Discussion
There are no detailed data regarding the reason why more than half of patients did not visit our clinic again on post-treatment phase. Therefore, we cannot tell how many patients were improved and finished treatment or not improved and stopped treatment.
There were several reasons including patient’s wish, introduction to other medical institutions based on patient’s signs and symptoms, and no need for revisit because of mild pain.
We found that changes in anxiety and depression were not associated with disability, but a change in self-efficacy was associated with a reduction in disability, independent of pain severity. These findings suggest that improving self-efficacy may be an effective strategy to reduce disability among patients with chronic pain. This was consistent with the results of a previous cross-sectional study that revealed a significant association between self-efficacy and disability among Japanese patients with chronic pain [23]. The present study included Japanese outpatients with various types of chronic pain (e.g., neuropathic pain, headache, orofacial pain and visceral pain). We found that self-efficacy was a predictor of reduced disability. This was consistent with the results from previous longitudinal studies targeting musculoskeletal pain (e.g., chronic low back and shoulder pain) [17] [18] [19]. However, a 5-year follow-up study involving patients with chronic back pain showed other factors can be predictors of future disability, including sex (being female), duration of episode, and pain frequency [33]. A previous study among people with chronic low back pain reported that being a woman, pain duration, lower exercise level, and higher pain frequency appeared to play major roles in predicting disability at the 5-year follow-up [33]. In our study, sex differences and pain duration did not contribute to change in disability at the 3-month follow-up. The inconsistencies between the two studies might be attributable to differences in the follow-up period (5 years vs. 3 months) and pain site (low back vs. whole body).
Although numerous studies have reported associations between anxiety/depression and major treatment outcomes in chronic pain, such associations were not detected in the present study. This may be explained by participants in our study having less severe anxiety and depression symptoms. The mean HADS anxiety and depression scores were below the recommended cut-off values for screening anxiety and depression [27]. Although we did not control for medication, any medications (e.g., antidepressants and anxiolytics) taken before participating in this study might have reduced participants’ anxiety and depression. Further research is needed to clarify the influence of medications, especially those that affect the central nervous system.
Previous research reported that increased pain-related self-efficacy was a protective factor that helped prevent development of chronic pain [34] and had a positive influence on treatment adherence behavior [35]. These may be potential explanatory mechanisms in this study. Some previous studies demonstrated that cognitive behavioral therapy and interdisciplinary pain rehabilitation programs improved both self-efficacy and depression in patients with chronic pain [36], and suggested that activity pacing and interviews for enhancing motivation should be incorporated into clinical practice as effective therapeutic interventions [37] [38]. We did not focus on psychotherapy specifically targeting psychological factors such as self-efficacy. Therefore, it was unclear if our therapy directly affected patients’ improvement in self-efficacy. We conducted standard therapies that targeted pain reduction and improvement of ADL, according to clinical practice guidelines for chronic pain in Japan. However, there were no specific protocols for treatment. In the present study, we only described observational results and therefore cannot comment on the effectiveness of any specific therapies. Further studies are needed to identify which therapies are effective to maintain and improve self-efficacy beliefs.
Interestingly, pain duration and pain intensity were not associated with improvement of disability in this study. Improved self-efficacy may mean that patients, even those with severe persistent pain, may possibly get better and be able to maintain their social life. Measuring self-efficacy by changes in PSEQ scores may help healthcare providers manage patients’ self-efficacy in clinical practice. Our findings suggest that physicians should focus on patients’ self-efficacy as well as pain intensity, as self-efficacy may contribute to improvement in the ADL of patients with chronic pain, which in turn may help them return to their social activities. Development of therapies targeting improvement of self-efficacy may therefore be beneficial.
There were several limitations in the present study. First, this study was conducted in a single-center. A multi-centered study is needed to avoid a bias in terms of patients’ background. Second, we did not stratify patients by different chronic pain diagnoses because the number of participants was small. Third, we excluded patients who did not visit our clinic again, which might have resulted in a selection bias. More than half of the patients in the present study had not visited our clinic again at 3 months after their first visit, which might have introduced section bias. Our data did not include patients who were dissatisfied with treatment and discontinued visits to the clinic. Patients who had visited the clinic for 3 months or more were usually satisfied with their current treatment. Those patients might have shown more improvement in self-efficacy than patients who were dissatisfied with their current treatment. Fourth, this study investigated relatively few factors over a short-term (3-month) study period. Further studies are needed that explore more related factors over a longer period. Finally, we did not control therapeutic interventions, and did not use specific interventions targeting psychological factors (e.g., self-efficacy). Patients’ self-efficacy might have improved independent of therapy.
