Abstract
Purpose
Epigenetic regulating proteins like histone methyltransferases produce variations in several functions, some of them associated with the generation of oncogenic processes. Mutations of genes involved in these functions have been recently associated with cancer, and strategies to modulate their activity are currently in clinical development.
Methods
By using data extracted from the METABRIC study, we searched for mutated genes linked with detrimental outcome in invasive breast carcinoma (n = 772). Then, we used downstream signatures for each mutated gene to associate that signature with clinical prognosis using the online tool “Genotype-2-Outcome” (http://www.g-2-o.com). Next, we performed functional annotation analyses to classify genes by functions, and focused on those associated with the epigenetic machinery.
Results
We identified KMT2D, SETD1A and SETD2, included in the lysine methyltransferase activity function, as linked with poor prognosis in invasive breast cancer. KMT2D which codes for a histone methyltransferase that acts as a transcriptional regulator was mutated in 6% of triple negative breast tumors and found to be linked to poor survival. Genes regulated by KMT2D included RAC3, KRT23, or KRT14, among others, which are involved in cell communication and signal transduction. Finally, low expression of KMT2D at the transcriptomic level, which mirror what happens when KMT2D is mutated and functionally inactive, confirmed its prognostic value.
Conclusion
In the present work, we describe epigenetic modulating genes which are found to be mutated in breast cancer. We identify the histone methyltransferase KMT2D, which is mutated in 6% of triple negative tumors and linked with poor survival.
Introduction
Advances in the analyses of the genomic landscape of human cancers have permitted the identification of different molecular alterations, including mutations, copy number variations, or gene rearrangements, which may be linked with the genesis and maintenance of tumors [1,2]. Unfortunately, for most of the identified molecular alterations, limited druggable opportunities exist [1,2]. Very well-known exceptions include inhibition of protein kinase activity, when that alteration affects a kinase [2]. This has been the case for agents targeting mutated or amplified protein kinases, such as EGFR or HER2 in lung and breast cancers [3–5]. In a similar manner, chromosomal rearrangements can produce fusion proteins, like Trk fusion proteins, with kinase activity amenable for pharmacological inhibition [6,7].
Changes at the genome not directly produced by an alteration of the nucleotide sequence of the DNA are known as epigenetic modifications [8]. Alterations in proteins involved in epigenetic regulation can affect genetic programs that can in turn impact on several cellular functions. Ultimately, such genomic alterations can translate into different diseases, from cancer to neurological alterations or aging disorders, among others [8,9]. Epigenetic regulating proteins include enzymes involved in histone modifications, histone proteins, chromatin remodeling complexes or DNA methylation enzymes [8]. Mutations at genes coding for proteins involved in several of these functions have been already described, and some of them have been associated with cancer [10]. Therefore, inhibition of epigenetic proteins can have a wide effect impacting on the expression of multiple genes, affecting multiple pathways at the same time [10]. In this context, agents that target epigenetic enzymes have been recently described and are currently in clinical development [11]. An example is KMT2D that codes for a histone methyltransferase that methylates the Lys-4 position of histone H3, and is involved in the regulation of several transcription factors, like the estrogen receptor (ER) or FOXA1, among others [12,13]. Although not very well known, KMT2D can act in some circumstances as a tumor suppressor gene maintaining the expression of relevant proteins involved in genomic stability [14].
In this study, we evaluated the mutational status of genes involved in epigenetic control in breast cancer, identifying KMT2D as mutated in around 6% of triple negative tumors and linked with a particular detrimental prognosis.
Material and methods
Identification of breast cancer mutated genes
Data was extracted from the Breast Cancer METABRIC study (EGAS00000000083), contained at cBioPortal (http://www.cbioportal.org)[15]. This database contains cDNA microarray profiling of about 2000 samples (n = 2509). Briefly, METABRIC project aimed to classify breast tumors into subcategories depending on molecular signatures. To do so, DNA and RNA were isolated from samples and hybridized to the Affymetrix SNP 6.0 and Illumina HT-12 v3 platforms for genomic and transcriptional profiling, respectively. First, we searched for mutated genes in those samples from Invasive Breast Carcinoma patients (n = 772), including luminal A, luminal B, HER2+ and basal-like. Genes that were mutated in more than 2.5% of the patients were identified. The frequency of mutations was independently confirmed using the TCGA database (n = 818).
