Abstract
Habitual loading and resistance training (RT) can lead to changes in muscle and tendon morphology as well as in its mechanical properties which can be measured by Shear Wave Elastography (SWE) technique. The objective of this study was to analyze the Vastus Lateralis (VL) and patellar tendon (PT) mechanical properties adaptations to an 8-week RT protocol using SWE. We submitted 15 untrained health young men to an 8-week RT directed for knee extensor mechanism. VL and PT shear modulus (μ) were assessed pre and post intervention with SWE. PT thickness (PTT), VL muscle thickness (VL MT) and knee extension torque (KT) were also measure pre and post intervention to ensure the RT efficiency. Significant increases were observed in VL MT and KT (pre = 2.40 ± 0.40 cm and post = 2.63 ± 0.35 cm, p = 0.0111, and pre = 294.66 ± 73.98 Nm and post = 338.93 ± 76.39 Nm, p = 0.005, respectively). The 8-week RT was also effective in promoting VL μ adaptations (pre = 4.87 ± 1.38 kPa and post = 9.08.12 ± 1.86 kPa, p = 0.0105), but not in significantly affecting PT μ (pre = 78.85 ± 7.37 kPa and post = 66.41 ± 7.25 kPa, p = 0.1287) nor PTT (baseline = 0.364 ± 0.053 cm and post = 0.368 ± 0.046 cm, p = 0.71). The present study showed that an 8-week resistance training protocol was effective in adapting VL μ but not PT μ. Further investigation should be conducted with special attention to longer interventions, to possible PT differential individual responsiveness and to the muscle-tendon resting state tension environment.
Introduction
Skeletal muscles act as active and primary motors for the body segments movements [1], while tendons represent an important connective tissue with high resistance to tensile loading, responsible for muscle force transmission to the bone [2]. Both tendon tensile environment and muscle demand levels will determine adaptations in these structures [3,4]. According to the overload environment, tendons can increase resistance and thickness or undergo inflammation and structural disorganization [5]. Increased muscle demand and training regime, especially against an high external resistance, can result in hypertrophy, changes in architecture or, sometimes, lesions and degeneration [1]. Due to its fundamental function for standing in upright position, walking and running and significant impairment in normal movement during pathologic situations, knee extensor mechanism (quadriceps and patellar tendon (PT)) mechanical properties has received particular interest recently [6–10].
In the last decades, changes in PT mechanical properties have been object of many studies. Its adaptations to overload regimen, resistance training (RT) protocols, ageing and pathologic conditions were extensively documented [6,11–13]. Yet, the PT structural and histological adaptations to overloading are still not fully understood. Much of the knowledge about its mechanical changes in vivo is derived from indirect analysis based on B-mode ultrasound (US) imaging combined with dynamometry. This method, however, is subject to inaccuracies due to possible calibration errors and misinterpretations of the structures displacement [9,14]. In the other hand, muscle mechanical properties analysis is not feasible using this strategy due the dynamic nature of muscle contraction, even in isometric actions, and the absence of intrinsic anatomical landmarks to calculate strain and displacement. Other methodologies, as an application of a durometer to describe a muscle hardness index are also very limited and the reproducibility of this specific device has not been systematically evaluated [15].
Shear Wave Elastography (SWE) received particular interest in US imaging routine, allowing a static evaluation of tissues mechanical properties [8,16]. Supersonic Shearwave Imaging (SSI) is able to determine the shear modulus (μ) in a determined region of interest (ROI) based on the combination of a radiation force and an ultrafast acquisition imaging system [17–19]. The μ is therefore computed from the velocity of the propagating shear waves assuming an isotropic and homogeneous medium [20,21]. Analyzing the PT, SWE has revealed PT μ reducing after stretch-shortening regimens, ageing, tendinopathy and surgical procedures [19,22–24]. SWE was also externally validated and although μ and tensile elastic modulus represent different measures, PT μ showed a strong positive correlation to longitudinal Young´s Modulus (E), ultimate force to failure and resistance to tensile loading in in vitro models [25,26]. In muscle study, SWE has showed a strong positive correlation to the muscle loading environment passively or during active contraction (R2> 0.9) [27–29]. SWE also detected reductions in muscle μ after long term stretching protocols [30], eccentric resistance training [31], aging [32], musculoskeletal pathologies [33] and μ increases during acute muscle damage [34].
Although PT mechanical adaptations and quadriceps structural changes after resistance training monitored by US are extensively documented [35,36], to our knowledge no previous studies evaluated the PT or quadriceps μ changes promoted by a RT protocol using SSI. Changes in tendon collagen cross-linking as well as muscle architecture can impact ultimate force to failure [25,26]. These adaptations could be reflected in variations of tendon stiffness. Therefore, our initial hypothesis was that the PT stiffening and quadriceps hypertrophy after RT observed in previous studies using US evaluation, would be reflected as an increase in μ on SSI evaluation. Therefore, our main objective was to assess the changes in PT and quadriceps μ using SWE, after a progressive RT protocol.
Materials and methods
Ethics statement
The University Hospital Ethics Committee approved this study (registration number 2.811.595). The experimental procedures were conducted in accordance with the Declaration of Helsinki. All participants received instructions about the study procedures and provided informed written consent before testing.
