Abstract
The Association of University Radiologists Radiology Research Alliance (AUR RRA) Task Force on 3-dimensional (3D) printing presents a review of the logistic considerations for establishing a clinical service using this new technology, specifically focused on implications for radiology. Specific topics include printer selection for 3D printing, software selection, creating a 3D model for printing, providing a 3D printing service, research directions, and opportunities for radiologists to be involved in 3D printing. A thorough understanding of the technology and its capabilities is necessary as the field of 3D printing continues to grow. Radiologists are in the unique position to guide this emerging technology and its use in the clinical arena.
Introduction
Producing three-dimensional (3D) physical prototypes from digital models became popular in the late 1990s (1). Over the past decade, the heathcare sector has shown increased interest in this technology for clinical use. Radiology has a longstanding history of acquiring and maintaining volumetric anatomic data. This precedent leaves our specialty at an advantage to unlock the true potential of medical 3D printing for mainstream use.
The process of creating 3D models from digital data can be categorized into four major steps: image acquisition, image segmentation, creation of a 3D model, and transfer of model data to a 3D printer (1, 2). Image acquisition is most commonly in the form of computed tomography (CT), due to rapid acquisition and relative ease of image post processing for 3D printing (1). The volumetric data is analyzed to ensure that there are no gaps in anatomy during acquisition (3, 4). Volumetric data from CT, magnetic resonance imaging (MRI), or ultrasound (US) images are rendered in digital imaging and communication in medicine (DICOM) format. DICOM data cannot be 3D printed, and thus image data requires conversion using specialized software into one of several output file types amenable for 3D printing. The most common of these is Standard Tessellation Language or STereoLithography (STL) format, which helps 3D printers to define objects by surfaces that enclose a region of space (1). These surfaces are defined as collections of triangles called facets.
Segmentation is the process of extracting region of interest (ROI) specific data and refining the STL representation of the selected anatomy (2). This step requires specialized software to ensure model integrity, preferably with software programs that are approved by the Food and Drug Administration (FDA) (5, 6). Parts of segmentation can be automated or manual, which can serve as a challenge for radiologists who may be unfamiliar with the application software.
After segmentation, acquired STL data is sent to another program for creation of a 3D model (1, 3). Open source and commercially available computer-aided design (CAD) or computer-aided manufacturing (CAM) software packages include many post-processing techniques, such as smoothing and wrapping, that must be used to ensure printability of a 3D model (Table 1). Lastly, the completed STL file is transferred to a 3D printer for production. The various steps of creating a 3D model are illustrated in Figure 1.
Table 1.
Examples of various free software platforms available for the creation of 3D printed models.
| CURA | Beginner | Slicer Software to prepare STL files for 3D Printing | Free | PC, Mac, Linux |
| CRAFTWARE | Beginner | Slicer Software to prepare STL files for 3D Printing | Free | PC, Mac |
| OSIRIX | Intermediate | To create 3D model and prepare STL file | Osirix lite (32 bit) is free. Osirix MD (64 bit) costs about $600 USD | Mac only |
| HOROS | Intermediate | To create 3D model and prepare STL file | 64-bit Free, Donation to User Group requested | Mac only |
| NETFABB | Intermediate | Slicer to prepare STL files for 3D Printing. | Basic Free, Professional edition Paid | PC, Mac, Linux |
| REPETIER | Intermediate-advanced | Slicer Software to prepare STL files for 3D Printing. | Free | PC, Mac, Linux |
| 3-D TOOL | Intermediate | To View and Check STL Files | Free | PC |
| MESHFIX | Intermediate | Check STL Files | Free | PC |
| SLIC3R | Professional | Slicer Software to prepare STL files for 3D printing | Free | PC, Mac, Linux |
| BLENDER | Professional | Edit STL Files for 3D Printing | Free | PC, Mac |
Figure 1.
Flowchart demonstrating the workflow for printing of a 3D model. After acquiring the images, DICOM files are initially segmented to extract the anatomy of interest. An STL file is created and post-processed with computer aided design (CAD) software. Support scaffolds can also be created to hold the model parts in place. The completed STL file is then sent to the 3D printer for printing.
3D print production relies on fusing successive 2D layers of material as guided by the STL data set. Selection of the 3D printer and materials depends on the clinical task at hand, as well as other factors such as cost, time of production, and familiarity with the corresponding hardware and software of a printer (1, 7). Preparing the 3D print for clinical use typically requires cleaning, curing, polishing and/or sterilization (1).