Functional analyses
For the functional annotation analysis of the set of mutated genes, the gene list enrichment analyses tool DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/) was used. To do so, genes with a mutation frequency greater than 2.5% and linked with poor prognosis were selected (S1 Table).
For the functional analysis of the KMT2D-associated gene signature (S2 Table), the online Enrichr tool was used (http://www.amp.pharm.mssm.edu/Enrichr/). An adjusted p-value <0.05 was applied to select enriched gene-sets. Genes were separated into overexpressed and underexpressed and "KEGG 2015" option was chosen for the analyses and the calculation of the "combined score".
Outcome analyses
To evaluate the relationship between the presence of mutated genes and patient clinical outcome, the Genotype-2-Outcome online tool (http://www.g-2-o.com) [16] was used. This publicly available database allows the evaluation of clinical outcome for all breast cancer subtypes (All, Triple Negative Breast Cancer, Luminal A, Luminal B and HER2+) by exploring the association with prognosis of a specific transcriptomic signature associated with that mutation. In brief, the expression of each gene is compared between the mutated and wild type patients and those genes reaching significance are designated as the signature for the mutation. Then, the mean expression of all these genes is computed and is used as a surrogate of mutational status. The continuous spectra of the signature is used to define “high” and “low” expression cohorts, and these are compared using a Cox proportional hazards regression analysis. In the survival analysis, the median expression is used as a cutoff to discriminate “high” and “low”. The prognostic endpoint was relapse-free survival.
To evaluate the relationship between the expression of the genes and patient clinical prognosis, the KM Plotter Online Tool (http://www.kmplot.com) [17,18] was used. This database permits the evaluation of overall survival (OS) and relapse-free survival (RFS) in basal-like, luminal A, luminal B, HER2+ and triple negative breast cancers.
For both outcome analyses, patients were separated according to median values. Patients above the threshold were considered to have a “high” expression while patients below the threshold were defined as those with “low” expression.
Evaluation of KMT2D mutations
Data contained at cBioportal (http://www.cbioportal.org) was used to identify mutations in KMT2D. Mutation Assessor (http://www.mutationassessor.org), SIFT (http://sift.bii.a-star.edu.sg/) and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) databases were used to evaluate the effect of the mutation on KMT2D functionality.
Results
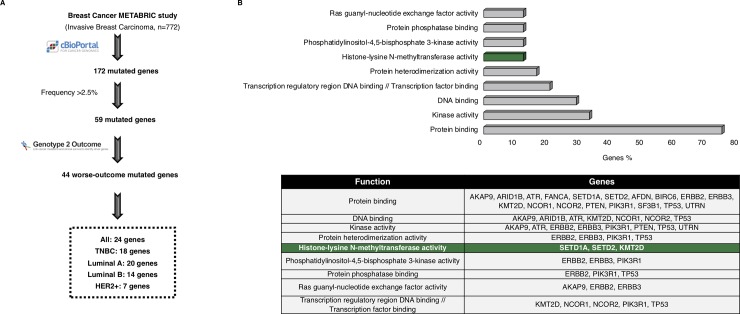
By using the METABRIC database, we identified 172 mutated genes in the analyses of the 772 samples from invasive breast tumors. We found that 59 out of the 172 genes were mutated in more than 2.5% of the samples. Next, we evaluated the impact of these genes on patient outcome using the online tool Genotype-2-Outcome (http://www.G-2-O.com/)[16] (Fig 1A). This application identifies the transcriptomic signature associated with the presence of the mutation in patients. Using this approach, 44 of the mutated genes had an associated signature linked to detrimental prognosis in breast cancer (Fig 1A). S1 Table shows the list of all mutated genes, including those associated with outcome and those not.
Fig 1. Whole genome mutational profiling and identification of histone-lysine methyltransferase activity as deregulated in breast cancer.
A. Flow chart of the study, in which the METABRIC dataset was used to identify breast cancer mutated genes associated with worse outcome. B. Functional analyses of the mutated genes associated with worse outcome, using DAVID Bioinformatics Resources 6.8 tool, and found in more than 2.5% of the breast cancer samples analyzed. The table shows the list of the mutated genes contained in each function.