Conclusion
Our findings provide evidence that higher levels of self-efficacy are highly correlated with greater improvements in pain-related disability. In addition to pain relief, raising self-efficacy may be a primary target in chronic pain treatment.
Acknowledgments
The authors are grateful to all participants in this study. The authors would like to thank all staff from the Pain Clinic at Juntendo University Hospital for help with data collection. We also thank Audrey Holmes, MA, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Data Availability
Data cannot be shared publicly because data contain potentially identifying or sensitive patient information. Based on regulations for ethical guidelines in Japan, the Institutional Review Board for Clinical Research of Juntendo University Hospital imposed restrictions on the data collected in this study. All enquiries should be addressed to the Data Management Committee via email: kenkyu5858@juntendo.ac.jp.
Funding Statement
Pfizer provided support in the form of salaries for authors (YK and MY), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors (YK and MY) are articulated in the ‘Author contributions’ section.
References
- 1.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. Jama. 2003;290(18):2443–54. Epub 2003/11/13. 10.1001/jama.290.18.2443 . [DOI] [PubMed] [Google Scholar]
- 2.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis—an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(4):415–22. Epub 2008/02/26. 10.1016/j.joca.2007.12.017 . [DOI] [PubMed] [Google Scholar]
- 3.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. Jama. 2008;299(6):656–64. Epub 2008/02/14. 10.1001/jama.299.6.656 . [DOI] [PubMed] [Google Scholar]
- 4.Kingsbury SR, Gross HJ, Isherwood G, Conaghan PG. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology. 2014;53(5):937–47. 10.1093/rheumatology/ket463 [DOI] [PubMed] [Google Scholar]
- 5.March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best practice & research Clinical rheumatology. 2014;28(3):353–66. Epub 2014/12/08. 10.1016/j.berh.2014.08.002 . [DOI] [PubMed] [Google Scholar]
- 6.Gaskin DJ, Richard P. The economic costs of pain in the United States. The journal of pain: official journal of the American Pain Society. 2012;13(8):715–24. Epub 2012/05/23. 10.1016/j.jpain.2012.03.009 . [DOI] [PubMed] [Google Scholar]
- 7.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–32. Epub 2000/04/27. . [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Díaz S, Rodríguez L. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: An overview of epidemiologic studies published in the 1990s. Archives of Internal Medicine. 2000;160(14):2093–9. 10.1001/archinte.160.14.2093 [DOI] [PubMed] [Google Scholar]
- 9.Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. The Lancet. 2013;382(9894):769–79. 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadivelu N, Kai AM, Kodumudi V, Sramcik J, Kaye AD. The Opioid Crisis: a Comprehensive Overview. Current pain and headache reports. 2018;22(3):16 Epub 2018/02/25. 10.1007/s11916-018-0670-z . [DOI] [PubMed] [Google Scholar]
- 11.Lee TH. Zero Pain Is Not the Goal. Jama. 2016;315(15):1575–7. Epub 2016/03/16. 10.1001/jama.2016.1912 . [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–30. Epub 2017/02/14. 10.7326/M16-2367 . [DOI] [PubMed] [Google Scholar]
- 13.Clinical Practice Guideline for Chronic Pain. First Edition ed Japan: Shinko Trading Co. Ltd.; 2018. [Google Scholar]
- 14.Tripp DA, VanDenKerkhof EG, McAlister M. Prevalence and Determinants of Pain and Pain-Related Disability in Urban and Rural Settings in Southeastern Ontario. J Pain Research and Management. 2006;11(4). 10.1155/2006/720895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steenstra IA, Munhall C, Irvin E, Oranye N, Passmore S, Van Eerd D, et al. Systematic Review of Prognostic Factors for Return to Work in Workers with Sub Acute and Chronic Low Back Pain. Journal of occupational rehabilitation. 2017;27(3):369–81. Epub 2016/09/21. 10.1007/s10926-016-9666-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RM, Kaiser RS. Psychological Characteristics of Chronic Pain: a Review of Current Evidence and Assessment Tools to Enhance Treatment. Current pain and headache reports. 2018;22(3):22 Epub 2018/03/16. 10.1007/s11916-018-0663-y . [DOI] [PubMed] [Google Scholar]
