Abstract
Objective:
This study examined whether diabetic morbidity mediates the relationship of food insecurity with depression among older adults with diabetes.
Methods:
Data came from the 2010–2014 waves of the Health and Retirement Study and analyses were limited to respondents with diabetes (n=2,951). Depression was indexed by the 8-item Centers for Epidemiologic Studies Depression Scale. Weighted logistic regression was used to examine relationships of food insecurity and diabetic morbidity with depressive symptoms, both cross-sectionally and longitudinally. Path analysis quantified the contribution of diabetic morbidity as a mediation of the relationship of food insecurity with depressive symptoms.
Results:
Food insecurity was associated with having poor diabetes control (odds ratio (OR) = 1.7; 95% confidence interval (CI) = 1.1-2.5) and diabetes-related kidney problems (OR = 1.6; 95% CI = 1.1-2.5). Additionally, food insecurity was associated with depression contemporaneously (OR = 2.0, 95% CI = 1.7-2.4) and longitudinally (OR = 1.5, 95% CI = 1.3-1.8). However, food insecurity was no longer associated with depression when adjusting for diabetic morbidity. In path analyses, diabetic morbidity explained 12.7% (p-value=0.04) of the association of food insecurity with depressive symptoms in 2012 and 18.5% (p-value=0.09) of the association with depressive symptoms in 2014.
Conclusion:
The relationship of food insecurity with depression was attributable to worse diabetes morbidity. Interventions that reduce food insecurity among older adults with diabetes may improve disease management and reduce depression severity.
Keywords: Depression, Diabetes, Epidemiology, Multiple Chronic Conditions, Older Adults, Social Determinants
INTRODUCTION
Food insecurity refers to a state of uncertain or restricted access to food of sufficient quantity or quality due to financial constraint (1)—affecting approximately 8% of older United States residents in 2016 (2). Determinants of food insecurity in the U.S. include poverty, unemployment and a lack of social capital (3,4). Food insecurity is a complex psychosocial stressor that has dietary (5), nutritional (6), psychological (7), and social (8) implications. As a result, addressing food insecurity may be an important means of improving mental health disparities across the life course (9). Reducing food insecurity among older adults may be particularly important due to their high prevalence of medical conditions for which nutrition is part of ongoing disease management, such as diabetes. In the U.S., the proportion of those ≤45 years with diabetes will be as high as 30% by 2050(10).
Mood disorders, particularly depression, are highly comorbid with diabetes in a bi-directional manner (11). Diabetes distress, including illness duration, number of diabetes symptoms and poor self-care, is also associated with depression (12). Appropriate self-management of diabetes is partly dependent on maintaining a healthy diet (13) and making time for self-care (14), factors likely strained by food insecurity. Older adults who are food insecure may not have consistent access to healthy food, or may spend a substantial portion of time and energy navigating food assistance programs, such as the Supplemental Nutrition Assistance Program (SNAP, formerly known as food stamps) (15). Food insecurity has been associated with poorer diabetes self-management and nutritional status (16), as well as poor glycemic control (17), among adults with diabetes. In recognition of this evidence, the American Diabetes Association Standards of Care began recommending that clinicians screen diabetes patients for food insecurity in 2016 (18). While food insecurity has been associated with measures of diabetic morbidity, it is not clear what role this association may play in subsequent onset of depression or depression severity.
Mood disorders, particularly depression, are highly comorbid with diabetes, however current understanding is complicated by the bidirectional (11) and/or cyclical (19) nature of this relationship. Diabetes distress, including illness duration, number of diabetes symptoms and poor self-care is associated with depression (12). However, depression is also associated with impaired functioning and poor health behaviors (e.g., physical inactivity, smoking, poor sleep), including poor diabetes management behaviors (20), which could speed the progression of prediabetes or diabetes. Further empirical evidence is needed to determine whether diabetic morbidity is an underlying pathway by which food insecurity increases risk of depression among those with diabetes. Improved understanding of the linkages between food insecurity, depression, and diabetes morbidity may help to identify modifiable factors that could inform personalized treatment for persons with both depression and diabetes.
This study examined the relationships between food insecurity, depression and diabetic morbidity in a nationally representative sample of older U.S. adults with diabetes. Additionally, this study aimed to quantify the degree to which the relationship between food insecurity and depression is mediated by diabetic morbidity. The primary hypothesis was that part of the association between food insecurity and greater depressive symptoms is explained by diabetic morbidity.
METHODS
Sample
Data come from the Health and Retirement Study (HRS), an ongoing, nationally-representative longitudinal survey of ~20,000 U.S. adults aged ≥50 (21). The HRS has been in the field continuously since 1992. Described in detail elsewhere, the HRS assesses a wide range of social, economic, and health characteristics. The HRS Core interview is conducted every two years and includes information on demographics, employment, assets, income and health status. Since 2008 the HRS also conducts “Enhanced face-to-face” interviews on an alternating 50% of the cohort, in which respondents are asked to provide a blood spot. Informed consent is collected prior to data collection for each wave. The HRS is approved by the University of Michigan Institutional Review Board.
This analysis was restricted to HRS respondents who reported a lifetime history of diabetes in 2012 (n=4,936). The sample was further limited to respondents who were interviewed during the 2010, 2012 and 2014 waves (n=3,855) in order to determine both whether diabetes had worsened since 2010, and to assess how diabetes morbidity contributed to incident depression status in 2014. Analyses were further limited to those who provided a blood spot in either 2012 or 2014 to assess glycemic control based on HbA1C (n=3,084). Among those who met these criteria, 2,951 (96%) respondents had complete-case data on all covariates and were included in the analytic sample. When comparing those in the analysis sample (i.e. complete-case data) to those meeting various study criteria, sample distributions for variables of interest remained stable (data not shown). No other exclusion criteria were applied (Supplemental Figure 1).