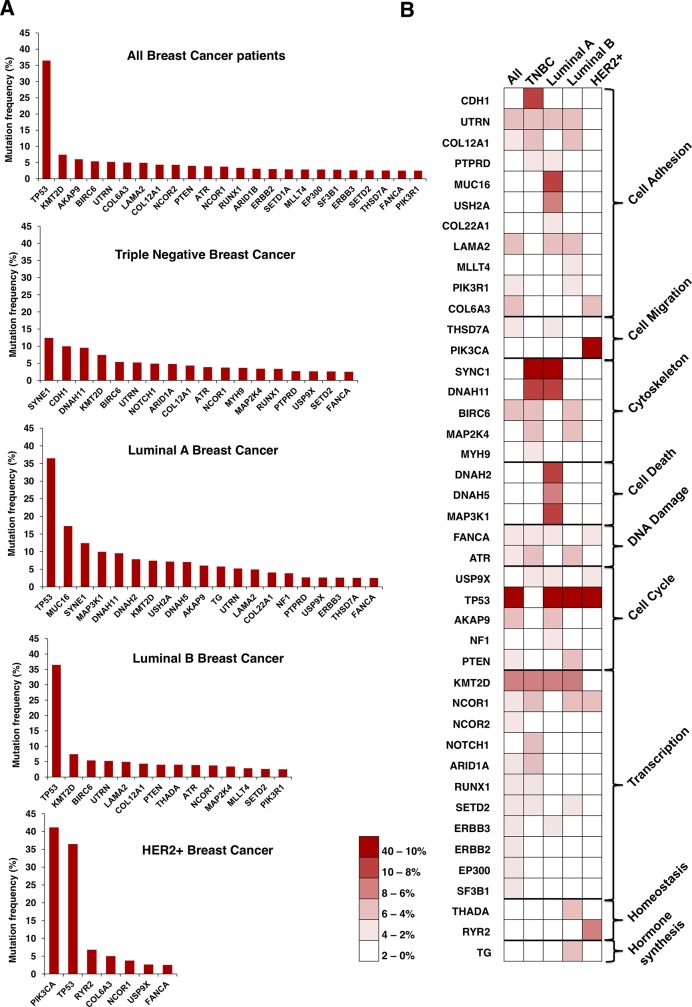
To get insights into the biological function of the mutated genes, we performed a functional annotation analysis. Protein binding, kinase activity, DNA binding and transcription factor binding were among the identified functions which grouped more genes (Fig 1B). Then, the mutational frequency of the identified genes for all breast cancer subtypes was studied. Mutations in some of the genes have been widely described in breast cancer, as is the case for TP53, in luminal and HER2+ tumors (Fig 2A). In the case of TNBC, mutated genes displaying higher frequency, more than 8%, included SYNE1, CDH1 and DNAH11 (Fig 2A). In HER2+ disease, PIK3CA was mutated in more than 40% of tumors. Of note, mutated genes found in TNBC tumors showed a broader range of functions than the other subtypes (Fig 2B).
Fig 2. Mutational profile by breast cancer subtype, and association with biological functions.
A. Graphs displayed the mutation frequency of those genes mutated in more than 2.5% of patients for all and each breast cancer subtype. B. Heat map of the mutation frequency and the functions of the identified genes for each breast cancer subtypes. The percentage of mutated cases is displayed in the legend.
