Abstract
Context
Delayed gastric emptying (GE) is common but often asymptomatic in diabetes. The relationship between symptoms, glycemia, and neurohormonal functions, including glucagonlike peptide 1 (GLP-1), are unclear.
Objectives
To assess whether GE disturbances, symptoms during a GE study, and symptoms during enteral lipid infusion explain daily symptoms and whether GLP-1 mediates symptoms during enteral lipid infusion.
Design
In this randomized controlled trial, GE, enteral lipid infusion, gastrointestinal (GI) symptoms during these assessments, autonomic functions, glycosylated hemoglobin (HbA1c), and daily GI symptoms (2-week Gastroparesis Cardinal Symptom Index diary) were evaluated. During enteral lipid infusion, participants received the GLP-1 antagonist exendin 9-39 or placebo.
Setting
Single tertiary referral center.
Participants
24 healthy controls and 40 patients with diabetic gastroenteropathy.
Main Outcome Measures
GE, symptoms during enteral lipid infusion, and the effect of exendin 9-39 on the latter.
Results
In patients, GE was normal (55%), delayed (33%), or rapid (12%). During lipid infusion, GI symptoms tended to be greater (P = 0.06) in patients with diabetes mellitus (DM) than controls; exendin 9-39 did not affect symptoms. The HbA1c was inversely correlated with the mean symptom score during the GE study (r = −0.46, P = 0.003) and lipid infusion (r = −0.47, P < 0.01). GE and symptoms during GE study accounted for 40% and 32%, respectively, of the variance in daily symptom severity and quality of life.
Conclusions
In DM gastroenteropathy, GE and symptoms during a GE study explain daily symptoms. Symptoms during enteral lipid infusion were borderline increased but not reduced by a GLP-1 antagonist.
Gastric emptying (GE) and symptoms during a GE study explain daily symptoms in diabetic gastroenteropathy. A glucagon-like peptide 1 antagonist did not reduce symptoms during an intestinal lipid infusion.
In secondary referral diabetic clinics, ≤50% of patients with type 1 and type 2 diabetes mellitus (DM) have delayed gastric emptying (GE) (1–3); rapid GE is less common (4). Between 5% and 12% of patients with diabetes have gastrointestinal (GI) symptoms (5). The upper GI manifestations of DM, henceforth referred to as diabetic gastroenteropathy, may be categorized into three groups: gastroparesis, which is a syndrome characterized by delayed GE and severe upper GI symptoms that suggest but are not associated with gastric outlet obstruction; dyspepsia (i.e., indigestion), which is characterized by less severe upper GI symptoms and normal, mildly delayed, or rapid GE; and paucisymptomatic or asymptomatic delayed GE (6). Although selected symptoms (e.g., early satiety) are associated with delayed GE in DM (7), the relationship between GI symptoms and delayed GE is variable. Indeed, many patients with markedly delayed GE are asymptomatic, prompting skepticism about the contribution of delayed GE to symptoms and the clinical utility of measuring GE (8).
By contrast, more severe symptoms observed during a GE study were associated with the more severe daily symptoms in 409 patients with dyspepsia, of whom 73 had DM (9). The latter study appraised univariate associations of symptoms during a GE study with daily symptoms. Whether symptoms during a GE study augment the utility of GE itself for predicting the severity of daily symptoms is unknown.
Current concepts of the pathogenesis of diabetic gastroenteropathy in humans focus on delayed GE and its relationship to acute hyperglycemia, autonomic neuropathy, and enteric neuromuscular inflammation and injury (5). Abnormal gastric accommodation and perception have also been described (10). In the only study that evaluated symptoms during enteral lipid infusion in DM, the perception of duodenal nutrients (90 kcal over 330 minutes) was not different between eight patients with diabetes and nine controls (11). However, this low caloric load was probably insufficient to evoke symptoms. Therefore, the contribution of enteral nutrient sensitivity to symptoms in diabetic gastroenteropathy is unclear. By comparison, ~60% of patients with functional dyspepsia but only 10% of controls had severe symptoms during duodenal nutrient infusion of a higher caloric load (300 kcal over 120 minutes) (12). In that and another study, the severity of symptoms after oral and enteral lipid ingestion was associated with plasma glucagonlike peptide 1 (GLP-1) levels (13). DM is also associated with disturbances of GLP-1, which induces nausea and satiety and delays GE by vagally mediated mechanisms (14). Whether GLP-1 contributes to symptoms during enteral lipid infusion in healthy people and DM gastroenteropathy is unknown.
Therefore, the aims of this study were to compare GE, symptoms during a GE study, autonomic functions, and sensitivity to duodenal nutrient infusion in patients with diabetic gastroenteropathy and healthy people and to evaluate whether GI sensorimotor dysfunctions can predict day-to-day symptoms and quality of life (QOL); evaluate the contribution of GLP-1 to symptoms during enteral lipid infusion; and evaluate the relationship between control of glycemia and GI sensorimotor functions (i.e., delayed GE and symptoms during a GE study and enteral nutrient infusion). Our hypotheses were that GE and symptoms during a GE study predict the severity of daily symptoms and QOL in diabetic gastroenteropathy, patients with diabetes with upper GI symptoms have more severe symptoms than healthy controls during enteral nutrient infusion, autonomic neuropathy is associated with delayed GE and more severe symptoms during a GE study in DM, a GLP-1 antagonist reduces the severity of symptoms during enteral infusion, and hyperglycemia is associated with delayed GE and less severe symptoms during a GE study and during intestinal nutrient infusion.
Materials and Methods
Study participants
Twenty-four healthy asymptomatic controls (mean ± SE age 40 ± 3 years; 14 women) with a body mass index (BMI) of 26.1 (±1.0) kg/m2 and 40 patients with DM and upper GI symptoms (age 45 ± 2 years; 31 women) with a BMI of 27.8 (±0.9) kg/m2 were studied (Table 1). Participants were recruited by public advertisement (controls) and from the clinical practice (patients). The main exclusion criteria for all participants were age <18 or >70 years; another condition or GI structural disorder that may cause GI symptoms; DM (controls only); clinically significant systemic (e.g., cardiovascular, respiratory, renal) disease that may interfere with study objectives or pose safety concerns; GI surgery other than appendectomy, cholecystectomy, hysterectomy, tubal ligation, or inguinal hernia repair; and opioids or anticholinergic agents. All women of childbearing potential had to have a negative pregnancy test within 48 hours of study participation. The study was approved by the Mayo Clinic Institutional Review Board, registered on clinicaltrials.gov (NCT02170870), and conducted between June 2014 and May 2017 at the Mayo Clinic, Rochester, Minnesota. All participants signed informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Table 1.
