Abstract
Management of diabetes insipidus (DI) is usually facilitated by an intact thirst mechanism prompting water ingestion in times of rising osmolality. Maintenance of eunatremia can be quite difficult in patients with DI and adipsia because of the absence of this homeostatic mechanism. Few published protocols for management of these complex cases exist. We report a case of a 16-year-old girl who had a diagnosis of craniopharyngioma with preoperative hypopituitarism and central DI. She underwent transsphenoidal resection in 2013 and additionally developed postoperative cognitive impairment and hypothalamic dysfunction, including adipsia. She subsequently experienced widely dysregulated sodium levels, necessitating inpatient care ∼30% of days in 2014 and 2015. We created a protocol for this patient that uses a fixed daily dose of subcutaneous DDAVP combined with daily modulation of fluid intake based on daily serum sodium measurement. The protocol provides guidance for the day’s fluid intake based on both the current sodium result and the rate of change from the previous day. Since the adoption of the protocol in June 2016, the patient has had a dramatic reduction in hospitalizations. Use of a protocol for providing recommendations for fluid intake based on the sodium level and rate of change may help to maintain normal sodium levels in such patients, decreasing hospitalization and improving quality of life.
Keywords: adipsia, diabetes insipidus, sodium, fluid intake
Extracellular osmolality is tightly regulated in the human body under two principal mechanisms: arginine vasopressin (AVP) and thirst. AVP is produced in the supraoptic and paraventricular nuclei of the hypothalamus and is stored in the posterior pituitary. Rising osmolality triggers release of AVP, which promotes water conservation at the kidney and also generates a sensation of thirst [1]. Although the thirst mechanism is spared in most cases of diabetes insipidus (DI), because the structures involved in thirst and AVP production are anatomically proximate, an insult to one could damage the other as well, resulting in both central DI and adipsia. Adipsic DI can be catastrophic because these patients have no drive toward fluid intake to compensate for hyperosmolar states, and severe hypernatremia can develop. Fortunately, this condition is rare, with only ∼100 reports of adipsic DI in the literature [2]. Patients who have undergone craniopharyngioma resection are disproportionately affected [3].
1. Case Presentation
In February 2012, an academically high-performing 16-year-old woman sustained a minor head injury while playing basketball. She had a history of primary amenorrhea that had not been further evaluated, but she was otherwise healthy. On evaluation with MRI, she was incidentally noted to have a sellar mass measuring 5 × 3.5 × 2.8 cm without noted involvement of the hypothalamus. She was found to have hypogonadotropic hypogonadism and subsequently developed secondary adrenal insufficiency and DI. Because of progressive visual field deficits, she underwent transsphenoidal resection of the sellar mass in July 2013. Pathology demonstrated adamantinomatous craniopharyngioma. Postoperatively, she had severe complications including meningitis, panhypopituitarism, ongoing DI, hypothermia, polyphagia, adipsia, and cognitive deficits that later prevented self-management of her condition.
She needed prolonged hospitalization and rehabilitation and, after returning home, needed frequent rehospitalization for both hypernatremia and hyponatremia. Efforts to track and match intake with output were unsuccessful because of urinary incontinence. In 2014, the patient was an inpatient in an acute care hospital for 99 of 365 (27%) days, and in 2015 she was an inpatient for 120 of 365 (33%) days. It was suggested to the patient’s family that they consider permanent hospitalization in a long-term acute care hospital or transition to palliative care.
The patient entered the care of our institution in November 2015, after having been hospitalized at an outside facility nearly continuously for several months. As before, discharge home necessitated daily sodium monitoring; any attempts at less frequent monitoring (checks 2 to 5 times weekly) led to readmission. Similarly, adjusting the dosage of DDAVP on a day-to-day basis resulted in widely variable sodium levels and recurrent hospital admission. Therefore, for several months the on-call endocrinology team reviewed daily sodium values (drawn via central venous port access) and advised on fluid intake based on clinical judgement. This process revealed that fluid intake changes much smaller than would be predicted by standard free water deficit calculations resulted in substantial changes in her sodium levels. It was also noted that the amount of fluid needed to maintain eunatremia fluctuated gradually over time (days to weeks) without clear cause, and she demonstrated occasional abrupt changes in sodium levels. In May 2016, to facilitate more uniform management, these empiric data were used to create a protocol to accommodate both patterns of fluctuation (Table 1).