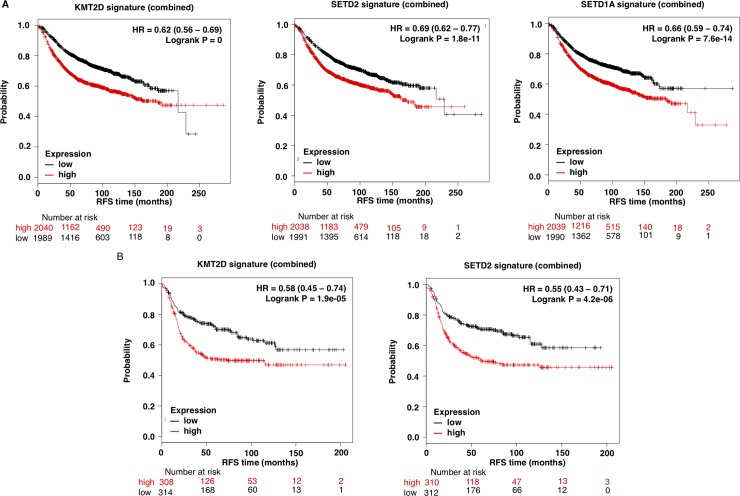
Because epigenetic enzymes are currently under evaluation as druggable targets, we focused on genes that had this function. Therefore, we selected the three genes included in the functional analyses under the “Histone-lysine N-methyltransferase activity” function, KMT2D, SETD2 and SETD1A, (Fig 1B). Next, we confirmed the presence of these mutations in the different breast cancer subtypes, using data contained at TCGA (Table 1). According to TCGA data, mutations of KMT2D were observed in 6% of TNBC and mutations of SETD2 in 1.2%, confirming the data obtained with METABRIC. Unfortunately, the data contained at METABRIC does not divide tumors by subtype. The presence of mutation in the other breast cancer subtypes was not confirmed or was too low compared to the percentage found in METABRIC. The proportion of SETD1A mutations was not confirmed in TCGA for any of the subtypes (Table 1). Next, we aimed to further explore the impact of the mutations of these two genes in patient prognosis, by exploring the effect of their associated transcriptomic signature in breast cancer (All subtypes). The complete list of deregulated genes included in the KMTD2 associated transcriptomic signature is shown in S2 Table. S3 Table and S4 Table shows the transcriptomic signatures for SET1DA and SETD2, respectively. KMT2D transcriptomic signature was linked with detrimental outcome (HR 0.62 CI: 0.56–0.69; log rank p = 0), as well as SETD1A (HR 0.66 CI: 0.59–0.74; logrank p = 7.6E-14) and SETD2 (HR 0.69, CI: 0.62–0.77; logrank p = 1.8e-11) transcriptomic signatures (Fig 3A).
Table 1. Proportion of mutations in the TCGA and METABRIC databases.
| Breast cancer subtype | Database | KMT2D | SETD2 | SETD1A |
|---|---|---|---|---|
| All | METABRIC | 7,43% | 2,62% | 2,91% |
| TNBC | TCGA | 6,00% | 1,2% | - |
| Luminal A | TCGA | 0,32% | - | - |
| Luminal B | TCGA | 0,81% | 0,00% | - |
| HER2+ | TCGA | - | - | - |
Proportion of mutations in KMT2D, SETD2 and SETD1A using data from the METABRIC and TCGA studies contained in cBioportal. METABRIC does not provide data by breast cancer subtype.
Fig 3. KMT2D, SETD2 and SETD1A mutational signature and clinical outcome.
A. Association of KMT2D, SETD2, and SETD1A mutational signature with patient outcome in all breast tumors. B. Association of KMT2D and SETD2 mutational signatures with prognosis in triple negative breast tumors. The online tool Genotype-2-Outcome was used for both analyses.
As the presence of KMT2D and SETD2 mutations were consistent in TNBC, we next explored if KMT2D and SETD2 mutational signatures were associated with detrimental prognosis in this specific tumor subtype. Notably, the presence of the associated transcriptomic signatures for both, KMTD2 and SETD2, were associated with poor prognosis (HR 0.58 CI: 0.45–0.74; log rank p = 1.9e-05 and HR 0.55 CI: 0.43–0.71; log rank p = 4.2e-0.6; respectively) (Fig 3B). Next we explored if treatment with chemotherapy influenced outcome in patients harboring the described signatures. As can be seen in S1A Fig for all breast patients and in S1B Fig for triple negative patients, administration of chemotherapy did not influence outcome for KMT2D and SETD1A. However for SETD2 a slightly effect was observed.