Demographic and Baseline Clinical Characteristics
| Characteristic | Controlsa (n = 24) | Patients With T1DM (n = 23) | Patients With T2DM (n = 17) |
|---|---|---|---|
| Age, y | 40 (3) | 38 (3) | 54 (2) |
| BMI, kg/m2 | 26.1 (1.0) | 25.4 (1.0) | 31.0 (1.3) |
| Female sex, no. (%) | 14 (58) | 19 (83) | 12 (71) |
| Borderline, definite anxiety, no. | 0, 0 | 4, 3 | 4, 2 |
| Borderline, definite depression, no. | 0, 0 | 4, 1 | 4, 1 |
| NDI dyspepsia symptom severity score, median (IQR) | 12.95 (12.5, 13.0) | 11.1 (10.6, 11.8) | 10.6 (9.9, 12.2) |
| NDI QOL score, median (IQR) | 100 (100,100) | 52.2 (26.5, 79.4) | 39.43 (22.1, 83.6) |
| PAGI-SYM subscores, median (IQR) | |||
| Heartburn subscore | 0 (0, 0.14) | 2.4 (0.75, 3.1) | 2.8 (1.8, 3.9) |
| Nausea, vomiting, and regurgitation subscore | 0 (0, 0) | 1.3 (0.6, 3.3) | 1.3 (0.3, 2.9) |
| Early satiety subscore | 0 (0, 0.25) | 2.4 (0.8, 3.1) | 2.8 (1.8, 3.9) |
| Bloating subscore | 0 (0, 0.13) | 2.5 (1, 4) | 3.3 (0.6, 4.4) |
| Upper abdominal pain subscore | 0 (0, 0) | 2.3 (1, 3.1) | 2 (1, 3.5) |
| HbA1c (%) | 5.2 (0.07) | 9.2 (0.5) | 8.1 (0.4) |
| Colonic filling at 6 h (%) | 56 (4) | 47 (6) | 42 (7) |
| Cardiovagal dysfunction, no. (%) | 1 (4) | 13 (57) | 2 (12) |
| Cardiovascular sympathetic dysfunction, no. (%) | 1 (4) | 11 (48) | 4 (24) |
| Sudomotor dysfunction, no. (%) | 4 (17) | 12 (52) | 7 (41) |
Abbreviations: IQR, interquartile range; NDI, Nepean Dyspepsia Index.
Values are presented as mean (SE) unless specified otherwise.
Assessment of dyspepsia symptoms
At baseline, the Nepean Dyspepsia Index questionnaire assessed symptoms and QOL related to dyspepsia in the past 3 months (15, 16). QOL was averaged for five domains to obtain an overall QOL that was then subtracted from 100; lower scores reflect poorer QOL. GI symptoms over the preceding 2 weeks were evaluated with the Patient Assessment of Upper Gastrointestinal Disorders–Symptom Severity (PAGI-SYM) index and summarized as subscores (17). The Hospital Anxiety and Depression Scale (HADS) questionnaire was used to identify anxiety and depression (18).
After patients completed the GI and autonomic assessments, they recorded their GI symptoms every day in the Gastroparesis Cardinal Symptom Index–Daily Diary (GCSI-DD) for 2 weeks (19, 20). The nausea, vomiting, fullness, and pain (NVFP) score was computed by averaging these four symptoms (21).
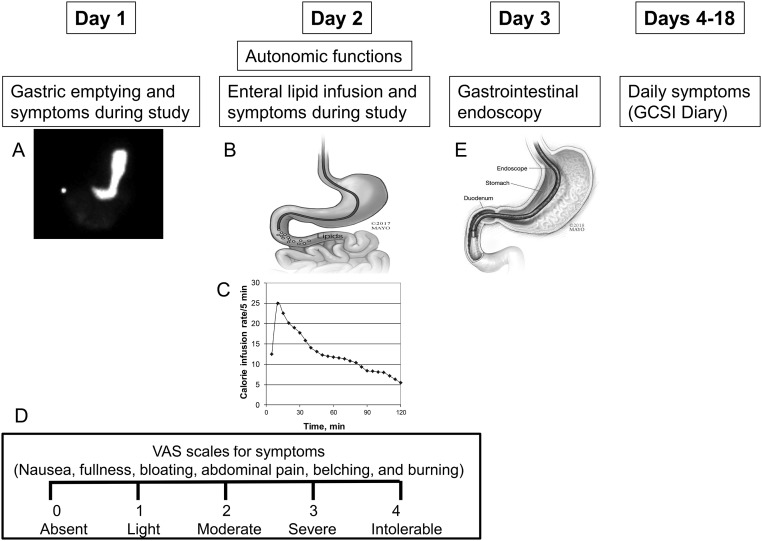
Gastric and small bowel transit
Study procedures (i.e., GE and enteral nutrient infusion) were performed on consecutive days (Fig. 1). After a meal (296 kcal; 32% proteins, 35% fats, and 33% carbohydrates) consisting of two eggs labeled with technetium sulfur colloid (1 mCi) served on one slice of bread with milk (240 mL; 1% fat) labeled with indium diethylenetriaminepentaacetate (0.1 mCi), GE of solids and liquids and small bowel transit were simultaneously assessed with scintigraphy (12). Based on data in 319 healthy participants studied with the same techniques, rapid and delayed emptying were defined as ≥36% emptied at 1 hour and <76% emptied at 4 hours, respectively (22). Small bowel transit time was calculated as the percentage of isotopically labeled meal filling the colon at 6 hours (23).
Figure 1.
Study design. (A) GE scintigraphy, (B, C) enteral lipid infusion at a variable rate, and (E) GI endoscopy were performed over 3 days. (D) Symptoms during the GE study and lipid infusion were assessed with VAS scales. Thereafter, participants recorded their symptoms daily for 2 weeks. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Autonomic functions
Cardiovagal, adrenergic, and sudomotor functions were evaluated with standardized techniques (21, 24). We calculated a semiquantitative composite autonomic severity score (CASS) ranging from 0 to 10 by combining sudomotor (range, 0 to 3), cardiovagal (range, 0 to 3), and adrenergic (range, 0 to 4) scores that were adjusted for age and sex.