Experimental procedure
The study was conducted at the Biomedical Engineering Department in our University between july 2017 and august 2018. The body weight and height of all subjects were measured and body mass index (BMI) was calculated. Age and dominant leg were informed. All subjects’ PT and VL were submitted to SSI evaluation pre and post intervention. As a form to assure that the RT protocol was effective, Vastus Lateralis muscle thickness (VL MT) and knee extensor torque (KT) were measured at baseline and after the eight weeks of RT. PT thickness (PTT) was measured pre and post intervention to detect possible tendon structural adaptations. All post intervention measures were performed at one week after the last training session.
The intervention and data acquisition protocol is available in dx.doi.org/10.17504/protocols.io.ykwfuxe.
Subjects
In this longitudinal study, 15 untrained male volunteers (28.6 ± 3.26 years old, 177.3 ± 6.88 cm height, 91.8 ± 17.25 kg of body mass and 28.84 kg/m2 of body mass index) had the right knee examined. Age was set between 25 and 40 years old to eliminate any variation of PT properties due to age or gender. None of the subjects had participated in any systematic training or physical activity for at least 6 months. Any clinical history or report of knee pain/injuries, systemic disease or previous knee surgery was considered as exclusion criteria. All subjects were right handed.
Resistance training protocol
Participants were designated to eight-week resistance training for the quadriceps femoris muscle consisting of free-weight Squats (SQ) and Knee Extensions (KE) in a knee extension machine (MatFitness, São Paulo, Brazil) in this precise exercise order. RT protocol was designed based on the ACSM recommendations for healthy individuals and adapted based on previous studies with similar design [37]. The frequency of the training program was 2 sessions per week with at least 72 hours rest between sessions. A total of 16 sessions were performed in the 8-week training period with all the sessions occurring between 8 and 10 AM.
At baseline, 10RM testing was performed for both exercises. All subjects were submitted to a familiarization before testing during which the subjects performed the same exercises as used in the 10RM tests with the aim of standardizing the technique of each exercise. The 10 RM tests and retest were then performed on 2 nonconsecutive days separated by 48–72 hours. The heaviest resistance load achieved on either of the test days was considered the pre-training 10RM of a given exercise. The 10RM was determined in no more than five attempts, with a rest interval of five minutes between attempts and a 10-minute recovery period was allowed before the start of the testing of the next exercise.
The 10RM tests were used to set the initial training load. Subjects were instructed to perform both exercises to failure in all sets and the weighs were continually adjusted to keep the exercises in an 8–12 repetitions range, with a two-minute rest interval between sets. Full range of motion was used in both SQ and KE. The RT program followed a linear periodization and progressive volume with four sets per exercise in weeks 1–4 and six sets per exercise in weeks 5–8. Before each training session, the participants performed a specific warm-up, consisting of 20 repetitions at approximately 50% of the resistance used in the first exercise of the training session (SQ). Contraction time was self-determined as individuals were instructed to perform both exercises until concentric failure in the 8–12 repetition range with the highest load possible. Adherence to the program was superior to 90% in all individuals and a strength and conditioning professional and a physician supervised all the training sessions. Verbal encouragement was provided during all training sessions.
Measurement of patellar tendon shear modulus and thickness
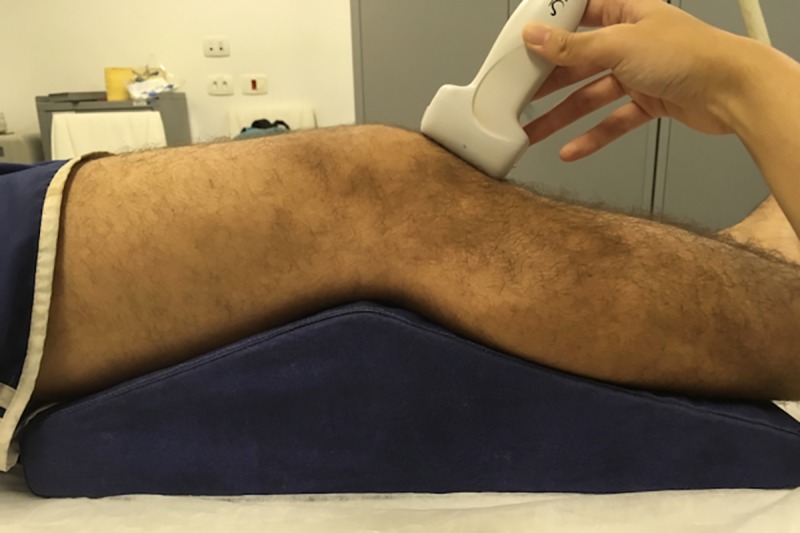
An Aixplorer US (v.11, Supersonic Imaging, Aix-en-Provence, France) with a 60-mm linear-array transducer at 4–15 MHz frequency was used in this study. The transducer was positioned at the inferior pole of patella and aligned with the patellar tendon, with no pressure on top of a generous amount of coupling gel. B-mode was used to locate and align the PT longitudinally. When a clear image of the PT was captured, the shear wave elastography mode was then activated. The transducer was kept stationary for approximately 10 seconds during the acquisition of the SWE map. A total of four images were acquired and saved for off-line processing analysis. Scanning of PT was performed with the subject in supine lying and the knee at 30° of flexion [38]. The knee was supported on a custom-made knee stabilizer to keep the leg in neutral alignment on the coronal and transverse planes (Fig 1). Prior to testing, the subjects were allowed to have a 10-min rest to ensure the mechanical properties of PT were evaluated at resting status. The room temperature was controlled at 20° C for all image acquisitions and the same experienced operator performed all exams.