Measures
Food insecurity—
The HRS Core interview asks participants “Have you always had enough money to buy the food you need” since their last interview, recorded as yes, no, don’t know or refused. Respondents who do not give an affirmative answer were then asked, “In the last 12 months, did you ever eat less than you felt you should because there wasn’t enough money to buy food.” These two questions were used to create a binary variable to categorize food insecurity status in 2012. Those who did not have enough money to buy food or ate less due to a lack of money were considered food insecure. Those who always had enough money to buy the food they needed since the last interview were considered food secure and served as the reference group.
Elevated depressive symptoms—
The 8-item Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess elevated depressive symptoms in 2012 and 2014. Prior work demonstrates that the 8-item version of the CES-D performs equivalent to the original 20-item version of this scale among older adults (22,23). While the CES-D was initially designed to assess current depression symptomatology within the general population (24,25); it is commonly used to identify depression cases as a binary measure (26–28). We used a threshold of ≥ 3 to indicate clinically-significant depressive symptoms, which has a 71% sensitivity and 79% specificity relative to a fully-structured interview for diagnosis major depressive episode (29). Additionally, we treated the CES-D scale as a continuous measure (with scores ranging from 0 to 8) of depression symptom severity for the path analysis.
Diabetic morbidity—
Four indicators of diabetic morbidity were examined. First, respondents were asked, “Is your diabetes generally under control?” Responses were dichotomized as “not under control” versus “under control” (reference group). Second, respondents were asked, “Compared to when we interviewed you last, has your diabetes gotten better, worse, or stayed about the same?” Responses were dichotomized as “worse” versus “better,” “about the same,” and “don’t know.” Additionally, for those who did not have diabetes in 2010 but reported having diabetes in 2012 for the first time (n=370, 12.5% of the analytic sample), diabetes was considered to have worsened. These questions have been used in prior research to assess change in chronic condition status—for diabetes as well as hypertension (30).
Third, respondents were asked, “Has your diabetes caused you to have trouble with your kidneys or protein in your urine?”. Responses were dichotomized as “yes” versus “no” and “don’t know.” Kidney problems associated with diabetes represent a significant health burden among those with diabetes and is considered an indicator of diabetes progression (31).
Fourth, hyperglycemia was assessed by HbA1C. As described above, blood spots were collected on a random 50% of the cohort as part of the HRS Enhanced Face-to-Face interview in 2012, and the other 50% in 2014. These blood spots were analyzed by the University of Washington (32,33) using an automated ion-exchange high-performance liquid chromatography system to measure percentage of glycosylated hemoglobin HbA1C. HbA1C levels ≥7% (53 mmol/mol) were used to indicate hyperglycemia; levels <7% indicated adequate glycemic control (34).
Covariates—
Demographic covariates included gender (male [reference group], female), age group (51 to < 65, 65 to < 75, ≥ 75 years old), race/ethnicity (non-Hispanic White [reference group], non-Hispanic Black, other) and marital status (married [reference group], separated or divorced, widowed, single or never married). Indicators of socioeconomic status (SES)were: work status (currently work for pay [reference group] vs. do not work for pay/disabled/not in the labor force), retirement status (completely or partially retired vs. not retired/homemaker/not in the labor force [reference group]), educational attainment (<high school, high school degree or equivalent, some college, college degree and above [reference group]) and income-to-poverty ratio (IPR). IPR is the ratio of an individual’s household income to the poverty level, which refers to the minimum income required to meet basic needs and adjusted for household size (35).
Finally, a continuous variable indicating number of medical comorbidities was created by summing the number of lifetime conditions from a list of 16: hypertension; cancer or malignant tumor of any kind except skin cancer; chronic lung disease except asthma such as chronic bronchitis or emphysema; heart attack, coronary heart disease, angina, congestive heart failure or other heart problem; stroke or transient ischemic attack; emotional, nervous, or psychiatric problems, and; arthritis or rheumatism. Using a continuous measure to approximate comorbidity severity has been included in prior analyses among older adults (36), including within HRS samples limited to those with diabetes (37)—as in this study.
Analysis
Initially X2 and F tests compared the weighted distribution of diabetic morbidity, depressive symptoms, and demographic and socioeconomic covariates by food insecurity. Next, weighted multivariable logistic regression was used to examine the association of food insecurity with each of the four measures of diabetic morbidity (i.e. whether diabetes was generally under control, whether diabetes had gotten worse, having kidney problems associated with diabetes or hyperglycemia). These models adjusted for gender, age group, race/ethnicity, marital status, educational attainment, income-to-poverty ratio, work status, retirement status and number of medical comorbidities.
Next, weighted multivariable logistic regression was used to evaluate the relationship of food insecurity with cross-sectional depression status (using only the 2012 data) and longitudinal depression status (assessed in 2014). We fit a series of nested logistic models: Model 1 adjusted for gender, age group, race/ethnicity, marital status, educational attainment, income-to-poverty ratio, work status, retirement status and number of comorbidities; and Model 2 added the four indicators of diabetic morbidity to generate an initial estimate of mediation. For the longitudinal analysis, similar models were used to assess the relationship between food insecurity and odds of elevated depressive symptoms in 2014, additionally adjusting for elevated depressive symptoms status in 2012. All regression analyses were fit using SAS, v. 9.4 with survey procedures to account for the complex survey design of the HRS.