Table 1.
Protocol Devised to Determine Day-to-Day Fluid Intake
| Sodium (mEq/L) | Δ From Previous Day (mEq/L) | Oral Fluid Dosage Adjustment |
|---|---|---|
| >149 | n/a | Seek emergency care |
| 148––149 | n/a | 1× bolus of 300 mL+ maintenancea |
| 146––147 | n/a | 1× bolus: 200 mL + maintenancea |
| 142–145 | ↑3–4 | Increase maintenance by 200 mL |
| Δ0–2 | No change | |
| ↓3–4 | Decrease maintenance by 100 mL | |
| 139–141 | ↑3–4 | Increase maintenance by 100 mL |
| Δ0–2 | No change | |
| ↓3–4 | Decrease maintenance by 100 mL | |
| 135–138 | ↑3–4 | Increase maintenance by 100 mL |
| Δ0–2 | No change | |
| ↓3–4 | Decrease maintenance by 200 mL | |
| 132–134 | n/a | 1× decrease in maintenance of 300 mLa |
| 130–131 | n/a | 1× decrease in maintenance 400 mLa |
| <130 | n/a | Seek emergency care |
Abbreviation: n/a, not applicable.
Home care team to consider possible etiologies and contact the clinic or seek emergency care if concerning symptoms are present. If clinically stable and sodium is improving the next day, continue to follow the protocol. Once the sodium result falls between 135 and 145 mEq/L, resume the previous maintenance dose without any rate-of-change adjustment. If sodium is not improving the next day, the on-call endocrinology team will recommend a fluid dose based on clinical judgment.
The patient takes a fixed dose of subcutaneous DDAVP (1 μg) twice daily. A protocol recommends a running daily “maintenance” dose of oral fluid that is constantly changing according to the sodium levels. On days that her serum sodium falls within the normal range, the maintenance dose is titrated based on the serum sodium result and the rate and direction of change compared with the previous day. This method addresses the gradual fluctuations in fluid requirements. On days that her serum sodium falls outside the normal range, the protocol makes recommendations for a one-time change to the current maintenance fluid dose. This one-time change is expected to correct the sodium to normal, at which point the maintenance dose can be resumed (no rate-of-change adjustment is incorporated because the fall or rise is attributable to the one-time change in fluid intake). This method provides a stepwise approach to addressing hypernatremia and hyponatremia by first correcting the sodium back into the normal range, where ongoing fine-tuning of the maintenance dose can then occur. Doing so avoids changing her ongoing maintenance fluid dose in response to an acute event and risking overcorrection or cycling hyponatremia and hypernatremia.
Although the protocol could theoretically be followed independently by her in-home care team, because of delegation restrictions, her nurses relay the sodium result daily to the on-call endocrinology fellow, who advises on fluid intake per the protocol each day.
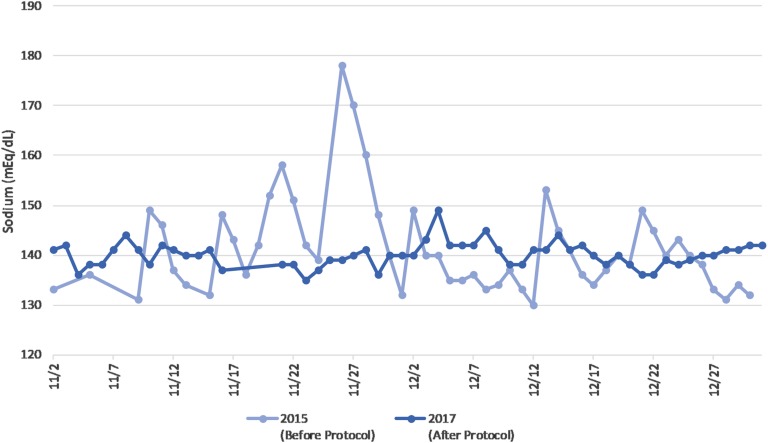
Over the past year, the patient’s sodium level has been relatively stable and has ranged from 129 to 148 mEq/L, although she has had fluctuations of up to 11 mEq/L between sodium measurements on two sequential days. Figure 1 illustrates the relative stability in sodium seen during use of the protocol, as compared with her erratic baseline 2 years earlier. Her prescribed fluid intake has ranged from 800 to 2600 mL/d. In the year after institution of the protocol, the patient was hospitalized <5% of days.