Fig 1. Imaging acquisition with the knee resting over a custom-made support at 30o.
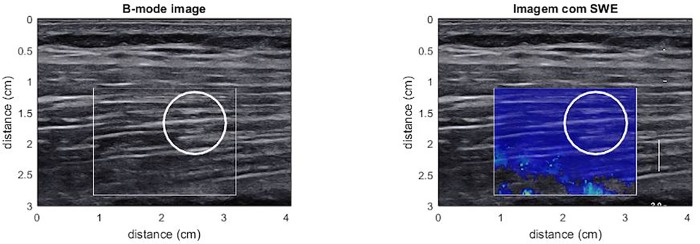
The Q-box selected was the larger possible rectangle in order to consider more PT elasticity information. The μ values were obtained by a custom MatLab routine and ROI limits were defined as the area between 5 and 25 mm from the inferior pole of the patella excluding the paratendon (Fig 2) [39]. The custom routine calculated the μ by dividing the mean E generated from the system by 3 [21].
Fig 2. MatLab custom routine and ROI defined between 5 and 25 mm from the patella tip.
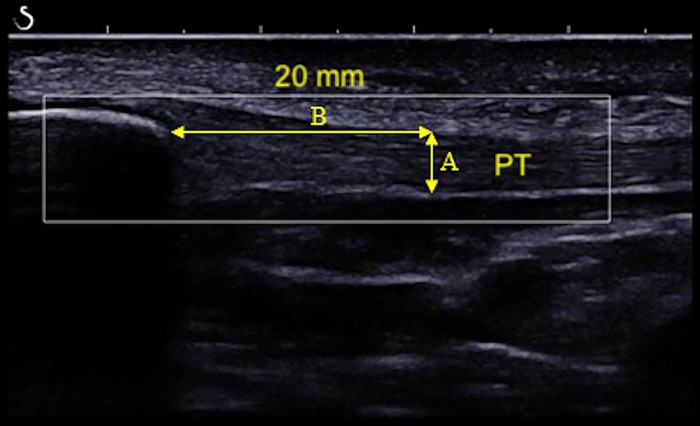
Off-line analysis using ImageJ 1.43u (National Institutes of Health, Bethesda, MD, USA) was performed with using two B-mode recorded images and the mean values were considered for analysis. PTT was measured at 20 mm from the inferior pole of the patella. The measure was limited by the PT deep and superficial paratendon and oriented transversely to the tendon fibers (Fig 3).
Fig 3. ImageJ measure of PTT at 20 mm from the inferior pole of the patella.
Measurement of vastus lateralis shear modulus
The same equipment was used for VL μ measurement. A longitudinal line was drawn between the most superficial and palpable portion of the great trochanter and the lateral epicondyle. Scans were taken at 50% of the length of the line [40]. The line length and distance from the great trochanter where the imaging was performed was registered for every volunteer to ensure that the post intervention analysis was made in the same exact location in the. B-mode was used to locate and align the probe with the VL. The images were recorded with subjects lying supine with their knee fully extended and their muscles fully relaxed [40]. When a clear image of the VL was captured, the SWE mode was then activated. The ROI was selected avoiding any detectable vascular structure within the muscle and the deep fascia and based on the quality map (Fig 4).
Fig 4. VL μ measurement and selected ROI.
Measurement of vastus lateralis muscle thickness
The images acquisition was performed by an experienced researcher, using a US (GE LogiqE, Healthcare, EUA), frequency of 8 MHz, for longitudinal scans of the VL muscle. The US probe was centered and the images were recorded with subjects on the same position and location used for VL SWE. The VL images were obtained on longitudinal plane laterally and the MT was determined as the mean of three distances (proximal, middle and distal) between superficial and deep aponeurosis for each image [41] (Fig 5). The images were processed with publicly available software (ImageJ 1.43u; National Institutes of Health, Bethesda, MD, USA). For each image, two consecutive measurements were performed and the mean values were considered for analysis.
Fig 5. VL MT measurement with B-mode US.
Measurement of knee extension torque
The maximal isometric extension KT was measured with an isokinetic dynamometer (BIODEX®, Biodex Medical Systems, Shirley, NY, USA) at 80° of knee flexion [40]. Subjects were positioned seated with inextensible straps fastened around the waist, trunk and distal part of the thigh. The backrest inclination and seat translation as well as the dynamometer height were adjusted for each subject, to ensure proper alignment of the rotation axis of the dynamometer with the lateral condyle of the femur. The right knee was fixed to the dynamometer lever arm 5 cm above the lateral malleolus. Settings were recorded for re-test reproducibility. After a specific warm-up consisting of two submaximal isometric knee extensions, the subjects performed two 5-s maximal voluntary isometric contractions (MVIC) with one-minute rest between trials. Subjects were verbally encouraged to reach maximal effort while a visual feedback of the torque level was provided. The highest peak torque among trials (corrected for gravity) was recorded for analysis.
Statistical analysis
Descriptive data such as mean ± standard deviation (SD) were calculated. The software GraphPad Prism 7 was used for statistical analysis. After the normality distributions were verified using the Shapiro-Wilk tests, paired t-tests were used to compare the PT μ, PT thickness, the VL μ, the VL MT and the KT at baseline and after the resistance training protocol. A value of p < 0.05 was adopted as statistically significant.