Finally, path analysis was used to test the degree to which diabetic morbidity mediated the association between food insecurity and depressive symptoms. Path analysis tests both direct and indirect paths of associations, and is considered a type of structural equation model composed of observed variables (38). This analysis treated depressive symptoms as a continuous variable (ranging from 0 to 8 CES-D items) to meet the assumptions of the path estimation procedures.
The path analysis consisted of two main paths: (1) food insecurity → indicators of diabetic morbidity, and (2) diabetic morbidity → depressive symptoms. The paths between food insecurity and diabetic morbidity accounted for gender, age group, race/ethnicity, marital status, educational attainment, income-to-poverty ratio, work status, retirement status and number of comorbidities. The paths between diabetic morbidity and depressive symptoms also adjusted for these covariates. Path analyses were conducted both cross-sectionally (using only 2012 data) and longitudinally (using the 2012 to 2014 data). The path analysis was fit using STATA S/E (v15.1) and accounted for the complex sampling design of HRS using the sem and nlcom command procedures. In path analyses, parameter estimates (β’s) indicate direct effects adjusted for covariates. Direct effects represent the influence of a one-unit change of one variable on another variable when mediating variables are held constant (39).
RESULTS
Table 1 provides descriptive characteristics for the total sample of older adults with diabetes and by level of food insecurity; 15.2% were food insecure. Food insecurity was more common among those who were female, aged 51 to <65 years, non-Hispanic Black or other (i.e. non-Hispanic Asian, Hispanic, or multiple races/ethnicities) and those who were separated, divorced or widowed. Additionally, those with less than a college degree, a lower IPR and greater medical comorbidities were more likely to be food insecure. Poor diabetes control was more common among those who were food insecure (18.8%) than those who were not food insecure (9.8%). Diabetes-related kidney problems were over twice as common among those who were food insecure (19.1%) than those not food insecure (8.0%). Hyperglycemia (HbA1C ≥7%) was marginally more common among those who were food insecure (40.9%) than those who were not food insecure (35.4%). Diabetes getting worse over the past two years did not appear to vary across by food insecurity status, affecting 25.8% of those who were food insecure and 22.1% of those who were not food insecure.
Table 1.
| Food Insecurec |
||||
|---|---|---|---|---|
| Total | No | Yes |
P
valued |
|
| Characteristics | n = 2,951 | n = 2,505 | n = 446 | |
| Female | 1,631 (51.9) | 1,343 (50.1) | 288 (63.3) | <0.001 |
| Age Category | <0.001 | |||
| 51 to < 65 | 1,249 (47.7) | 981 (45.7) | 268 (61.1) | |
| 65 to < 75 | 943 (32.7) | 823 (33.0) | 120 (30.2) | |
| 75 to 106 | 759 (19.6) | 701 (21.3) | 58 (8.7) | |
| Race/Ethnicity | <0.001 | |||
| Non-Hispanic White | 1,615 (68.8) | 1,471 (71.9) | 144 (48.6) | |
| Non-Hispanic Black | 712 (13.7) | 537 (11.7) | 175 (26.4) | |
| Other | 624 (17.5) | 497 (16.4) | 127 (25.0) | |
| Marital Status | <0.001 | |||
| Married | 1,690 (58.1) | 1,505 (60.3) | 185 (44.2) | |
| Separated or divorced | 444 (15.5) | 326 (13.9) | 118 (26.3) | |
| Widowed | 543 (15.4) | 457 (15.1) | 86 (17.3) | |
| Single or never married | 274 (11.0) | 217 (10.8) | 57 (12.3) | |
| Baseline educational attainmente | <0.001 | |||
| < High school | 731 (19.1) | 564 (17.4) | 167 (30.1) | |
| High school degree or equivalent | 1,023 (34.8) | 872 (34.3) | 151 (38.1) | |
| Some college | 679 (24.1) | 595 (25.2) | 84 (17.5) | |
| College degree and above | 518 (21.9) | 474 (23.1) | 44 (14.3) | |
| Work for pay | 915 (36.0) | 808 (37.7) | 107 (24.7) | <0.001 |
| Retired | 2,020 (64.0) | 1,730 (64.2) | 290 (62.9) | 0.12 |
| Income-to-Poverty Ratio (mean, 95% CI) | 4.4 (4.1, 4.7) | 4.7 (4.4, 5.0) | 2.3 (1.7, 2.9) | <0.001 |
| Number of comorbiditiesf (mean, 95% CI) | 2.4 (2.3, 2.4) | 2.3 (2.2, 2.3) | 2.9 (2.7, 3.1) | <0.001 |
| Diabetic morbidity | ||||
| Poor diabetes control | 328 (11.0) | 250 (9.8) | 78 (18.9) | <0.001 |
| Diabetes got worse | 628 (22.6) | 515 (22.1) | 113 (25.8) | 0.13 |
| Associated kidney problems | 305 (9.5) | 233 (8.1) | 72 (18.9) | <0.001 |
| Hyperglycemia (HbA1C ≥7%) | 1,117 (36.2) | 930 (35.4) | 187 (40.8) | 0.08 |
| Depressive symptoms score (mean, 95% CI) | ||||
| 2012 CES-Dg | 1.7 (1.6, 1.9) | 1.6 (1.4, 1.7) | 2.8 (2.4, 3.1) | <0.001 |
| 2014 CES-D | 1.7 (1.6, 1.8) | 1.6 (1.4, 1.7) | 2.7 (2.3, 3.0) | <0.001 |
| Met criteria for elevated depressive symptoms | ||||
| 2012 CES-D | 816 (26.2) | 605 (23.6) | 211 (42.5) | <0.001 |
| 2014 CES-D | 841 (25.8) | 631 (23.1) | 210 (43.4) | <0.001 |
Health and Retirement Study
All variables measured in 2012 wave and table values represent weighted column percentages unless otherwise indicated
Assessed over the prior 24 months; those who reported that they worried about food or skipped meals due to lack of financial resources were considered food insecure.