Enteral nutrient infusion
Enteral nutrients were administered through an 8-Fr vinyl nasoduodenal feeding tube placed under fluoroscopic guidance with the tip in the second part of the duodenum (Fig. 1B). To avoid increasing hyperglycemia in DM, only lipids were administered. The lipid infusion (Nestle HealthCare, Bridgewater, NJ) (66.7 mL diluted to 222 mL, for 0.5 g/mL, 300 kcal) was administered at a rate that mimicked the systemic delivery of glucose after glucose ingestion, over 2 hours (Fig. 1C) (12).
Exendin 9-39
During enteral lipid infusion, participants were randomly assigned in a parallel manner to placebo or the GLP-1 antagonist exendin 9-39 (C.S. Bio, Menlo Park, CA), at a ratio of 1:1. Under the aegis of an investigator-initiated Investigational New Drug authorization from the Food and Drug Administration, the exendin 9-39 was administered intravenously as a bolus (1200 pmol/kg) followed by infusion at 300 pmol/kg/min throughout the lipid infusion (25, 26). This dosage blocks the effects of GLP-1 infused at supraphysiologic dosages and the effects of endogenous GLP-1 on GI motility and insulin secretion (27–29). Exendin 9-39 is safe and well tolerated and has modest effects on blood glucose even in type 1 diabetes mellitus (T1DM) (30).
Symptoms during GE study and enteral nutrient infusion
During both studies, participants reported the severity of six symptoms—nausea, fullness, bloating, abdominal pain, belching, and burning—at 15-minute intervals on a Likert scale marked absent (0), light (1), moderate (2), severe (3), and intolerable (4) (Fig. 1D) (12). For each study, data were analyzed as the mean symptom score, which was the average of scores for nausea, fullness, bloating, and abdominal pain over the 2-hour infusion; and the proportion of participants with severe or intolerable symptoms during the study.
Upper GI endoscopy
The same investigator (A.E.B.) performed an upper GI endoscopy in all participants to identify structural causes for symptoms.
Study endpoints
The primary outcome measure was the mean of the symptom scores during the enteral lipid infusion. The secondary outcome variables were GE t1/2, mean symptom score during the GE study, CASS scores, NVFP score calculated from GCSI-DD, PAGI-SYM subscores, and QOL score computed from Nepean Dyspepsia Index questionnaire.
Blood glucose and hormones
Blood samples for plasma glucose, C-peptide, GLP-1, cholecystokinin (CCK), ghrelin, and protein YY (PYY) were collected at 5-minute intervals for 30 minutes, at 10-minute intervals from 30 to 60 minutes, and at 15-minute intervals from 60 to 120 minutes. Arterialized venous plasma samples were obtained from a hand or forearm vein and were placed in a Perspex hot box heated to 55°C. Samples were placed in ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose was measured by the Hitachi 912 assay (Roche Diagnostics) (12). GLP-1 enzyme-linked immunosorbent assay (Linco Research, Inc.) measures biologically active GLP-1 (7-36 amide, 7-37) levels with no cross-reactivity to GLP-1 (9-36) amide, GLP-2, or glucagon. The CCK immunoassay (Alpco Diagnostics) uses rabbit antiserum to a synthetic cholecystokinin 26-33 sulfate (CCK 8 sulfate) and has no cross-reactivity with gastrin. Radioimmunoassays were used for PYY (Linco Research Inc.), which detects two molecular physiologically active forms (1-36, 3-36) and for total ghrelin (Linco Research, Inc).
Sample size and statistical analysis
The sample size was based on the ability to detect the effects of exendin 9-39 on symptoms during enteral lipid infusion. Previously, 56% of patients with dyspepsia reported severe symptoms during enteral nutrient infusion (12). Assuming that a comparable proportion of patients with DM have severe symptoms during enteral infusion, the sample size of 40 patients and 24 controls provided 80% power to detect an absolute difference of 38% between the prevalence of symptoms in patients with DM treated with placebo and exendin 9-39 (e.g., 18% vs 56%).
An Excel spreadsheet of treatment assignments (balanced on sex and GE using a block size of four), was generated by computer (by A.R.Z.) and sent to the research pharmacy. Study personnel were blinded until the study was completed.
All analyses used SAS software (version 9.3; SAS Institute, Cary, NC), and continuous data are reported as mean ± SEM and discrete data as frequencies (%). The associations between symptoms during nutrient infusion and participant status (controls vs patients) were evaluated with Fisher exact test. The association between GE and separate symptoms during a GE study with other variables [i.e., fasting blood glucose, glycosylated hemoglobin (HbA1c), autonomic functions, and GLP-1 secretion during enteral lipid infusion] was evaluated with the Kruskal-Wallis test and Spearman correlations. An analysis of covariance compared symptoms during enteral lipid infusion and exendin’s effects between controls and patients with DM. Multiple linear regression models assessed the extent to which GE and symptoms during the GE study predicted daily symptoms summarized by the NVFP score (21) and overall quality of life calculated from the Nepean Dyspepsia Index questionnaire.