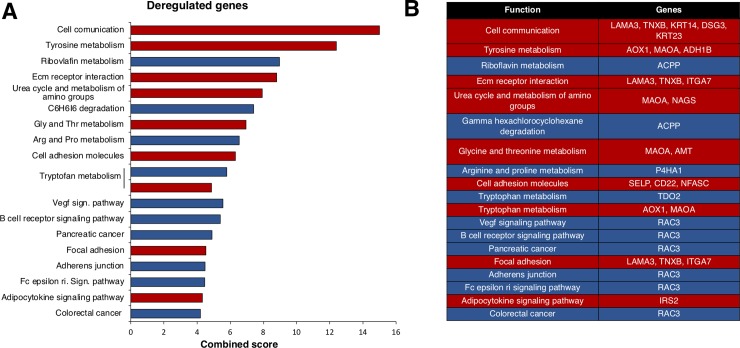
From here, we focused on KMT2D, as it was the most prevalent mutated gene in both datasets and was strongly associated with poor outcome. KMT2D is a histone methyltransferase that acts as a transcriptional regulator. The functions of the trascriptomic signature of KMT2D, determined with the online tool Enrichr, are displayed in Fig 4. Most down-regulated genes were included in the cell communication function, followed by tyrosine metabolism or extracellular matrix receptor interaction (S2 Table). Genes that code for Keratins, KRT23 or KRT14, were among the most relevant genes included in the cell communication function (Fig 4). The most relevant upregulated gene included the GTPase RAC3, that belongs to the RAS family of small GTPases involved in cell proliferation (S2 Table and Fig 4) [19].
Fig 4. Functional analysis of deregulated genes included in the KMT2D mutated signature.
A. Percentage of deregulated genes included in the KMT2D mutated signature by biological function. Overexpressed genes are displayed in blue and down-expressed genes in red. For functional annotation analysis, the online tool Enrichr was used as described in material and methods. B. Deregulated genes included in each function.
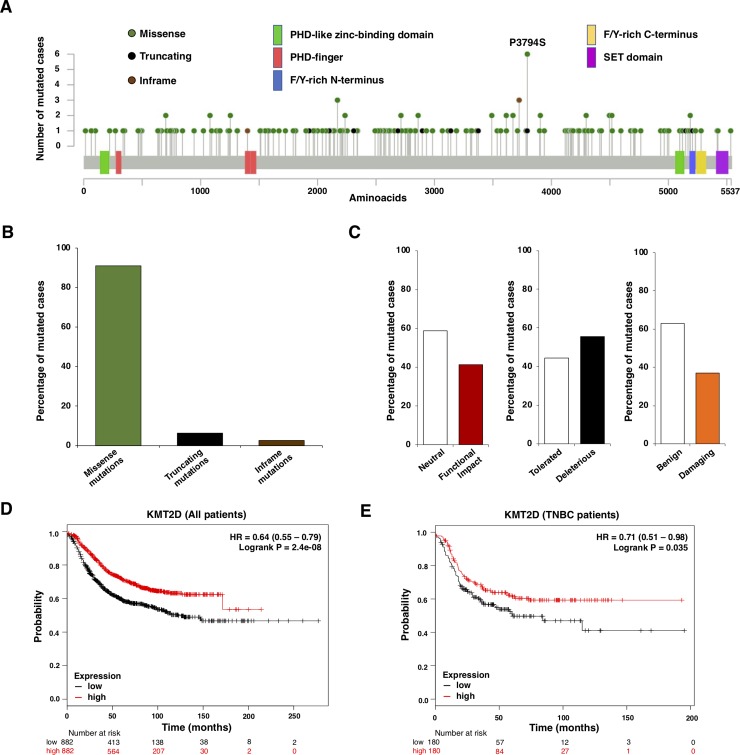
Last, we explored the functional consequences of the mutations present in KMT2D in the samples of the METABRIC database. To identify these mutations, we used the online tool cBioportal (Fig 5A). Missense mutations were scattered along the full length of the protein, and were the most abundant molecular alterations, followed by truncating mutations (Fig 5B). The functional impact of all these different mutations, evaluated with three different databases (Mutation Assessor, SIFT and PolyPhen-2), are displayed in Fig 5C. As shown, between 40–55% of KMT2D mutations had a functional impact. This indicated that those mutations lead to an abnormal protein, unable to participate in their normal function, mimicking a lack of expression of the gene. To confirm this hypothesis, we decided to explore if a low expression level of this gene could recapitulate the outcome observed at a mutational level, when we explored the effect of mutated KMT2D. Using the online tool KMplotter, that links the transcriptional expression of a gene with patient outcome [18], we found that low transcriptomic levels of KMT2D were associated with detrimental prognosis (relapse free survival) in all breast tumors (HR 0.64 CI: 0.55–0.79; log rank p = 2.4e-08) (Fig 5D), in addition to the triple negative subtype (HR 0.71 CI: 0.51–0.98; log rank p = 0.035) (Fig 5E).