X2 or F test
Measured the first year a respondent participated in HRS
Includes high blood pressure; cancer or malignant tumor of any kind except skin cancer; chronic lung disease except asthma such as chronic bronchitis or emphysema; heart attack, coronary heart disease, angina, congestive heart failure or other heart problem; stroke or transient ischemic attack; emotional, nervous, or psychiatric problems, and; arthritis or rheumatism
The Center for Epidemiologic Studies Depression Scale; score ≥3 indicates major depression
Table 2 shows the relationship between food insecurity and the four indicators of diabetic morbidity. In fully adjusted models, food insecurity was associated with higher odds of poor diabetes control and of diabetes-related kidney problems. Those who were food insecure had a 1.7 (95% confidence interval (CI) = 1.1 to 2.6) times higher odds of poor diabetes control and 1.6 (95% CI = 1.1 to 2.5) times higher odds of kidney problems. Food insecurity was not associated with diabetes worsening (odds ratio (OR) = 1.1; 95% CI = 0.8 to 1.5; p-value=0.44) or hyperglycemia (OR = 1.0; 95% CI =0.7 to 1.3; p-value=0.99).
Table 2.
| Food Insecurec |
|||
|---|---|---|---|
| Yes | No | ||
| Diabetic morbidity | n = 2,505 |
n = 446 OR (95% CI) |
P valued |
| Poor diabetes control | Ref. | 1.7 (1.1, 2.5) | 0.02 |
| Diabetes got worse | Ref. | 1.1 (0.8, 1.5) | 0.44 |
| Associated kidney problems | Ref. | 1.6 (1.1, 2.5) | 0.02 |
| Hyperglycemia (HbA1C ≥7%) | Ref. | 1.0 (0.7, 1.3) | 0.99 |
Health and Retirement Study
Logistic regressions account for gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio and number of comorbidities
Assessed over the prior 12 months; those who reported that they worried about food or skipped meals due to lack of financial resources were considered food insecure.
Type III F test
In cross-sectional analysis (Table 3), the odds of having elevated depressive symptoms were two times higher for those who were food insecure (OR = 2.0; 95% CI = 1.7 to 2.4). After adjustment for the indicators of diabetic morbidity, food insecurity was no longer associated with elevated depressive symptoms (OR = 1.3; 95% CI = 0.9 to 1.8).
Table 3.
The influence of food insecurity on depression and mediation by diabetic morbidity, HRS a
| Depressionb in 2012 | Depressionb in 2014 | |||
|---|---|---|---|---|
| Direct effectd | Adjusting
for mediation by diabetic morbiditye |
Direct effectf | Adjusting for mediation by diabetic morbidityg |
|
| Food Insecurec | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.0*** (1.7, 2.4) | 1.3 (0.9, 1.8) | 1.5*** (1.3, 1.8) | 1.4 (1.0, 1.9) |
Health and Retirement Study
The Center for Epidemiologic Studies Depression Scale, those with a score ≥ 3 meet criteria for elevated depressive symptoms
Assessed over the prior 12 months at the time when the HRS 2012 survey was administered to respondents; those who reported that they worried about food or skipped meals due to lack of financial resources were considered food insecure.
Adjusts for gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio and number of comorbidities
Adjusts for whether diabetes got worse and having kidney problems associated with diabetes, in addition to gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio and number of comorbidities
Adjusts for gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio, number of comorbidities and 2012 CESD score
Adjusts for all covariates in Model 2 plus whether diabetes got worse and having kidney problems associated with diabetes, in addition to gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio, number of comorbidities and 2012 CESD score
p-value<0.05; p-value<0.01;
p-value<0.001
Findings from the longitudinal analyses predicting elevated depressive symptoms were similar (Table 3). Food insecurity was associated with a 50% higher odds of elevated depressive symptoms in 2014, when accounting for baseline depressive symptomology in 2012 (OR = 1.5; 95% CI = 1.3 to1.8). After accounting for diabetic morbidity, food insecurity was no longer associated with elevated depressive symptoms (OR = 1.4; 95% CI = 1.0 to 1.9).