Results
Patellar tendon and vastus lateralis thickness
No statistically significant changes in PTT were observed after the eight weeks of RT (baseline = 0.364 ± 0.053 cm and post = 0.368 ± 0.046 cm, p = 0.71). A statistically significant increase was observed in VL MT after the resistance training protocol (baseline = 2.40 ± 0.40 cm and post = 2.63 ± 0.35 cm, p = 0.0111) (Fig 6).
Fig 6. A- VL MT at baseline and after eight weeks of resistance training. B- PTT at baseline and after eight weeks of resistance training.

Patellar tendon and vastus lateralis shear modulus
No statistically significant changes in PT μ were observed after the eight weeks of RT (baseline = 78.85 ± 7.37 kPa and post = 66.41 ± 7.25 kPa, p = 0.1287). A statistically significant increase in VL μ was observed after the eight weeks of RT (baseline = 4.87 ± 1.38 kPa and post = 9.08.12 ± 1.86 kPa, p = 0.0105) (Fig 7).
Fig 7. A- PT μ at baseline and after eight weeks of resistance training. B- VL μ at baseline and after eight weeks of resistance training.

Knee extensor torque
A statistically significant increase was observed in KT after the resistance training protocol (baseline = 294.66 ± 73.98 Nm and post = 338.93 ± 76.39 Nm, p = 0.005).
Discussion
Our study aimed to assess the effects of a progressive RT protocol directed for the knee extensor mechanism on the PT and VL μ measured by SWE. Our findings indicate that the proposed intervention was effective in promoting quadriceps hypertrophy and strength gains. VL MT and KT increased significantly (p = 0.005). Also, the intervention was effective in promoting VL μ adaptations (p = 0.0105), but not in significantly affecting PT μ (p = 0.1287) nor PTT (p = 0.71)
Effects of resistance training on patellar tendon shear modulus
The study of the tendons and muscle adaptation process to progressive overload is fundamental to design optimal strategies to injury prevention and rehabilitation [42]. Two recent literature reviews investigated the PT mechanical properties changes after short-term (6–14 weeks) RT protocols [35,43]. Significant increase in PT stiffness and E were observed routinely as a consequence of RT [10,44–46]. It is important to notice that these previous literature addressing the changes in PT mechanical properties secondary to RT were based exclusively on stress and strain estimation derived from B-mode US measures and dynamometry during isometric quadriceps contraction [35]. Although extensively documented, there is no consensus related to the acquisition protocol and this methodology can jeopardize results previously found [9,14,47]. Many technical details can limit these results reliability such as limited scanning from narrow fields of view [48,49], desynchronization between force production and elongation registration [50], limited three dimensional tracking of anatomical landmarks during muscle contraction [49,51,52], tendon force estimation inaccuracies [53] and others.
We used SWE to observe the changes PT mechanical properties expressed by PT μ, before and after a RT protocol. To our knowledge, this is the first work directed to PT with this design. Although, it is well documented the association between μ and E in isotropic and homogeneous materials [20], PT do not represent this ideal isotropic and homogeneous medium. Nevertheless, SWE was externally validated and PT μ showed a strong positive correlation to longitudinal Young´s Modulus (E), ultimate force to failure and resistance to tensile loading in in vitro models [25,26]. Another significant limitation using SWE for PT mechanical properties estimation is the guided waves effect, which is directly affected by the tendon thickness [54]. Therefore, changes in PT structure and thickness after RT could represent a bias in PT μ changes analysis pre and post intervention. In our study however, PTT analysis revealed no significant changes after the 8-week RT. This results are in accordance with previous literature that revealed that larger tendons were observed only after longer interventions (months, years) [3,35,53]. With the unaltered PTT pre and post intervention showed in our study, we can assume that the guiding effect should have impacted similarly the PT μ at baseline and after the RT protocol.
The 8 weeks intervention could be not sufficient to trigger changes in tendon mechanical properties at an extent to make the μ detectable by SWE. It has been previously reported that tendon remodeling process secondary to RT protocols can take longer periods [35], comparing to muscle adaptations. Although literature on the topic uses RT protocols lasting 6–14 weeks, a longer intervention could be necessary to make μ changes detectable by SWE. Our sample size and characteristics (15 individuals) was compatible to other studies previously published investigating PT adaptations to RT protocols with B-mode US [45,55], nevertheless, the known wide range of normal PT shear modulus values [7,8,39] may require a large sample to be tested.
Lastly, the changes in resting state passive tension in the muscle-tendon unit deserves particular attention. It was previously reported that the shear modulus presents strong correlation to the tangent traction modulus at the time of SWE image acquisition [38]. It is also documented that RT can increase flexibility [56] and that static stretching was able to reduce de E and μ acutely [57,58]. This could mask the resistance training effects on PT μ. In one hand the expected increased collagen synthesis and tendon stiffening would increase shear modulus, while in the other hand the relaxation in the muscle-tendon unit and reduction in passive tension applied to the tendon could reduce it.