Results
Participants, study conduct, and completion
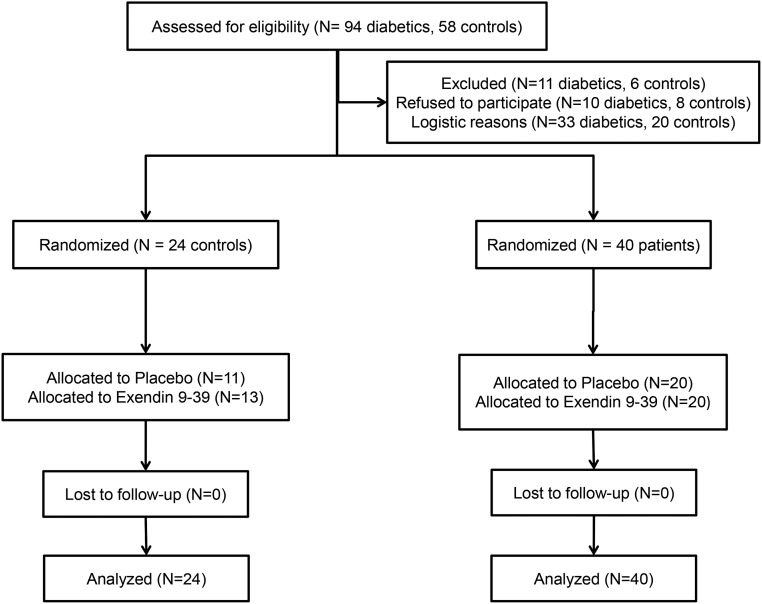
All 64 participants completed the GE and autonomic function studies. Enteral lipid infusion was not performed for four participants because a nasoduodenal tube could not be placed (three controls) or because of a computer malfunction (one patient) (Fig. 2). Of 21 controls and 39 patients who received the enteral infusion, the infusion was terminated prematurely because of GI symptoms in one control and one patient.
Figure 2.
Consolidated Standards of Reporting Trials flow diagram.
Demographic and clinical characteristics
The sex distribution, BMI, and age were not significantly different between controls and patients (Table 1). Among patients, fasting and peak plasma C-peptide concentrations were ≥200 pmol/L in 17 patients (43%), suggesting that endogenous insulin reserve was relatively preserved, consistent with type 2 diabetes mellitus (T2DM). Complications of DM were as follows: peripheral neuropathy (30 patients), nephropathy (16 patients), and retinopathy (16 patients). Medications for DM included insulin (32 patients) and biguanides (i.e., metformin, 11 patients), sulfonylureas (2 patients), dipeptidyl peptidase 4 inhibitors (2 patients), GLP-1 receptor analog (1 patient), and sodium-glucose cotransporter-2 inhibitor (1 patient).
Among patients, the predominant GI symptoms were dyspepsia (n = 26), nausea and vomiting (n = 10), and abdominal pain or bloating with or without bowel disturbances (n = 4). The upper GI endoscopy was normal or disclosed cystic fundic gland polyps (20 patients and 14 controls), antral erythema or minor erosions that were not deemed sufficient to explain symptoms (10 patients and 4 controls), mild esophagitis (2 patients and 3 controls), and retained food (8 patients). Three controls did not have an upper endoscopy. No controls and 4 patients had a cholecystectomy.
Five patients had definite anxiety, one had definite depression, and one had both. No controls had anxiety or depression.
GE and small bowel transit
GE of solids was normal in 18 controls (75%) and 22 patients (55%), including the patient who was taking a GLP-1 agonist. Two controls (8%) and 13 patients (33%, P = 0.03 vs controls) had delayed GE, and 4 controls (17%) and 5 patients (12%) had rapid emptying of solids. GE of liquids was normal in 23 controls (96%) and 22 patients (55%). One control and 8 patients had a prolonged gastric emptying half time (GE t1/2) (i.e., delayed GE of liquids). Of these 8 patients, all had delayed GE of solids. The colonic filling at 6 hours, which is a surrogate marker for small bowel transit, was not significantly different between controls and patients with DM.
Symptoms during GE study
During the GE study, no controls but 23 of 40 patients (58%) (P = 0.0001), reported one or more severe (Likert score 3) or intolerable (Likert score 4) symptoms [i.e., fullness (20 patients), bloating (15 patients), nausea (11 patients), abdominal pain (12 patients), belching (7 patients), and heartburn (4 patients)]. Among these patients, 12 had normal, 3 had rapid, and 8 had delayed GE. Hence, similar proportions [i.e., 12 of 22 patients (55%) with normal, 8 of 13 patients (62%) with delayed, and 3 of 5 patients (60%) with rapid GE] had severe symptoms during the GE study.
Enteral nutrient infusion
Twelve patients and three controls reported at least one symptom of severe or intolerable intensity during lipid infusion (P = 0.2). These patients had normal (n = 6), delayed (n = 5), or rapid (n = 1) GE. Of 17 patients who did not have severe symptoms during the GE study, 15 did not have severe symptoms during the enteral lipid infusion either. The mean symptom scores during the GE study and enteral infusion were correlated (r = 0.58, P < 0.0001).
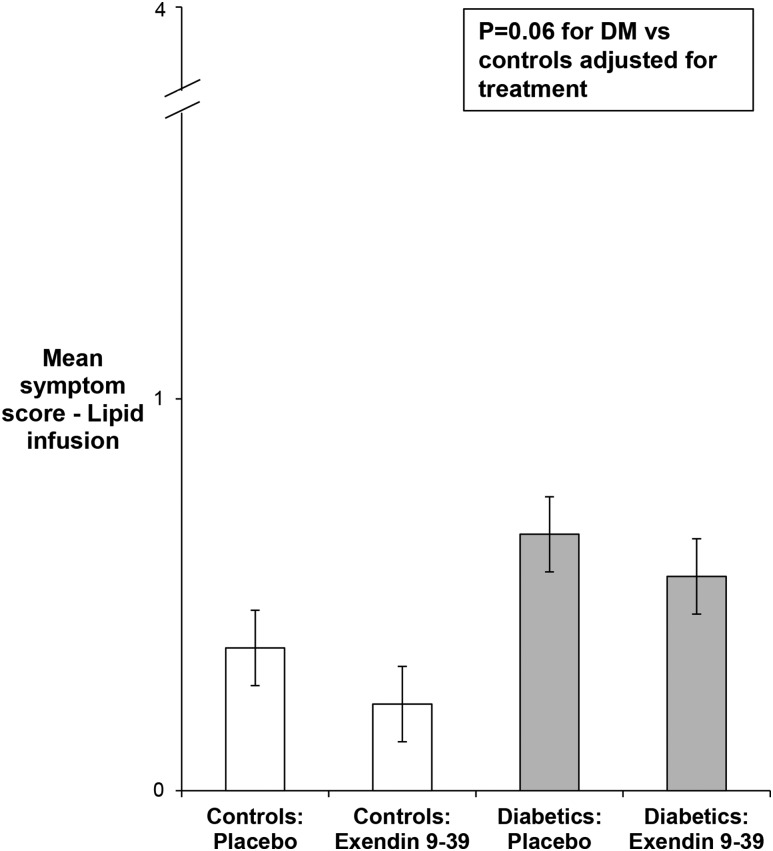
Of 30 women and 9 men, 16 women and 4 men were randomly assigned to exendin 9-39. Among patients with DM, the HADS anxiety and depression scores, severity of daily symptoms, and GE t1/2 were not significantly different between patients randomly assigned to exendin 9-39 and placebo. Exendin did not significantly affect symptoms during enteral infusion. Six of 12 patients and 2 of 3 controls with severe symptoms received exendin 9-39. Among controls randomly assigned to placebo and exendin 9-39, the mean symptom scores during lipid infusion were 0.36 ± 0.13 and 0.22 ± 0.05, respectively. Similarly, among patients, these scores were 0.65 ± 0.17 and 0.55 ± 0.11 for placebo and exendin 9-39, respectively. After adjustment for treatment, the mean GI symptom score during lipid infusion tended to be greater (P = 0.06) in patients with DM than in controls (Fig. 3).