Fig 5. Assessment of mutations at KMT2D.
A. Diagram showing each aminoacid (aa) which can be found to be mutated in the KMT2D gene. B. Type of mutations from the included cases. C. Functional impact of KMT2D mutations in the included cases. D. Relapse free survival (RFS) of breast cancer patients based on the transcriptomic expression of KMT2D. Low expression is associated with poor outcome. E. Relapse free survival (RFS) of triple negative breast cancer patients based on the transcriptomic expression of KMT2D. KM plotter online tool was used for these prognosis analyses. Low expression is associated with poor outcome.
Discussion
In the present article we report the identification of genes that are mutated in breast cancer and associated with detrimental outcome. After functional analysis of the identified genes, we focused on the “Histone-lysine N-methyltransferase activity” function and found that the histone methyltransferase gene KMT2D was mutated in around 6% of the TNBCs samples evaluated; in addition to be associated with poor prognosis in this breast cancer subtype.
KMT2D is a histone methyltransferase that methylates the Lys-4 position of the histone H3 [13]. The codified protein belongs to a large protein complex termed ASCOM, which is one of the transcriptional regulators of the estrogen receptor genes [12,13].
KMT2D mutations have been associated with the development of different tumors, including small cell lung cancer [13], esophageal squamous cell carcinoma, and large B-cell lymphoma [13]. Although there are many other tumors where mutations in this gene have been described [13,20], limited information about the presence of this mutation has been reported in breast cancer.
Recent data suggest that KMT2D is involved in the recruitment and activation of relevant breast cancer genes including FOXA1, PBX1, and ER [12]. As described in the present article and other reports, most of the mutations in KMT2D are frameshift and nonsense mutations in the SET and PHD domains, respectively [12]. Most of the described mutations result in the protein loss or in a reduction of the methyltransferase activity [21]. Therefore, this can produce defective enhancer regulation and, subsequently, modifications in the transcription of several genes or increase in genomic instability [8,14]. This is demonstrated in our study by the transcriptomic signature associated with the gene mutation, which will be discussed later, particularly with the upregulation of RAC3. Of note, KMT2D displays different effects depending on the cellular context, due to the recruitment of different transcription factors [13].
When evaluating the transcriptomic signature linked to KMT2D mutations, we found that RAC3 was one of the most significantly upregulated transcripts. This transcript codes for a GTPase which belongs to the RAS superfamily of small GTP-binding proteins, and it has been linked with the pathophysiology of many solid tumors, including breast cancer [19,22,23]. In breast cancer RAC3 regulates invasion and migration participating in the metastatic process[19].
Finally, we confirmed that the expression level of the KMT2D gene was associated with clinical outcome in a similar manner as we observed for the presence of the gene mutations, which mostly produce a reduction or loss of protein expression or a decrease in its activity. This result indirectly confirms the robustness of the mutational gene signature in relation to outcome.
In conclusion, in the present work, we identify that the histone methyltransferase gene KMT2D is mutated in a number of TNBC patients and associated with detrimental outcome in TNBC. Therefore, modulation of the expression or activity of downstream genes, or KMT2D itself, might have relevant consequences from a therapeutic point of view.
Supporting information
A. all breast cancer patients. B. Triple negative breast cancer patients.
(TIFF)
Genes with worse outcome for each cancer subtype are shown in the table.
(TIFF)
Genes found to be upregulated or downregulated in the KMT2D mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Genes found to be upregulated or downregulated in the SETD1A mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Genes found to be upregulated or downregulated in the SETD2 mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Data Availability
Data used in this study is from the Breast Cancer METABRIC study (EGAS00000000083), contained at cBioPortal (http://www.cbioportal.org). Data may directly be found here: https://www.cbioportal.org/study?id=brca_metabric.
Funding Statement
Instituto de Salud Carlos III (PI16/01121), ACEPAIN; Diputación de Albacete and CRIS Cancer Foundation (to AO). Ministry of Economy and Competitiveness of Spain (BFU2015-71371-R), the Instituto de Salud Carlos III through the Spanish Cancer Centers Network Program (RD12/0036/0003) and CIBERONC, the scientific foundation of the AECC and the CRIS Foundation (to AP). The work carried out in our laboratories receive support from the European Community through the regional development funding program (FEDER). E.M. Galan-Moya is funded by the implementation research program of the UCLM (UCLM resolution date: 31/07/2014), with a contract for accessing the Spanish System of Science, Technology and Innovation-Secti (co-funded by the European Commission/FSE funds).