- 17.Campbell P, Foster NE, Thomas E, Dunn KM. Prognostic indicators of low back pain in primary care: five-year prospective study. The journal of pain: official journal of the American Pain Society. 2013;14(8):873–83. Epub 2013/06/25. 10.1016/j.jpain.2013.03.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chester R, Jerosch-Herold C, Lewis J, Shepstone L. Psychological factors are associated with the outcome of physiotherapy for people with shoulder pain: a multicentre longitudinal cohort study. Br J Sports Med. 2018;52(4):269–75. Epub 2016/07/23. 10.1136/bjsports-2016-096084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster NE, Thomas E, Bishop A, Dunn KM, Main CJ. Distinctiveness of psychological obstacles to recovery in low back pain patients in primary care. Pain. 2010;148(3):398–406. Epub 2009/12/22. 10.1016/j.pain.2009.11.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa Lda C, Maher CG, McAuley JH, Hancock MJ, Smeets RJ. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. European journal of pain (London, England). 2011;15(2):213–9. Epub 2010/07/27. 10.1016/j.ejpain.2010.06.014 . [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Hubscher M, Moseley GL, Kamper SJ, Traeger AC, Mansell G, et al. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. 2015;156(6):988–97. Epub 2015/03/12. . [DOI] [PubMed] [Google Scholar]
- 22.Osborne RH, Wilson T, Lorig KR, McColl GJ. Does self-management lead to sustainable health benefits in people with arthritis? A 2-year transition study of 452 Australians. The Journal of rheumatology. 2007;34(5):1112–7. Epub 2007/03/09. . [PubMed] [Google Scholar]
- 23.Adachi T YK, Nishigami T, Sasaki J, Shibata M. Effects of pain self-efficacy and other cognitive-emotional factors on Health-related Quality of Life, and Pain Interference in Japanese chronic pain patients. The Journal of the Japanese Society for the Study of Chronic Pain. 2015;34:107–12. [Google Scholar]
- 24.Jackson T, Wang Y, Wang Y, Fan H. Self-efficacy and chronic pain outcomes: a meta-analytic review. The journal of pain: official journal of the American Pain Society. 2014;15(8):800–14. Epub 2014/06/01. 10.1016/j.jpain.2014.05.002 . [DOI] [PubMed] [Google Scholar]
- 25.Arimura T, Komiyama H M H. Pain disability assessment scale: a simplified scale for clinical use. Jpn J Behav Ther. 1997;23:7–15. [Google Scholar]
- 26.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. European journal of pain (London, England). 2007;11(2):153–63. Epub 2006/02/01. 10.1016/j.ejpain.2005.12.008 . [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. Epub 1983/06/01. . [DOI] [PubMed] [Google Scholar]
- 28.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378–81. Epub 1978/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7. Epub 2015/04/07. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JH, Williams AC. What affects return to work for graduates of a pain management program with chronic upper limb pain? Journal of occupational rehabilitation. 2003;13(2):91–106. Epub 2003/04/24. . [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of psychosomatic research. 2002;52(2):69–77. Epub 2002/02/08. . [DOI] [PubMed] [Google Scholar]
- 32.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. Epub 2012/12/05. 10.1038/bmt.2012.244 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enthoven P, Skargren E, Carstensen J, Oberg B. Predictive factors for 1-year and 5-year outcome for disability in a working population of patients with low back pain treated in primary care. Pain. 2006;122(1–2):137–44. Epub 2006/03/11. 10.1016/j.pain.2006.01.022 . [DOI] [PubMed] [Google Scholar]
- 34.Bérubé M, Choinière M, Laflamme YG, Gélinas C. Acute to chronic pain transition in extremity trauma: A narrative review for future preventive interventions (part 2). International Journal of Orthopaedic and Trauma Nursing. 2017;24:59–67. 10.1016/j.ijotn.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Thompson EL, Broadbent J, Bertino MD, Staiger PK. Do Pain-related Beliefs Influence Adherence to Multidisciplinary Rehabilitation?: A Systematic Review. 2016;32(2):164–78. 10.1097/ajp.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 36.Nash VR, Ponto J, Townsend C, Nelson P, Bretz MN. Cognitive behavioral therapy, self-efficacy, and depression in persons with chronic pain. Pain Manag Nurs. 2013;14(4):e236–e43. Epub 2013/01/01. 10.1016/j.pmn.2012.02.006 . [DOI] [PubMed] [Google Scholar]
- 37.Nielson WR, Jensen MP, Karsdorp PA, Vlaeyen JW. Activity pacing in chronic pain: concepts, evidence, and future directions. The Clinical journal of pain. 2013;29(5):461–8. Epub 2012/12/19. 10.1097/AJP.0b013e3182608561 . [DOI] [PubMed] [Google Scholar]
- 38.Jensen MP. Enhancing motivation to change in pain treatment Psychological approaches to pain management: A practitioner's handbook. New York, NY, US: Guilford Press; 1996. p. 78–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because data contain potentially identifying or sensitive patient information. Based on regulations for ethical guidelines in Japan, the Institutional Review Board for Clinical Research of Juntendo University Hospital imposed restrictions on the data collected in this study. All enquiries should be addressed to the Data Management Committee via email: kenkyu5858@juntendo.ac.jp.