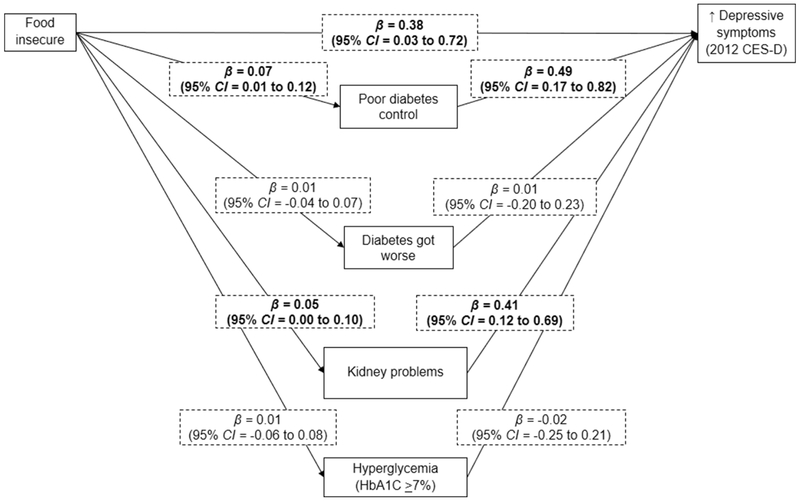
Figure 1 presents direct effects of the cross-sectional path analysis testing whether the association between food insecurity and depressive symptoms is mediated by diabetic morbidity. Food insecurity was associated with greater depressive symptoms (β = 0.38; 95% CI = 0.04 to 0.74), as well as poor diabetes control (β = 0.07; 95% CI = 0.01 to 0.12) and kidney problems (β = 0.05; 95% CI = 0.01 to 0.10). Both poor diabetes control (β = 0.49; 95% CI = 0.17 to 0.82) and kidney problems (β = 0.40; 95% CI = 0.12 to 0.68) were related to greater depressive symptoms. Diabetic morbidity explained 12.7% (95% CI = 0.8 to 24.6%) of the relationship between food insecurity and contemporaneous symptoms of depression.
Figure 1. Mediation of the cross-sectional association between food insecurity and depressive symptoms by diabetic morbidity.
Data came from older adults (>50 years) in the Health and Retirement Study, 2010-2012
Diabetic morbidity explained 12.7% (95% CI =0.8-24.6%) of the association between food insecurity and depressive symptoms (p-value=0.04)
Parameter estimates (β”s) represent direct effects and were adjusted for gender, age group, race/ethnicity, marital status, educational attainment, work status, retirement status income-to-poverty ratio and number of comorbidities
CES-D=The Center for Epidemiologic Studies Depression Scale
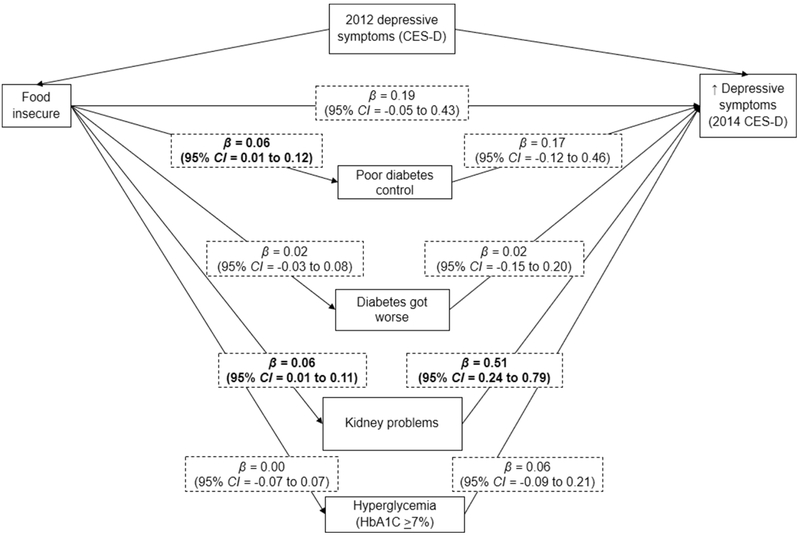
Figure 2 presents direct effects of the longitudinal path analysis testing whether diabetic morbidity mediated the relationship between food insecurity in 2012 and depressive symptoms in 2014, adjusting for 2012 depressive symptoms. Again, food insecurity was associated with poor diabetes control (β = 0.06; 95% CI = 0.01 to 0.12) and kidney problems (β = 0.06; 95% CI = 0.01 to 0.11). However, food insecurity was not associated with greater depressive symptoms two years later (β = 0.18; 95% CI = −0.05 to 0.42). Kidney problems was the only indicator of diabetic morbidity that was related to greater depressive symptoms (β = 0.51; 95% CI = 0.24 to 0.79). Diabetic morbidity marginally mediated the relationship of food insecurity and subsequent symptoms of depression, explaining 18.5% (95% CI = −2.6 to 39.6%) of the association.
Figure 2. Mediation of the association between food insecurity and depressive symptoms 2 years later by diabetic morbidity.
Data came from the Health and Retirement Study, 2010-2014
Diabetic morbidity explained 18.5% (95% CI=−2.6-39.6%)of the association between food insecurity and depress symptoms (p-value=0.09)
Parameter estimates (β”s) represent direct effects and were adjusted for 2012 CES-D score, gender, age group, race/ethnicity, marital status, baseline educational attainment, work status, retirement status, income-to-poverty ratio and number of comorbidities.
CES-D=The Center for Epidemiologic Studies Depression Scale
DISCUSSION
This study examined the relationship between food insecurity and depressive symptoms among older adults with diabetes. Further, it assessed whether this relationship was mediated by diabetic morbidity. Results suggest that food insecurity is associated with poor diabetes control and diabetes-related kidney problems. Additionally, logistic regression and path analyses indicate that the influence of food insecurity on diabetic morbidity may, at least in part, explain the association of food insecurity with subsequent depression status among older adults with diabetes. Future research should test whether interventions that reduce food insecurity in this population reduce diabetes morbidity and depressive symptoms. Findings may inform the development of innovative clinical approaches to address social determinants of health, such as food insecurity.
There were slight differences in longitudinal results when assessing mediation of the association between food insecurity and depression—possibly due to how depression was characterized. When depressive symptoms were treated as a continuous measure in path analyses, food insecurity was not associated with depression, which is likely why mediation in longitudinal path analyses did not meet statistical significance. However, in logistic regression analyses, food insecurity was associated with a higher odds of depression, and; when indicators for diabetic morbidity were included in logistic regression models, food insecurity was no longer associated with depression. Thus, indicating mediation by diabetic morbidity.