Figure 3.
Comparison of symptoms during enteral lipid infusion in controls and patients with DM.
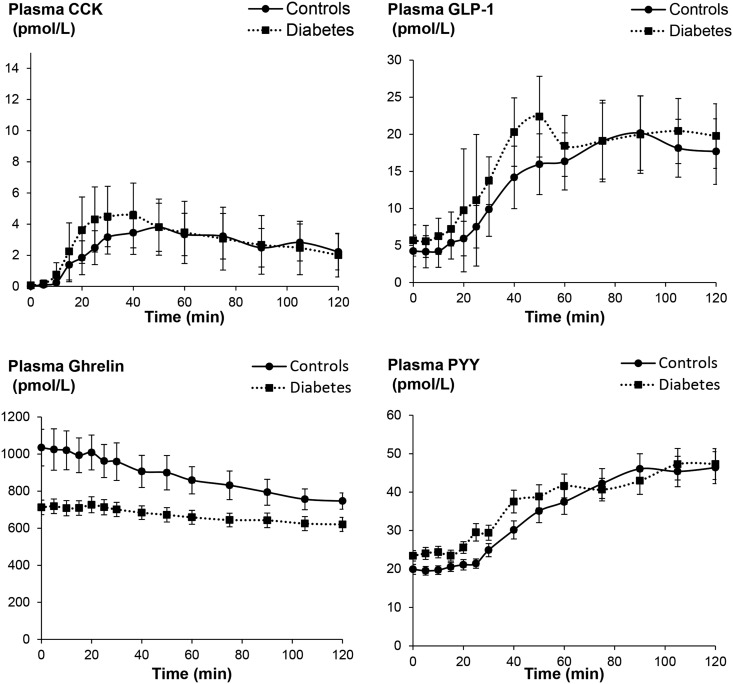
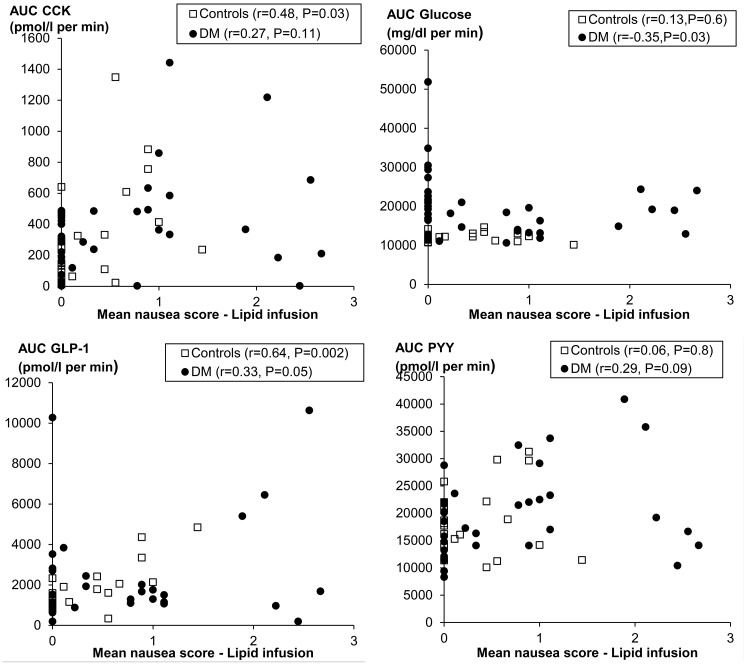
Of the gut hormones, only ghrelin levels were different (P = 0.009), being greater in controls than patients with DM (Table 2, Fig. 4). During lipid infusion, nausea was the symptom most strongly correlated with plasma hormone levels. Among all participants, the mean nausea score was correlated with plasma concentrations of CCK (r = 0.35, P < 0.01) and GLP-1 (r = 0.37, P < 0.005). Likewise, the mean symptom score was correlated with plasma concentrations of CCK (r = 0.30, P = 0.02) and GLP-1 (r = 0.31, P = 0.02) (Fig. 5). Plasma insulin, PYY, and ghrelin levels were not correlated with nausea (data not shown).
Table 2.
Comparison of Glucose and Plasma Hormones in Controls and Diabetic Enteropathy
| Controls | Diabetes Mellitus | |
|---|---|---|
| Glucose | 12,130.8 (10,705, 14,203) | 19,715 (11,579, 31,590) |
| Insulin | 1003 (396, 2354) | 999 (19, 3324) |
| GLP-1 | 1854 (817, 4364) | 2160 (132, 7411) |
| CCK | 330 (23, 883) | 367 (3, 949) |
| Ghrelin | 110,449 (66,550, 150,720) | 82,215 (54,425, 117,715)a |
| PYY | 4572 (2776, 7359) | 4840 (2327, 8453) |
Values are presented as area under the curve mean (5th percentile, 95th percentile) as pmol/L/min (CCK, glucagon, and PYY), mg/dL/min (glucose), and pg/mL/min (ghrelin).
P = 0.009 vs controls.
Figure 4.
Changes in plasma hormone (CCK, GLP-1, ghrelin, and PYY) levels measured during enteral lipid infusion. Values are mean ± SEM.
Figure 5.
Relationship between nausea during lipid infusion and plasma hormones.