Figure 1.
Sodium variability before and after protocol institution.
2. Discussion
Over the past two decades, recommendations for management of adult adipsic DI have largely followed tenets originally described by Ball et al. in 1997 [4], and these guidelines continue to be referenced in the literature as the mainstay of therapy [1, 5, 6]. These recommendations emphasize the following general approach:
Monitor fluid intake closely.
Replace native desmopressin with DDAVP and titrate the dosage to achieve urine output of ∼1.5 to 2 L daily.
Measure patient weight when eunatremic to determine target weight, and then daily.
Recommend intake of ∼1.5 to 2 L of fluid daily; titrate as appropriate to ascertain a maintenance dose.
If patient is under the target weight on a given day, subtract the current weight from the weight (in kilograms) and add the equivalent volume (in liters) to the fluid recommendation for the day.
Increase fluid intake in times of increased exercise or increased ambient temperature.
Monitor sodium regularly.
In providing conceptual guidance rather than specific numeric recommendations, these guidelines appropriately acknowledge that an individualized approach to therapy may be needed.
We performed a literature search and found reports of patients for whom the above protocol had limitations. Modawi et al. [7] described a patient whose central DI was caused by astrocytoma. Because of persistent tumor, the risk of hyponatremia with resultant cerebral edema was deemed unacceptably high; thus, he was not treated with DDAVP. His DI was successfully managed with a purely weight-based protocol guiding fluid intake. Hameed et al. [8] reported on a pediatric patient (age 16 months) whose sodium levels varied dramatically, with both hypernatremia and hyponatremia. This fluctuation was thought to be partially related to an infantile drive to drink that is motivated not only by thirst but by hunger. The patient was successfully treated with a fixed daily dose of DDAVP, daily sodium monitoring, and a fluid prescription that was determined daily based on her calculated free water deficit.
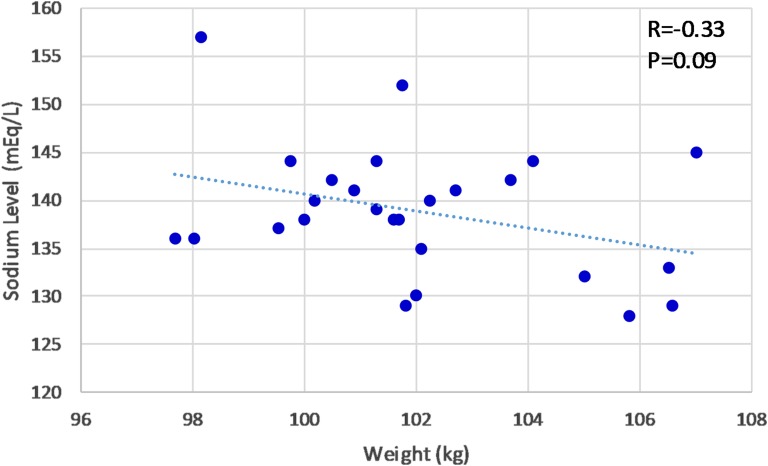
Several factors made following the mainstay published protocol challenging in our case. First, incontinence makes it difficult to measure our patient’s urine output accurately in the home setting. Second, the patient appears to have complete adipsia, in contrast to patients with partial adipsia who have an osmolar setpoint that will eventually trigger thirst with a strong enough degree of hyperosmolality and are thus protected against extreme hypernatremia. Finally, her daily weights and sodium levels are not closely correlated, as shown in Fig. 2. Although there is a nonsignificant trend toward lower sodium concentrations with higher weights, large day-to-day variation is present, and she does not demonstrate a stable “eunatremic weight.” As indicated in Fig. 2, with a sodium level of 140 mEq/L, her weight was 100.2 kg on one day and 102.2 kg on another; sodium values associated with this same weight range varied from 130 to 152 mEq/L.