Results of this study are consistent with prior evidence indicating that food insecurity is associated with greater difficulty managing diabetes (16). Studies in children and middle-aged adults report that people who are food insecure are more likely to reuse needles for injecting insulin and engage in less frequent blood glucose monitoring (40,41). Additionally, food insecurity can lead to individuals making trade-offs between purchasing healthy foods that are helpful for managing diabetes and purchasing diabetes medication and supplies, such as blood glucose test strips (42).
Prior work demonstrates that those who are food insecure potentially have much to gain from diabetes interventions. Lyles and colleagues (2013) evaluated an educational intervention that aimed to improve self-management of diabetes. Intervention participants who were food insecure reported greater improvement in self-efficacy and reduction in blood sugar levels than those who were not food insecure (43). However, engaging individuals who are food insecure in diabetes interventions may be a significant challenge. For example, a recent randomized control trial assessed the potential benefits of targeting individuals with diabetes who are also at risk of food insecurity by recruiting participants from food banks across the U.S. (54). Following 6 months of a comprehensive intervention--which included food provision, health care referral, glucose monitoring and diabetes education--no improvement in depressive symptoms or HbA1c concentrations was observed. Authors of this study noted that participant engagement was low (36%), which may have contributed to the lack of intervention efficacy. Looking ahead, whether delaying diabetes onset or reducing patient morbidity also lowers risk of depression among those who are food insecure will require further study.
Healthcare approaches that systematically account for diabetes in a broader psychosocial context, such as the Collaborative Care Model (CCM), may be particularly effective for addressing food insecurity among those with comorbid depression and diabetes. Collaborative care for depression in the context of medical comorbidity aims to proactively manage depression, as opposed to treating acute symptoms, using teams of nurse care managers and consulting psychiatrists overseen by primary physicians. A meta-analysis of randomized control trials of the CCM showed that this treatment model has clinically-significant benefits for both depression and diabetes outcomes (44). However, integrating social determinants of health, such as food insecurity, within collaborative care models or more traditional primary care settings has limited success to date. This is in part due to fee-for-service payment structures and a culture focused on treating single diseases (often in specialty care) rather than overall health promotion (45). There are a select few programs that are effective on a small scale (46)—although likely difficult to expand to diverse patient populations. Yet, efforts that target social determinants of health could reduce health disparities more so than other medical advances (47). More work is needed to determine whether it is possible to ameliorate the health effects of food insecurity through primary care intervention.
Limitations
Findings should be interpreted in light of study limitations. Although these results are generalizable to older adults with diabetes in the US, they may not be applicable to younger age groups. The experience of food insecurity in the US and the American healthcare system may differ in important ways compared to other countries, such that results may not be applicable elsewhere. It is also possible that HRS respondents underreported both food insecurity and symptoms of depression due to social desirability. Depending on how underreporting was distributed within the HRS sample, this could bias results toward or away from the null. The potential for unobserved confounding should also be considered when interpreting results of this study.
The HRS Core interview does not include the US Department of Agriculture (USDA) Household Food Security Survey (HFSS), which is administered annually to estimate U.S. national food insecurity levels (2) and is considered the most valid tool for food insecurity measurement (48). Instead, HRS collects information from 2 questions that assess whether respondents could afford food or skipped meals. The questions included in the HRS are similar to a common 2-item screener for food insecurity, which has 97% sensitivity and 83% specificity when compared to the HFSS (49). Additionally, the 2-item food insecurity screener in the HRS has been used in previous research to assess associations of food insecurity with depression (50).
While a number of diabetic morbidity measures were included in our analyses, the inability to consider potential mediation due to hypoglycemia is a limitation. Previous findings indicate that food insecurity can increase the risk of hospitalization due to hypoglycemia (51), and; hypoglycemia is associated with depression symptoms (52). Lastly, three of the four measures of diabetic morbidity were assessed based on self-report, which can bias study findings (53). Future research would benefit from more clinically-informed measures of diabetes progress.
Conclusion
Depression and diabetes often co-occur, and food insecurity is considered a risk factor for both conditions. However, pathways by which food insecurity may influence depression among those with diabetes are not well understood. Findings indicate that diabetic morbidity, particularly kidney problems associated with diabetes, contribute to elevated depression symptoms among older adults. Future research is needed to determine whether reducing food insecurity within the context of diabetes care improves depression outcomes.
Supplementary Material
Supplemental Fig. 1 Study inclusion criteria among Health and Retirement Study (HRS) respondents.
HIGHLIGHTS.
The influence of food insecurity on depression within the context of diabetes management is not well understood.
This study examined whether diabetic morbidity mediates the relationship of food insecurity with depression among older adults with diabetes.
Food insecurity was associated with poorer diabetes control and diabetes-related kidney problems.
The relationship of food insecurity with depression was attributable to diabetic morbidity.