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science. 2013; 339: 1546–1558. 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocana A, Pandiella A. Personalized therapies in the cancer "omics" era. Mol Cancer. 2010; 9: 202 10.1186/1476-4598-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber TD, Vogelstein B, Kinzler KW, Velculescu VE. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med. 2004; 351: 2883 10.1056/NEJM200412303512724 [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364: 2507–2516. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344: 783–792. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 6.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990; 247: 1079–1082. [DOI] [PubMed] [Google Scholar]
- 7.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001; 344: 1038–1042. 10.1056/NEJM200104053441402 [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Epigenetics in cancer. N Engl J Med. 2008; 358: 1148–1159. 10.1056/NEJMra072067 [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006; 7: 21–33. 10.1038/nrg1748 [DOI] [PubMed] [Google Scholar]
- 10.Pfister SX, Ashworth A. Marked for death: targeting epigenetic changes in cancer. Nat Rev Drug Discov. 2017; 16: 241–263. 10.1038/nrd.2016.256 [DOI] [PubMed] [Google Scholar]
- 11.Ocana A, Nieto-Jimenez C, Pandiella A. BET inhibitors as novel therapeutic agents in breast cancer. Oncotarget. 2017; 8: 71285–71291. 10.18632/oncotarget.19744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets, et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science. 2017; 355: 1324–1330. 10.1126/science.aah6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froimchuk E, Jang Y, Ge K. Histone H3 lysine 4 methyltransferase KMT2D. Gene. 2017; 627: 337–342. 10.1016/j.gene.2017.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantidakis T, Saponaro M, Mitter R, Horswell S, Kranz A, Boeing S, et al. Mutation of cancer driver MLL2 results in transcription stress and genome instability. Genes Dev. 2016; 30: 408–420. 10.1101/gad.275453.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012; 486: 346–352. 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pongor L, Kormos M, Hatzis C, Pusztai L, Szabo A, Györffy B. A genome-wide approach to link genotype to clinical outcome by utilizing next generation sequencing and gene chip data of 6,697 breast cancer patients. Genome Med. 2015; 7: 104 10.1186/s13073-015-0228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyorffy B, Surowiak P, Budczies J, Lanczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013; 8: e82241 10.1371/journal.pone.0082241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010; 123: 725–731. 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- 19.Donnelly SK, Cabrera R, Mao SPH, Christin JR, Wu B, Guo W, et al. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J Cell Biol. 2017; 216: 4331–4349. 10.1083/jcb.201704048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013; 502: 333–339. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Dominguez-Sola D, Hussein S, Lee JE, Holmes AB, Bansal M, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015; 21: 1190–1198. 10.1038/nm.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai YJ, Tsai JC, Tseng YT, Wu MS, Liu WS, Lam HI, et al. Small G protein Rac GTPases regulate the maintenance of glioblastoma stem-like cells in vitro and in vivo. Oncotarget. 2017; 8: 18031–18049. 10.18632/oncotarget.14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg BJ, Gil-Henn H, Mader CC, Halo T, Yin T, Condeelis J, et al. Phosphorylated cortactin recruits Vav2 guanine nucleotide exchange factor to activate Rac3 and promote invadopodial function in invasive breast cancer cells. Mol Biol Cell. 2017; 28: 1347–1360. 10.1091/mbc.E16-12-0885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. all breast cancer patients. B. Triple negative breast cancer patients.
(TIFF)
Genes with worse outcome for each cancer subtype are shown in the table.
(TIFF)
Genes found to be upregulated or downregulated in the KMT2D mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Genes found to be upregulated or downregulated in the SETD1A mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Genes found to be upregulated or downregulated in the SETD2 mutational signature. Genotype-2-Outcome database was used for this analysis.
(TIFF)
Data Availability Statement
Data used in this study is from the Breast Cancer METABRIC study (EGAS00000000083), contained at cBioPortal (http://www.cbioportal.org). Data may directly be found here: https://www.cbioportal.org/study?id=brca_metabric.