Figure 2.
Patient weight vs sodium concentration.
The cause of this variation is probably multifactorial. Her hypothalamic dysfunction has led to eating habits that cause difficulty in maintaining a stable weight. Also, fluctuating substantial lower extremity edema leads to changes in weight that reflect variance in volume status rather than free water excess or deficit. The patient also engages in regular physical activity, which may contribute to increased insensible fluid losses on certain days. Finally, coexisting type 2 diabetes mellitus is a complicating factor, because hyperglycemia causes water shifts into the extracellular space, resulting in a decrease in the measured sodium concentration that does not represent total body free water excess. However, problems with management of DI persist even when her diabetes mellitus is well controlled, so this is less likely to be a major contributor in this case.
The patient has had a dramatic improvement in her health and quality of life since institution of her daily sodium monitoring with fixed-dose DDAVP administration. This type of protocol may be helpful in improving quality of life for other patients with adipsic DI. The actual fluid recommendation according to the protocol would need to be individualized for each patient based on his or her individual response to fluid. It might also incorporate daily weights and intake/output measurements, if possible, to decrease the frequency of sodium sampling. This case demonstrates the benefit of tailoring individual management for patients with adipsic DI and represents proof of concept that following a fluid intake protocol can improve management of DI complicated by adipsia, even in pathology so severe that it had been thought to necessitate indefinite hospitalization for a young woman.
Acknowledgments
Many thanks to the University of Wisconsin fellows who have managed this protocol: Drs. Sindhura Bandaru, Heenam Goel, Brad Julius, Kristin Stevenson, Tanya Blaty, Rowan Karaman, Andy Cheng, Marcos Lamas, Rahul Sharma, Preaw Hanseree, and Marita Fisher. Thanks to Dr. Raquel Tatar for assistance with statistical analysis.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AVP
arginine vasopressin
- DI
diabetes insipidus
References and Notes
- 1. Cuesta M, Hannon MJ, Thompson CJ. Adipsic diabetes insipidus in adult patients. Pituitary. 2017;20(3):372–380. [DOI] [PubMed] [Google Scholar]
- 2. Eisenberg Y, Frohman LA. Adipsic diabetes insipidus: a review. Endocr Pract. 2016;22(1):76–83. [DOI] [PubMed] [Google Scholar]
- 3. Smith D, Finucane F, Phillips J, Baylis PH, Finucane J, Tormey W, Thompson CJ. Abnormal regulation of thirst and vasopressin secretion following surgery for craniopharyngioma. Clin Endocrinol (Oxf). 2004;61(2):273–279. [DOI] [PubMed] [Google Scholar]
- 4. Ball SG, Vaidja B, Baylis PH. Hypothalamic adipsic syndrome: diagnosis and management. Clin Endocrinol (Oxf). 1997;47(4):405–409. [DOI] [PubMed] [Google Scholar]
- 5. Crowley RK, Sherlock M, Agha A, Smith D, Thompson CJ. Clinical insights into adipsic diabetes insipidus: a large case series. Clin Endocrinol (Oxf). 2007;66(4):475–482. [DOI] [PubMed] [Google Scholar]
- 6. Mavrakis AN, Tritos NA. Diabetes insipidus with deficient thirst: report of a patient and review of the literature. Am J Kidney Dis. 2008;51(5):851–859. [DOI] [PubMed] [Google Scholar]
- 7. Modawi I, Barger GR, Rossi NF. Central diabetes insipidus and adipsia due to astrocytoma: diagnosis and management. CEN Case Reports. 2013;2(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hameed S, Mendoza-Cruz AC, Neville KA, Woodhead HJ, Walker JL, Verge CF. Home blood sodium monitoring, sliding-scale fluid prescription and subcutaneous DDAVP for infantile diabetes insipidus with impaired thirst mechanism. Int J Pediatr Endocrinol. 2012;2012(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]