Acknowledgements
RSB is supported by the National Institute of Mental Health (T32 MH73553). RSB takes full responsibility for this work as a whole, including study design, access to data and the decision to submit and publish the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe WS, Frongillo EA, Valois P. Understanding the experience of food insecurity by elders suggests ways to improve its measurement. J Nutr. 2003. September; 133(9):2762–9. [DOI] [PubMed] [Google Scholar]
- 2.Coleman-Jensen A, Rabbitt M, Gregory C, Singh A. Household Food Security in the United States in 2016 [Internet]. U.S. Department of Agriculture, Economic Research Service; 2017. September [cited 2017 Dec 20], Report No.: ERR-237. Available from: https://www.ers.usda.gov/publications/pub-details/?pubid=84972 [Google Scholar]
- 3.Gundersen C, Kreider B, Pepper J. The Economics of Food Insecurity in the United States. Appl Econ Perspect Policy. 2011. September 1;33(3):281–303. [Google Scholar]
- 4.Holben D Position of the American Dietetic Association: Food Insecurity in the United States. J Am Diet Assoc. 2010. September 1;110(9): 1368–77. [DOI] [PubMed] [Google Scholar]
- 5.Bergmans RS, Palta M, Robert SA, Berger LM, Ehrenthal DB, Malecki K. Associations between food security status and dietary inflammatory potential within lower-income adults from the United States National Health and Nutrition Examination Survey (NHANES), cycles 2007 to 2014. J Acad Nutr Diet.2018; 118(6):994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, Frongillo EA. Nutritional and health consequences are associated with food insecurity among US elderly persons. J Nutr. 2001;131 (5):1503–1509. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery J, Lu J, Ratliff S, Mezuk B. Food Insecurity and Depression among Adults with Diabetes: Results from the National Health and Nutrition Examination Survey (NHANES). Diabetes Educ. 2017;43(3):260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean WR, Sharkey JR, Johnson CM. Food Insecurity is Associated With Social Capital, Perceived Personal Disparity, and Partnership Status Among Older and Senior Adults in a Largely Rural Area of Central Texas. J Nutr Gerontol Geriatr. 2011. April 1;30(2): 169–86. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, Clouste Gulbenkian Foundation. Social determinants of mental health. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 10.Narayan KMV, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of Recent Increase in Incidence on Future Diabetes Burden: U.S., 2005–2050. Diabetes Care. 2006. September 1;29(9):2114–6. [DOI] [PubMed] [Google Scholar]
- 11.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Roux AVD, et al. Examining a Bidirectional Association Between Depressive Symptoms and Diabetes. JAMA. 2008. June 18;299(23):2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ell K, Katon W, Lee PJ, Guterman J, & Wu S (2015). Demographic, clinical and psychosocial factors identify a high-risk group for depression screening among predominantly Hispanic patients with Type 2 diabetes in safety net care. General hospital psychiatry, 37(5), 414–419. [DOI] [PubMed] [Google Scholar]
- 13.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. The Lancet. 2014. June 7;383(9933): 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell LB, Suh D-C, Safford MA. Time requirements for diabetes self-management: too much for many. J Fam Pr. 2005;54(1):52–56. [PubMed] [Google Scholar]
- 15.Kamp B Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and Nutrition Programs for Community-Residing Older Adults. J Am Diet Assoc. 2010. March 1;110(3):463–72. [DOI] [PubMed] [Google Scholar]
- 16.Seligman HK, Davis TC, Schillinger D, Wolf MS. Food Insecurity is Associated with Hypoglycemia and Poor Diabetes Self-Management in a Low-Income Sample with Diabetes. J Health Care Poor Underserved. 2010. November;21(4):1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standards of Medical Care in Diabetes—2016 Abridged for Primary Care Providers. Clin Diabetes Publ Am Diabetes Assoc. 2016. January;34(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns RJ, Deschênes SS, Schmitz N. Cyclical relationship between depressive symptoms and diabetes distress in people with Type 2 diabetes mellitus: results from the Montreal Evaluation of Diabetes Treatment Cohort Study. Diabet Med. 2015. October 1;32(10): 1272–8. [DOI] [PubMed] [Google Scholar]
- 20.Lin EHB, Katon W, Korff MV, Rutter C, Simon GE, Oliver M, et al. Relationship of Depression and Diabetes Self-Care, Medication Adherence, and Preventive Care. Diabetes Care. 2004. September 1;27(9):2154–60. [DOI] [PubMed] [Google Scholar]
- 21.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014. April 1;43(2):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Halloran AM, Kenny RA, King-Kallimanis BL. The latent factors of depression from the short forms of the CES-D are consistent, reliable and valid in communityliving older adults. Eur Geriatr Med. 2014. April 1;5(2):97–102. [Google Scholar]
- 23.Karim J, Weisz R, Bibi Z, ur Rehman S. Validation of the Eight-Item Center for Epidemiologic Studies Depression Scale (CES-D) Among Older Adults. Curr Psychol. 2015. December 1;34(4):681–92. [Google Scholar]
- 24.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977. June 1;1(3):385–401. [Google Scholar]
- 25.Stommel M, Given BA, Given CW, Kalaian HA, Schulz R, McCorkle R. Gender bias in the measurement properties of the Center for Epidemiologic Studies Depression Scale (CES-D). Psychiatry Res. 1993. December;49(3):239–50. [DOI] [PubMed] [Google Scholar]
- 26.Capistrant BD, Berkman LF, Glymour MM. Does Duration of Spousal Caregiving Affect Risk of Depression Onset? Evidence from the Health and Retirement Study. Am J Geriatr Psychiatry. 2014. August 1;22(8):766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holwerda TJ, Tilburg TG van, Deeg DJH, Schutter N, Van R, Dekker J, et al. Impact of loneliness and depression on mortality: Results from the Longitudinal Ageing Study Amsterdam. Br J Psychiatry. 2016. August;209(2): 127–34. [DOI] [PubMed] [Google Scholar]