Relationship between glycemia and GI function
Among patients with DM, the HbA1c was correlated with the GE t1/2 of solids in T1DM (r = 0.50, P = 0.02) but not T2DM (r = −0.29, P = 0.23). The fasting blood glucose (i.e., 168 ± 16 mg/dL in T1DM and 150 ± 11 mg/dL in T2DM) was not correlated with the GE t1/2 of solids or liquids. Only one patient with delayed GE had a fasting blood glucose ≥275 mg/dL. The mean symptom score during GE was not correlated with fasting blood glucose (r = −0.15, P = 0.37).
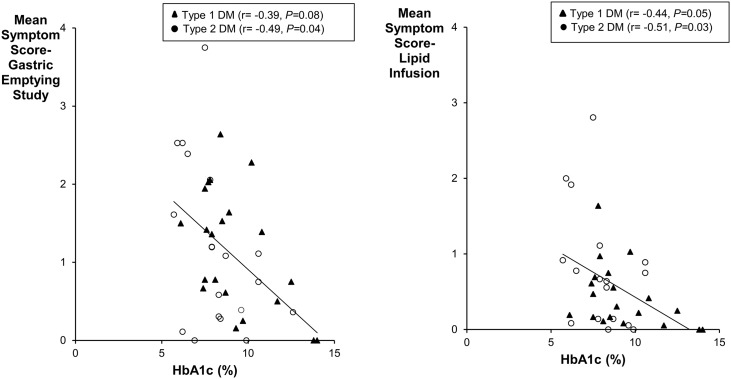
The HbA1c was inversely correlated with the mean symptom score during the GE study (r = −0.46, P = 0.003) and lipid infusion (r = −0.47, P < 0.01) (Fig. 6).
Figure 6.
Comparison of HbA1c with symptoms during GE study (left panel) and enteral lipid infusion (right panel).
Cardiovascular autonomic nervous functions
More patients than controls had cardiovagal (P = 0.003), adrenergic (P = 0.003), and sudomotor dysfunctions (P = 0.02) (Table 1). Among patients with DM, vagal dysfunction was not associated (P = 0.33, Kruskal-Wallis test) with slower GE of solids.
Among patients with DM and controls, the CASS total score was weakly correlated with the fullness (r = 0.34, P = 0.006), bloating (r = 0.31, P = 0.01), and abdominal pain (r = 0.29, P = 0.02) subscores during lipid infusion. Correlations between the CASS vagal score and individual GE symptom scores for bloating (r = 0.30, P = 0.01), fullness (r = 0.38, P = 0.002), and abdominal pain (r = 0.35, P = 0.004) but not nausea were significant.
Factors associated with daily symptom severity and QOL
The severity of the most severe symptom, as calculated by the average subscore from the PAGI-SYM questionnaire, was very mild (PAGI-SYM score of 1 to 1.99, 7 patients), mild (2 to 2.99, 10 patients), moderate (3 to 3.99, 2 patients), severe (4 to 4.99, 16 patients), or very severe (5, 3 patients).
Among patients with DM but not controls, the mean symptom score during GE strongly was correlated with the GCSI-DD score (r = 0.62, P < 0.0001). In the univariate analysis, the mean symptom score during the GE study explained 32% (P = 0.0001), and the mean symptom score during lipid infusion explained 11% (P = 0.03) of the variation in daily symptom severity in patients with DM.
In the multivariate model (Table 3), the GE t1/2 of solids and mean symptom score during GE study together explained 40% (P = 0.0001) of the variance in daily symptom severity in DM. Among controls and patients with DM, the GE t1/2 of solids and mean symptom score during GE study explained 60% (P < 0.0001) of the variance in symptoms. Symptoms scores during enteral lipid infusion were not significant and therefore not included in these models.
Table 3.
Multivariable Linear Regression Models for Predicting Symptoms and QOL in Diabetic Gastroenteropathy
|
Daily Symptoms
|
Daily QOL
|
||
|---|---|---|---|
| Patients With DM | Controls and Patients With DM | Patients With DM | |
| GE t1/2 | 0.19 (0.009) | 0.22 (0.0003) | 0.12 (0.06) |
| Mean symptom score during GE study | 0.38 (<0.0001) | 0.53 (<0.0001) | 0.30 (0.001) |
| Mean symptom score during enteral lipid infusion | 0.02 (0.41) | ||
| Total variance explained, % | 40 (0.0001) | 60 (<0.0001) | 32 (0.002) |
Unless stated otherwise, data are partial R2 (P values) values.
In DM, the mean symptom score during the GE study explained 19% (P = 0.004) of the variance in QOL. The mean symptom score during the GE study and lipid infusion and the GE t1/2 of solids explained 32% of variance in QOL in DM (P = 0.002). The anxiety and depression scores, HbA1c, and CASS scores did not predict the severity of daily symptoms or QOL in DM (data not shown).
Discussion
In this controlled study, 55% of 40 patients with diabetes had normal, 33% had delayed, and 12% had rapid GE. Fifty-five percent of patients with normal GE, 62% with delayed, and 60% with rapid GE had severe symptoms during the GE study. Likewise, 27% of patients with normal GE, 38% with delayed GE, and 20% with rapid GE reported severe symptoms during the intestinal lipid infusion. Hence, 31 of 40 (78%) patients had abnormal GE or increased perception based on symptoms during enteral nutrient infusion. Among 388 patients with dyspepsia, of whom 73 had diabetes, the severity of symptoms during a GE study was correlated with daily symptoms (9). In the current study, GE and symptoms during a GE study independently explained 40% of the variation in daily symptom severity and 32% of the variation in QOL among patients with DM. Taken together, these findings underscore the utility of evaluating GE and symptoms during a GE study in patients with diabetic gastroenteropathy. The correlation between severity of symptoms during the GE study and lipid infusion, which were performed on different days, supports the face validity of these assessments and suggests that symptoms after ingestion of oral nutrients partly reflect enteral nutrient sensitivity.
The concept that hyperglycemia affects gastric sensorimotor functions emanates primarily from small studies in which acute hyperglycemia (i.e., blood glucose of ~15 mmol/L) delayed GE and increased perception of gastric distension relative to euglycemia (6 mmol/L) in T1DM (31). Because the relationship between hyperglycemia and GE may be influenced by the duration of hyperglycemia (1), it is useful to characterize glycemic exposure as follows: remote HbA1c (e.g., 20 years ago), which was not available in this study, recent HbA1c (i.e., before the GE study), and immediate (i.e., fasting glucose before the study). As in a previous study, GE was not associated with fasting blood glucose (1), which challenges the concept that acute hyperglycemia predisposes to delayed GE (31). By contrast, in T1DM, a higher recent HbA1c was associated with slower GE.