Effects of resistance training on vastus lateralis shear modulus
Muscle mechanical properties seems to be much less explored, mainly by its limiting technical settings [59,60]. Using US plus dynamometry technique does not seems feasible due to the complex architecture when studying pennate muscles or due to the absence of reference anatomical landmarks in fusiform muscles to calculate strain. Until the advent of SWE, muscle mechanical properties were obtained by external mechanical analysis which is very limited, as the muscle hardness index using a durometer, whose reproducibility has not been systematically evaluated [15].
SWE values show a strong positive correlation with the muscle force production and activation for quadriceps, triceps surae, abductor minimum and others [27–29,61], showing that as muscle contract level rises, the more stiffer it becomes. However, the changes in muscle μ secondary to RT are far less studied. To our knowledge, only two studies addressed this topic. Akagi et al. (2016) reported no changes in the triceps brachii μ after a 6-week RT consisting of triceps extensions [16]. Differently from our study however, the authors report that transductor was positioned transversely to muscle fibers, which can actually exhibit lower μ values and blunt differences [62,63]. Furthermore, the study used a shorter intervention (6 weeks) consisting of only one exercise in lower training volume (5 sets), what could explain the undetectable changes.
Another study investigated the effects of a 6-week protocol consisting exclusively of eccentric RT (Nordic Curl) on biceps femoris [31]. Similarly, to Akagi et al. (2016) no statistically significant differences in biceps femoris μ were observed. However, in this study, stretching was also used in the protocol. It was already evidenced that stretching can reduce muscle μ [30], so increases in muscle μ secondary to the RT may have been counteracted by the addition of a stretching intervention. Increased stiffness in VL μ observed in our study could be resulted from collagen content, collagen linking and tissue fluid increasing [64]. Also changes in muscle architecture and pennation angle as the VL, can have some impact in the μ, although the magnitude of these changes in the SSI technique is still under investigation [18,65].
Limitations
Although SWE represents the state of the art in soft tissue mechanical properties evaluation [17,66], inferring structural adaptations in the musculoskeletal tissues after RT protocols is not trivial. Two different structures with similar composition can present different μ values on SWE evaluation if subjected to different tension during evaluation. The same applies to structures with different structural arrangement, that can present equal μ values on evaluation if subjected to different tension at the moment of testing [8,63,67]. We controlled the testing position as much as possible to avoid this influence, trying to guarantee that the muscle was fully relaxed, but it is not possible to guarantee that the relaxed muscle tension pre and post intervention was the same. Further studies researching muscle and tendon mechanical adaptations to RT with SWE should address this question and try to quantify the passive tension in the muscle-tendon unit in the resting state pre and post intervention.
Another relevant limitation of the study is the underestimation of the tendon μ by commercially available SWE equipment previously documented by Helfestein-Didier et al. [54]. Although the authors report high correlation (R = 0.84) between the conventional method and the “corrected” method quantifying shear wave velocities and dispersion analysis, the guided waves generated within the tendon due to its limited thickness can determine statistically significant underestimated μ values. Furthermore, the SWE assumes a transverse isotropic medium, which does not properly represent the complex tendon architecture [20]. In our study, no corrections through dispersion analysis were implemented to the SSI evaluation. When studying long term effects, these combined limitations can determine significant impact to results. Further research in the field should attempt to address this bias.
Lastly, the VL pennation angle was not determined pre and post RT. The Aixplorer SSI offers a relatively restricted field of view when compared to conventional US. In larger subjects (present group average = 91.8 ± 17.25 kg), many times it is not possible to visualize the deep fascia necessary to calculate the pennation angle. Previous literature has provided evidence that particular attention should be paid to interpreting the SWE data related to the pennate muscles [65] as it seems that the fascicle orientation can influence SWE measurements [68]. Also, it is well documented that RT protocols can determine changes in the pennation angle [69], so it is possible that SWE measurements in chronic studies addressing pennate muscle can suffer influence of the changes in muscle architecture promoted by RT. Further investigation using SWE to address pennate muscles μ changes after RT should give particular attention to the impact of fascicle orientation changes on SWE measurements.
To our knowledge, this is the first study analyzing the adaptation of the PT and VL mechanical properties to RT with SWE. Our initial hypothesis that RT would increase PT and VL μ was partially confirmed, as VL μ changes were observed, but not PT μ. These findings can help design further researches on the field and build new knowledge about the knee extensor mechanism remodeling process to mechanical overloading.
Conclusion
The present study showed that an 8-week resistance training protocol was effective in adapting VL μ but not PT μ, measured by SWE. Further investigation should be conducted with special attention to longer interventions, to possible PT structural changes set points and to the muscle-tendon resting state tension environment.
Supporting information
Patellar Tendon and Vastus Lateralis Shear modulus, Tendon Thickness, Muscle Thickness and Knee Torque pre and post intervention.
(XLSX)
Acknowledgments
The authors acknowledge Financiadora de Estudos e Projetos–FINEP, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro–FAPERJ and Comissão Nacional de Desenvolvimento Científico e Tecnológico–CNPq.