- 28.Houtjes W, Deeg D, Ven PM van de, Meijel B van, Tilburg T van, Beekman A. Is the naturalistic course of depression in older people related to received support over time? Results from a longitudinal population-based study. Int J Geriatr Psychiatry. 2017;32(6):657–63. [DOI] [PubMed] [Google Scholar]
- 29.Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study [Internet]. Ann Arbor, MI: Survey Research Center, University of Michigan; 2011. March [cited 2018 Dec 5] p. 13–4. Report No.: DR-005. Available from: http://hrsonline.isr.umich.edu/sitedocs/userg/dr-005.pdf [Google Scholar]
- 30.Mojtabai R, Olfson M. Medication Costs, Adherence, And Health Outcomes Among Medicare Beneficiaries. Health Aff (Millwood). 2003. July 1;22(4):220–9. [DOI] [PubMed] [Google Scholar]
- 31.Radcliffe NJ, Seah J, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017. January;8(1):6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crimmins E, Faul J, Kim J, Weir D. Documentation of Biomarkers in the 2010 and 2012 Health and Retirement Study. Ann Arbor, MI: University of Michigan, Survey Research Center; 2015. Report No.: DR-031. [Google Scholar]
- 33.Crimmins E, Faul J, Kim J, Weir D. Documentation of Blood-Based Biomarkers in the 2014 Health and Retirement Study. Ann Arbor, MI: University of Michigan, Survey Research Center; 2017. Report No.: 9387. [Google Scholar]
- 34.Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. Clin Diabetes Publ Am Diabetes Assoc. 2015. April;33(2):97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semega JL, Fontenot KR, Kollar MA. Income and Poverty in the United States: 2016 U.S. Government Printing Office, Washington, DC: U.S. Census Bureau; 2017. Sep. Report No.: P60–259. [Google Scholar]
- 36.Mier N, Ory MG, Towne SD, Smith ML. Relative Association of Multi-Level Supportive Environments on Poor Health among Older Adults. Int J Environ Res Public Health [Internet]. 2017. April [cited 2018 Dec 5]; 14(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5409588/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, et al. Beyond Comorbidity Counts: How Do Comorbidity Type and Severity Influence Diabetes Patients’ Treatment Priorities and Self-Management? J Gen Intern Med. 2007. December 1;22(12):1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon J The strengths and limitations of the statistical modeling of complex social phenomenon: Focusing on SEM, path analysis, or multiple regression models.World Acad Sci Eng Technoloy Int J Soc Behav Educ Econ Bus Ind Eng. 2015;9:1597–605. [Google Scholar]
- 39.Alwin DF, Hauser RM. The decomposition of effects in path analysis. Am Sociol Rev. 1975;37–47. [Google Scholar]
- 40.Chan J, DeMelo M, Gingras J, Gucciardi E. Challenges of Diabetes Self-Management in Adults Affected by Food Insecurity in a Large Urban Centre of Ontario, Canada. Int J Endocrinol. 2015;2015:903468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marjerrison S, Cummings EA, Glanville NT, Kirk SF, Ledwell M. Prevalance and associations of food insecurity in children with diabetes mellitus. J Pediatr.2011;158(4):607–11. [DOI] [PubMed] [Google Scholar]
- 42.Pilkington B, Daiski I, Bryant T, Dinca-Panaitescu M, Dinca-Panaitescu S, Raphael D. The experience of living with diabetes for low-income Canadians. Can J Diabetes. 2010;34(2):119–26. [Google Scholar]
- 43.Lyles CR, Wolf MS, Schillinger D, Davis TC, Dewalt D, Dahlke AR, et al. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36(6):1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atlantis E, Fahey P, Foster J. Collaborative care for comorbid depression and diabetes: a systematic review and meta-analysis. BMJ Open. 2014. April 1;4(4):e004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeVoe JE, Bazemore AW, Cottrell EK, Likumahuwa-Ackman S, Grandmont J, Spach N, et al. Perspectives in Primary Care: A Conceptual Framework and Path for Integrating Social Determinants of Health Into Primary Care Practice. Ann Fam Med. 2016. March 1;14(2):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fierman AH, Beck AF, Chung EK, Tschudy MM, Coker TR, Mistry KB, et al. Redesigning Health Care Practices to Address Childhood Poverty. Acad Pediatr. 2016. April;16(3 Suppl):S136–146. [DOI] [PubMed] [Google Scholar]
- 47.Woolf SH, Johnson RE, Phillips RL, Philipsen M. Giving everyone the health of the educated: an examination of whether social change would save more lives than medical advances. Am J Public Health. 2007. April;97(4):679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radimer KL, Kathy L. Measurement of household food security in the USA and other industrialised countries. Public Health Nutr. 2002. December;5(6A):859–64. [DOI] [PubMed] [Google Scholar]
- 49.Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010. July; 126(1):e26–32. [DOI] [PubMed] [Google Scholar]
- 50.Kim K, Frongillo EA. Participation in food assistance programs modifies the relation of food insecurity with weight and depression in elders. J Nutr. 2007. April;137(4): 1005–10. [DOI] [PubMed] [Google Scholar]
- 51.Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of Food Budgets at Month’s End and Hospital Admissions for Hypoglycemia. Health Aff (Millwood). 2014. January;33(1):116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green AJ, Fox KM, Grandy S. Self-reported hypoglycemia and impact on quality of life and depression among adults with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012. June 1;96(3):313–8. [DOI] [PubMed] [Google Scholar]
- 53.Althubaiti A Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016. May 4;9:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seligman HK, Smith M, Rosenmoss S, Marshall MB and Waxman E Comprehensive diabetes self-management support from food banks: A randomized controlled trial. Am J Public Health, 108(9), 2018. September, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 Study inclusion criteria among Health and Retirement Study (HRS) respondents.