Perception of GI distension may be reduced (32, 33) or increased (10, 34) in diabetic gastroenteropathy. Only one previous study evaluated symptoms during enteral nutrient infusion, which was not different in DM, perhaps partly because a low caloric load (90 kcal) was infused (35). In this study, after adjustment for treatment, symptoms during enteral lipid infusion were numerically greater (P = 0.06) in patients with DM than controls. Likewise, the severity of postprandial symptoms was correlated with plasma GLP-1 levels in nonulcer dyspepsia (12, 13), whereas maximum satiety was correlated with the plasma GLP-1 levels after a fatty meal in obese women (36). Although these studies suggest that GLP-1 may partly mediate postprandial symptoms, exendin, administered at a dosage that blocks the effects of endogenous GLP-1 on GI motility and insulin secretion (27–29), did not reduce symptoms during enteral lipid infusion in this study. Although a type II error cannot be excluded, these observations suggest that GLP-1 does not contribute substantially to symptoms during enteral nutrient infusion. Plasma CCK levels were also correlated with the severity of symptoms during enteral lipid infusion, which suggests that CCK may also contribute to increased enteral sensitivity. Indeed, CCK receptors mediate increased sensitivity to gastric distension during enteral lipid infusion (37).
A greater HbA1c was associated with less severe symptoms, not only during the lipid infusion but also during GE study, perhaps because increased HbA1c is associated with diabetic sensory neuropathy (38). Perhaps this explains, at least in part, why many patients with diabetes with delayed GE have few symptoms (1, 5, 31). Decreased GI perception has been attributed to autonomic neuropathy, based on an assessment of cerebral evoked potentials after esophageal stimulation in 10 patients with diabetes, all of whom had polyneuropathy, and 10 controls (32). In the current study, 78% of patients had a cardiovascular vagal or adrenergic neuropathy, which was associated with more severe symptoms during the GE study. These findings suggest that, similar to peripheral neuropathy, autonomic nervous dysfunction is associated not only with sensory loss (i.e., negative symptoms or “numbness,” as in peripheral neuropathy) but also increased symptoms (e.g., pain) (39). Similar to peripheral nerves, improvement in GI symptoms may signify progression of neuropathy with worsening of sensory function. In the National Institute of Diabetes and Digestive and Kidney Diseases gastroparesis cohort, loss of interstitial cells of Cajal, reflecting enteric neuropathology, was correlated with delayed GE but not with daily symptoms (40). Additional studies are necessary to elucidate the relative contributions of autonomic neuropathy and enteric neuromuscular pathology, which probably coexist, to delayed GE and symptoms in diabetic gastroenteropathy. Conceivably, damage to the vagus nerve, interstitial cell of Cajal, and enteric neurons delay GE, whereas autonomic dysfunctions influence the symptomatic expression of delayed GE.
This study comprehensively evaluated GI sensorimotor and autonomic functions, symptoms during these assessments, and daily symptoms in a representative patient population. To reduce selection bias, the eligibility criteria did not specify a threshold symptom severity. Therefore, the severity of daily symptoms ranged from very mild to severe, allowing the relationship between GI sensorimotor dysfunctions and symptom severity to be evaluated across a wide range. The average NVFP subscore was similar to or lower than in studies of relamorelin and hemin, respectively, for diabetic gastroparesis (21, 41). In this study, the prevalence of anxiety or depression in patients with DM was 15%, which is lower than the prevalence (i.e., 35%) in some studies of patients with dyspepsia (42, 43).This finding reduces the likelihood that psychosocial disturbances would overshadow peripheral GI sensorimotor dysfunctions. Indeed, by contrast to those studies in dyspepsia, anxiety and depression did not independently predict daily symptoms in DM. Visceral mechanosensitivity and gastric accommodation, which are abnormal in some patients with DM, were not evaluated in this study (10, 34, 44). Hence, the contributions of abnormal GE, accommodation, and sensation and intestinal chemosensitivity to symptoms during a GE study are unclear.
In summary, patients with diabetic gastroenteropathy have delayed or rapid GE and borderline increased symptoms during enteral lipid infusion. Taken together, GE and symptoms during the GE study explained 30% to 40% of the variation in daily symptom severity and QOL among patients with DM, confirming the utility of these measurements in clinical practice. The HbA1c was inversely correlated with symptoms during the GE study and intestinal lipid perfusion. During intestinal lipid perfusion, patients also reported more symptoms, which were not blocked by an antagonist of the hormone GLP-1.