Abbreviations
- E
Young´s Modulus
- KE
knee extension
- KT
knee extension torque
- MT
muscle thickness
- PT
patellar tendon
- PTT
patellar tendon thickness
- RM
repetitium maximum
- ROI
region of interest
- RT
resistance training
- SD
standard deviation
- SQ
squat
- SSI
supersonic shearwave imaging
- SWE
shear wave elastography
- US
ultrasound
- VL
vastus lateralis
- μ
shear modulus
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Lieber RL, Roberts TJ, Blemker SS, Lee SSM, Herzog W. Skeletal muscle mechanics, energetics and plasticity Daniel P Ferris. J Neuroeng Rehabil 2017;14:1–16. 10.1186/s12984-016-0214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: Attachment sites ('entheses’) in relation to exercise and/or mechanical load. J Anat 2006;208:471–90. 10.1111/j.1469-7580.2006.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, et al. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 2008;105:805–10. 10.1152/japplphysiol.90361.2008 [DOI] [PubMed] [Google Scholar]
- 4.Kubo K, Kanehisa H, Fukunaga T. <Effects of resistance and stretching training programmes on.pdf> 2002:219–26. 10.1113/jphysiol.2001.012703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galloway MT, Lalley AL, Shearn JT. The Role of Mechanical Loading in Tendon Development, Maintenance, Injury, and Repair. J Bone Jt Surgery-American Vol 2013;95:1620–8. 10.2106/JBJS.L.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couppé C, Kongsgaard M, Aagaard P, Vinther A, Boesen M, Kjær M, et al. Differences in tendon properties in elite badminton players with or without patellar tendinopathy. Scand J Med Sci Sport 2013;23:89–95. 10.1111/sms.12023 [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZJ, Ng GYF, Fu SN. Effects of habitual loading on patellar tendon mechanical and morphological properties in basketball and volleyball players. Eur J Appl Physiol 2015;115:2263–9. 10.1007/s00421-015-3209-6 [DOI] [PubMed] [Google Scholar]
- 8.Kot BCW, Zhang ZJ, Lee AWC, Leung VYF, Fu SN. Elastic Modulus of Muscle and Tendon with Shear Wave Ultrasound Elastography: Variations with Different Technical Settings. PLoS One 2012;7:2–7. 10.1371/journal.pone.0044348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson SJ, Burgess K, Onambele GNL. Creep and the in vivo assessment of human patellar tendon mechanical properties. Clin Biomech 2007;22:712–7. 10.1016/j.clinbiomech.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Grosset JF, Breen L, Stewart CE, Burgess KE, Onamb??l?? GL. Influence of exercise intensity on training-induced tendon mechanical properties changes in older individuals. Age (Dordr) 2014;36:9657 10.1007/s11357-014-9657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, et al. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 2008;105:1907–15. 10.1152/japplphysiol.00059.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. Mechanical properties of the patellar tendon in adults and children. J Biomech 2010;43:1190–5. 10.1016/j.jbiomech.2009.11.028 [DOI] [PubMed] [Google Scholar]
- 13.Taş S, Yilmaz S, Onur MR, Soylu AR, Altuntaş O, Korkusuz F. Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop Traumatol Turc 2017;51:54–9. 10.1016/j.aott.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess KE, Pearson SJ, Breen L, Onambélé GNL. Tendon structural and mechanical properties do not differ between genders in a healthy community-dwelling elderly population. J Orthop Res 2009;27:820–5. 10.1002/jor.20811 [DOI] [PubMed] [Google Scholar]
- 15.Niitsu M, Michizaki A, Endo A, Takei H, Yanagisawa O. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol 2011;52:99–105. 10.1258/ar.2010.100190 [DOI] [PubMed] [Google Scholar]
- 16.Akagi R, Shikiba T, Tanaka J, Takahashi H. A six-week resistance training program does not change shear modulus of the triceps brachii. J Appl Biomech 2016;32:373–8. 10.1123/jab.2015-0290 [DOI] [PubMed] [Google Scholar]
- 17.Bercoff J, Tanter M, Fink M. Supersonic Shear Imaging: A New Technique. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:396–409. 10.1109/TUFFC.2004.1295425 [DOI] [PubMed] [Google Scholar]
- 18.Lima K, Costa Júnior JFS, Pereira WC de A, Oliveira LF de. Assessment of the mechanical properties of the muscle-tendon unit by Supersonic Shearwave Imaging Elastography: a review. Ultrasonography 2017:1–13. 10.14366/usg.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZJ, Ng GYF, Lee WC, Fu SN. Changes in morphological and elastic properties of patellar tendon in athletes with unilateral patellar tendinopathy and their relationships with pain and functional disability. PLoS One 2014;9:1–9. 10.1371/journal.pone.0108337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol 2014;59:505–23. 10.1088/0031-9155/59/3/505 [DOI] [PubMed] [Google Scholar]
- 21.Royer D, Gennisson J-L, Deffieux T, Tanter M. On the elasticity of transverse isotropic soft tissues (L). J Acoust Soc Am 2011;129:2757–60. 10.1121/1.3559681 [DOI] [PubMed] [Google Scholar]
- 22.Botanlioglu H, Kaynak G, Kantarci F, Guven MF, Zengin G, Aydingoz O. Length, thickness, and elasticity of the patellar tendon after closed wedge high tibial osteotomy: A shear wave elastographic study. J Orthop Surg 2016;24:194–7. 10.1177/1602400215 [DOI] [PubMed] [Google Scholar]
- 23.Hsiao MY, Chen YC, Lin CY, Chen WS, Wang TG. Reduced patellar tendon elasticity with aging: In vivo assessment by shear wave elastography. Ultrasound Med Biol 2015;41:2899–905. 10.1016/j.ultrasmedbio.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Ooi CC, Richards PJ, Maffulli N, Ede D, Schneider ME, Connell D, et al. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J Sci Med Sport 2016;19:373–8. 10.1016/j.jsams.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Martin JA, Biedrzycki AH, Lee KS, DeWall RJ, Brounts SH, Murphy WL, et al. In Vivo Measures of Shear Wave Speed as a Predictor of Tendon Elasticity and Strength. Ultrasound Med Biol 2015;41:2722–30. 10.1016/j.ultrasmedbio.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh CL, Kuo PL, Gennisson JL, Brum J, Tanter M, Li PC. Shear Wave Measurements for Evaluation of Tendon Diseases. IEEE Trans Ultrason Ferroelectr Freq Control 2016;63:1906–21. 10.1109/TUFFC.2016.2591963 [DOI] [PubMed] [Google Scholar]
- 27.Ateş F, Hug F, Bouillard K, Jubeau M, Frappart T, Couade M, et al. Muscle shear elastic modulus is linearly related to muscle torque over the entire range of isometric contraction intensity. J Electromyogr Kinesiol 2015;25:703–8. 10.1016/j.jelekin.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Bouillard K, Hug F, Guevel A, Nordez A. Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J Appl Physiol 2012;113:1353–61. 10.1152/japplphysiol.00858.2012 [DOI] [PubMed] [Google Scholar]
- 29.Bouillard K, Nordez A, Hug F. Estimation of individual muscle force using elastography. PLoS One 2011;6 10.1371/journal.pone.0029261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akagi R, Takahashi H. Effect of a 5-week static stretching program on hardness of the gastrocnemius muscle. Scand J Med Sci Sport 2014;24:950–7. 10.1111/sms.12111 [DOI] [PubMed] [Google Scholar]
- 31.Seymore KD, Domire ZJ, DeVita P, Rider PM, Kulas AS. The effect of Nordic hamstring strength training on muscle architecture, stiffness, and strength. Eur J Appl Physiol 2017;117:943–53. 10.1007/s00421-017-3583-3 [DOI] [PubMed] [Google Scholar]
- 32.Akagi R, Yamashita Y, Ueyasu Y. Age-related differences in muscle shear moduli in the lower extremity. Ultrasound Med Biol 2015;41:2906–12. 10.1016/j.ultrasmedbio.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 33.Botanlioglu H, Kantarci F, Kaynak G, Unal Y, Ertan S, Aydingoz O, et al. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skeletal Radiol 2013;42:659–66. 10.1007/s00256-012-1520-4 [DOI] [PubMed] [Google Scholar]
- 34.Akagi R, Tanaka J, Shikiba T, Takahashi H. Muscle hardness of the triceps brachii before and after a resistance exercise session: A shear wave ultrasound elastography study. Acta Radiol 2015;56:1487–93. 10.1177/0284185114559765 [DOI] [PubMed] [Google Scholar]
- 35.Wiesinger HP, Kösters A, Müller E, Seynnes OR. Effects of Increased Loading on in Vivo Tendon Properties: A Systematic Review. Med Sci Sports Exerc 2015;47:1885–95. 10.1249/MSS.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. Progression Models in Resistance Training for Healthy Adults. Med Sci Sports Exerc 2009. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 37.American College of Sports Medicine. ACSM position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41:687–708. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZJ, Fu SN. Shear Elastic Modulus on Patellar Tendon Captured from Supersonic Shear Imaging: Correlation with Tangent Traction Modulus Computed from Material Testing System and Test-Retest Reliability. PLoS One 2013;8:1–9. 10.1371/journal.pone.0068216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannarino P, Lima KMM, Fontenelle CRC, Matta TT, de Salles BF, Simão R, et al. Analysis of the correlation between knee extension torque and patellar tendon elastic property. Clin Physiol Funct Imaging 2017. 10.1111/cpf.12424 [DOI] [PubMed] [Google Scholar]
- 40.Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol 2009;105:869–78. 10.1007/s00421-008-0972-7 [DOI] [PubMed] [Google Scholar]
- 41.Matta TT, Nascimento FX, Trajano GS, Simão R, Willardson JM, Oliveira LF. Selective hypertrophy of the quadriceps musculature after 14 weeks of isokinetic and conventional resistance training. Clin Physiol Funct Imaging 2017;37:137–42. 10.1111/cpf.12277 [DOI] [PubMed] [Google Scholar]
- 42.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res 2015;33:832–9. 10.1002/jor.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohm S, Mersmann F, Arampatzis A. Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sport Med—Open 2015;1:7 10.1186/s40798-015-0009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malliaras P, Kamal B, Nowell A, Farley T, Dhamu H, Simpson V, et al. Patellar tendon adaptation in relation to load-intensity and contraction type. J Biomech 2013;46:1893–9. 10.1016/j.jbiomech.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 45.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, et al. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol 2007;191:111–21. 10.1111/j.1748-1716.2007.01714.x [DOI] [PubMed] [Google Scholar]
- 46.Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, et al. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol 2009;107:523–30. 10.1152/japplphysiol.00213.2009 [DOI] [PubMed] [Google Scholar]