Acknowledgments
Financial Support: National Institute of Diabetes and Digestive and Kidney Diseases Grant DK68055 (to A.E.B.). This study was supported by US Public Health Service National Institutes of Health Grant R01 DK68055. This project was supported by Grant UL1 TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT02170870 (registered 23 June 2014).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CASS
composite autonomic severity score
- CCK
cholecystokinin
- DM
diabetes mellitus
- GCSI-DD
Gastroparesis Cardinal Symptom Index–Daily Diary
- GE
gastric emptying
- GE t1/2
gastric emptying half time
- GI
gastrointestinal
- GLP-1
glucagonlike peptide 1
- HADS
Hospital Anxiety and Depression Scale
- HbA1c
glycosylated hemoglobin
- NVFP
nausea, vomiting, fullness, and pain
- PAGI-SYM
Patient Assessment of Upper Gastrointestinal Disorders–Symptom Severity
- PYY
protein YY
- QOL
quality of life
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References
- 1. Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M, Bayless M, Wimmergren N, Herman W, Whitehouse F, Jones K, Kruger D, Martin C, Ziegler G, Zinsmeister AR, Nathan DM; Diabetes Control and Complications Trial–Epidemiology of Diabetes Interventions and Complications Research Group . Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology. 2015;149(2):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, Zinsmeister AR. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13(3):466–476.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf). 2009;70(3):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9(1):5–12; quiz e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bharucha AE, Prichard DO. Diabetic gastroparesis. Endocr Rev (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2018;02:02. [DOI] [PubMed] [Google Scholar]
- 8. Talley NJ. Editorial: moving away from focussing on gastric pathophysiology in functional dyspepsia: new insights and therapeutic implications. Am J Gastroenterol. 2017;112(1):141–144. [DOI] [PubMed] [Google Scholar]
- 9. Khayyam U, Sachdeva P, Gomez J, Ramzan Z, Smith MS, Maurer AH, Fisher RS, Parkman HP. Assessment of symptoms during gastric emptying scintigraphy to correlate symptoms to delayed gastric emptying. Neurogastroenterol Motil. 2010;22(5):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SSC. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20(6):635–642. [DOI] [PubMed] [Google Scholar]
- 11. Rayner CK, Schwartz MP, van Dam PS, Renooij W, de Smet M, Horowitz M, Wishart JM, Smout AJ, Samsom M. Upper gastrointestinal responses to intraduodenal nutrient in type 1 diabetes mellitus. Eur J Gastroenterol Hepatol. 2004;16(2):183–189. [DOI] [PubMed] [Google Scholar]
- 12. Bharucha AE, Camilleri M, Burton DD, Thieke SL, Feuerhak KJ, Basu A, Zinsmeister AR. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol. 2014;109(12):1910–1920, quiz 1909, 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witte AB, Hilsted L, Holst JJ, Schmidt PT. Peptide YY3-36 and glucagon-like peptide-1 in functional dyspepsia. Secretion and role in symptom generation. Scand J Gastroenterol. 2016;51(4):400–409. [DOI] [PubMed] [Google Scholar]
- 14. Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131(2):640–658. [DOI] [PubMed] [Google Scholar]
- 15. Talley NJ, Haque M, Wyeth JW, Stace NH, Tytgat GN, Stanghellini V, Holtmann G, Verlinden M, Jones M. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13(2):225–235. [DOI] [PubMed] [Google Scholar]
- 16. Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. Am J Gastroenterol. 1999;94(9):2390–2397. [DOI] [PubMed] [Google Scholar]
- 17. Rentz AM, Kahrilas P, Stanghellini V, Tack J, Talley NJ, de la Loge C, Trudeau E, Dubois D, Revicki DA. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13(10):1737–1749. [DOI] [PubMed] [Google Scholar]
- 18. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 19. Revicki DA, Camilleri M, Kuo B, Norton NJ, Murray L, Palsgrove A, Parkman HP. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30(6):670–680. [DOI] [PubMed] [Google Scholar]
- 20. Revicki DA, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index–Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24(5):456–463, e215–e216. [DOI] [PubMed] [Google Scholar]
- 21. Bharucha AE, Daley SL, Low PA, Gibbons SJ, Choi KM, Camilleri M, Saw JJ, Farrugia G, Zinsmeister AR. Effects of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesis. Neurogastroenterol Motil. 2016;28(11):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24(12):1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao SSC, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23(1):8–23. [DOI] [PubMed] [Google Scholar]
- 24. Loavenbruck A, Iturrino J, Singer W, Sletten DM, Low PA, Zinsmeister AR, Bharucha AE. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol Motil. 2015;27(1):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sathananthan M, Farrugia LP, Miles JM, Piccinini F, Dalla Man C, Zinsmeister AR, Cobelli C, Rizza RA, Vella A. Direct effects of exendin-(9,39) and GLP-1-(9,36)amide on insulin action, β-cell function, and glucose metabolism in nondiabetic subjects [published correction appears in Diabetes. 2013;62(12):4284–4284] Diabetes. 2013;62(8):2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah M, Law JH, Micheletto F, Sathananthan M, Dalla Man C, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63(2):483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101(7):1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Goke B. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil. 2009;21(6):609–618, e621–602. [DOI] [PubMed] [Google Scholar]
- 30. Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes. 2011;60(5):1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halland M, Bharucha AE. Relationship between control of glycemia and gastric emptying disturbances in diabetes mellitus. Clin Gastroenterol Hepatol. 2016;14(7):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care. 1991;14(11):1086–1089. [DOI] [PubMed] [Google Scholar]
- 33. Frøkjaer JB, Andersen SD, Ejskaer N, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Drewes AM. Gut sensations in diabetic autonomic neuropathy. Pain. 2007;131(3):320–329. [DOI] [PubMed] [Google Scholar]
- 34. Samsom M, Salet GA, Roelofs JM, Akkermans LM, Vanberge-Henegouwen GP, Smout AJ. Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci. 1995;40(9):2037–2042. [DOI] [PubMed] [Google Scholar]
- 35. Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24(2):371–381. [DOI] [PubMed] [Google Scholar]
- 36. Wikarek T, Chudek J, Owczarek A, Olszanecka-Glinianowicz M. Effect of dietary macronutrients on postprandial incretin hormone release and satiety in obese and normal-weight women. Br J Nutr. 2014;111(2):236–246. [DOI] [PubMed] [Google Scholar]
- 37. Feinle C, Meier O, Otto B, D’Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kazamel M, Dyck PJ. Sensory manifestations of diabetic neuropathies: anatomical and clinical correlations. Prosthet Orthot Int. 2015;39(1):7–16. [DOI] [PubMed] [Google Scholar]
- 40. Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, McCallum RW, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Unalp-Arida A, Hamilton FA, Farrugia G, NIDDK Gastroparesis Clinical Research Consortium . Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24(6):531–539, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shin A, Camilleri M, Busciglio I, Burton D, Smith SA, Vella A, Ryks M, Rhoten D, Zinsmeister AR. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11(11):1453–1459.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Fischler B, Demyttenaere K, Tack J. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57(12):1666–1673. [DOI] [PubMed] [Google Scholar]
- 43. Pohl D, Van Oudenhove L, Törnblom H, Le Nevé B, Tack J, Simrén M. Functional dyspepsia and severity of Psychologic symptoms associate with postprandial symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2018;16(11):1745–1753.e1. [DOI] [PubMed] [Google Scholar]
- 44. Søfteland E, Brock C, Frøkjær JB, Brøgger J, Madácsy L, Gilja OH, Arendt-Nielsen L, Simrén M, Drewes AM, Dimcevski G. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J Diabetes Complications. 2014;28(3):370–377. [DOI] [PubMed] [Google Scholar]