- 47.Seynnes OR, Bojsen-Møller J, Albracht K, Arndt A, Cronin NJ, Finni T, et al. Ultrasound-based testing of tendon mechanical properties: a critical evaluation. J Appl Physiol 2015;118:133–41. 10.1152/japplphysiol.00849.2014 [DOI] [PubMed] [Google Scholar]
- 48.Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech 2006;21:54–8. 10.1016/j.clinbiomech.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 49.Maganaris CN. Validity of procedures involved in ultrasound-based measurement of human plantarflexor tendon elongation on contraction. J Biomech 2005. 10.1016/j.jbiomech.2004.03.024 [DOI] [PubMed] [Google Scholar]
- 50.Finni T, Peltonen J, Stenroth L, Cronin NJ. On the hysteresis in the human Achilles tendon. J Appl Physiol 2012. 10.1152/japplphysiol.01005.2012 [DOI] [PubMed] [Google Scholar]
- 51.Iwanuma S, Akagi R, Hashizume S, Kanehisa H, Yanai T, Kawakami Y. Triceps surae muscle-tendon unit length changes as a function of ankle joint angles and contraction levels: The effect of foot arch deformation. J Biomech 2011. 10.1016/j.jbiomech.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 52.Peter Magnusson S, Aagaard P, Rosager S, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 2001. 10.1111/j.1469-7793.2001.0277j.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seynnes OR, Kamandulis S, Kairaitis R, Helland C, Campbell E-L, Brazaitis M, et al. Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties. J Appl Physiol 2013;115:84–9. 10.1152/japplphysiol.01417.2012 [DOI] [PubMed] [Google Scholar]
- 54.Helfenstein-Didier C, Andrade RJ, Brum J, Hug F, Tanter M, Nordez A, et al. In vivo quantification of the shear modulus of the human Achilles tendon during passive loading using shear wave dispersion analysis. Phys Med Biol 2016;61:2485–96. 10.1088/0031-9155/61/6/2485 [DOI] [PubMed] [Google Scholar]
- 55.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 2001;91:26 LP–32. 10.1152/japplphysiol.00658.2001 [DOI] [PubMed] [Google Scholar]
- 56.Morton SK, Whitehead JR, Brinkert RH, Caine DJ. Resistance training vs. static stretching: Effects on flexibility and strength. J Strength Cond Res 2011;25:3391–8. 10.1519/JSC.0b013e31821624aa [DOI] [PubMed] [Google Scholar]
- 57.Pamboris G, Noorkoiv M, Baltzopoulos V, Gokalp H, Marzilger R, Mohagheghi A. Effects of an acute bout of dynamic stretching oPamboris, Noorkoiv G, M, Baltzopoulos V, Gokalp H, Marzilger R, and Mohagheghi A. 2018. “Effects of an Acute Bout of Dynamic Stretching on Biomechanical Properties of the Gastrocnemius Muscle Determined by S. PLoS One 2018:1–19. 10.6084/m9.figshare.5919496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirata K, Miyamoto-Mikami E, Kanehisa H, Miyamoto N. Muscle-specific acute changes in passive stiffness of human triceps surae after stretching. Eur J Appl Physiol 2016;116:911–8. 10.1007/s00421-016-3349-3 [DOI] [PubMed] [Google Scholar]
- 59.Levinson SF, Shinagawa M, Sato T. Sonoelastic determination of human skeletal-muscle elasticity. J Biomech 1995;28:1145–54. 10.1016/0021-9290(94)00173-2 [DOI] [PubMed] [Google Scholar]
- 60.Murayama M, Nosaka K, Yoneda T, Minamitani K. Changes in hardness of the human elbow flexor muscles after eccentric exercise. Eur J Appl Physiol 2000. 10.1007/s004210000242 [DOI] [PubMed] [Google Scholar]
- 61.Koo TK, Guo JY, Cohen JH, Parker KJ. Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin Biomech 2014;29:33–9. 10.1016/j.clinbiomech.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 62.Eby Sarah F., Song Pengfei, Chen Shigao, Chen Qingshan, Greenleaf James F., An K-N. Validation of Shear Wave Elastography in Skeletal Muscle. J Biomech 2014;46 10.1016/j.jbiomech.2013.07.033.Validation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gennisson JL, Deffieux T, Macé E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol 2010. 10.1016/j.ultrasmedbio.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 64.Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech 1984;17:725–35. 10.1016/0021-9290(84)90103-9 [DOI] [PubMed] [Google Scholar]
- 65.Gennisson JL, Deffieux T, Macé E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol 2010;36:789–801. 10.1016/j.ultrasmedbio.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 66.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: Principles and techniques. Diagn Interv Imaging 2013;94:487–95. 10.1016/j.diii.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 67.Dubois G, Kheireddine W, Vergari C, Bonneau D, Thoreux P, Rouch P, et al. Reliable Protocol for Shear Wave Elastography of Lower Limb Muscles at Rest and During Passive Stretching. Ultrasound Med Biol 2015;41:2284–91. 10.1016/j.ultrasmedbio.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 68.Miyamoto N, Hirata K, Kanehisa H, Yoshitake Y. Validity of measurement of shear modulus by ultrasound shear wave elastography in human pennate muscle. PLoS One 2015;10:1–11. 10.1371/journal.pone.0124311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawakami Y, Abe T, Kuno SY, Fukunaga T. Training-induced changes in muscle architecture and specific tension. Eur J Appl Physiol Occup Physiol 1995. 10.1007/BF00964112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patellar Tendon and Vastus Lateralis Shear modulus, Tendon Thickness, Muscle Thickness and Knee Torque pre and post intervention.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.