This article, by the 3D Printing Task Force of the RRA reviews the logistics of 3D printing and their implications for radiologists. Specific topics include printer selection for 3D printing, software selection, creating a 3D model for printing, providing a 3D printing service, research directions, and opportunities for radiologists to be involved in 3D printing.
Printer Selection for 3D Printing
Most 3D printers use data encoded in STL files to deposit and fuse successive two-dimensional layers of material to create a 3D model layer by layer. Although certain printers may require other file types, STL files are the most commonly used (1).
Several terms are used to describe the different types of 3D printing technologies, often creating confusion when attempting to understand the options available. The American Society for Testing and Materials (ASTM) International Standards have been developed to classify 3D printing technologies into 7 major groups to help standardize and simplify the description of available 3D printing techniques: 1) material extrusion, 2) powder bed fusion, 3) vat photopolymerization, 4) material jetting, 5) binder jetting, 6) sheet lamination, and 7) directed energy deposition (1).
The choice of the 3D printing method and materials depend on the cost and availability of the printer and materials, time required, color and transparency requirements, sterilization and temperature resistance, and flexibility or molding properties of the material. The printer costs range from $150 to $500,000 while the material costs range from a few to several thousand dollars (8, 9). Print times can range from minutes to days, depending on the model complexity and materials used.
Material Extrusion
Material Extrusion, also known as Fused Deposition Modeling (FDM), is the most widespread technique used in the manufacturing of 3D models. Material such as metal, plastic, or polymer is wound on a coil. There is controlled release of the material from the coil onto an extrusion head, which heats up the material and deposits successive layers of it onto the build platform (1). The material hardens upon cooling and the subsequent layer is then created. This technique is analogous to the mechanism behind a hot glue gun (8).
The advantages of this method are its cost-effectiveness (often less than $100/kg of material), the models created are hard and durable, and different colors can be used (10). Disadvantages are that the resolution for fine details is limited and the model is initially soft until the material hardens, so overhanging parts need to be supported until hardening (10). Additionally, printing times can vary depending on material used since each layer must partially cool before the successive layer is applied (8). The cost also varies depending on the printer used, with large scale commercial printers often requiring more expensive materials (>$100/kg) but producing higher quality models than smaller desktop FDM printers. Toxic fumes can also be produced during the manufacturing process, and adequate ventilation is required, which can add to the expense of the printing process (8, 11).
Powder Bed Fusion
Powder Bed Fusion uses a laser or electron beam to fuse particles of metal, plastic, ceramic, or glass powder. The energy source is applied to a thin bed of powder on the build tray, causing the particles to melt and fuse (8). The powder bed then lowers and the subsequent layer is created.
This method allows for the construction of support lattices and models with overhanging edges, as the model is continually supported by the surrounding un-sintered powder. The powder used as support material can then be recycled, reducing the overall waste during production. This method also allows for multiple parts to be built simultaneously within the powder bed. Additional advantages of this method include the production of fast, accurate, and reliable models with high tensile strength (8). The glue used to bind the 3D print releases strong fumes and requires special storage. Therefore, these types of printers require dedicated infrastructure with good airflow to dissipate the fumes. The disadvantages are that the materials are expensive (>$200/kg) and the metal models often require further processing to obtain a smooth surface (1).
Vat Photopolymerization
Vat Photopolymerization has three basic components: a vat of photo-curable liquid resin, a high-intensity light source (usually a laser), and a controlling system (1). Layers of resin are successively applied and exposed to the light source, which causes the resin to solidify. The print then undergoes final curing in a UV chamber. These models are often used for bone or dental models, as well as for dental implant guides and hearing aids (1).
The advantages of this technique are that it is accurate and allows for the creation of models in a variety of colors and degrees of transparency (8). The disadvantages are that some but not all of the material tends to be expensive ($210/kg), the models can be fragile, and often require more complex processing after printing. This includes smoothing of edges, removal of support materials, and curing in an oven to fully harden the resin (1, 8). Additionally, only one type of material can be used for printing at a time. The cost and overall time required for printing using this method is highly variable depending on the type of printer used.
Material Jetting
Material Jetting is analogous to ink-jet printing; but instead of ink, a liquid photopolymer is jetted onto a build tray to create the model. Often, two or more jetting heads are used at the same time, one to build the model and one for the surrounding support material that will later be removed (1). After the material is jetted onto the build tray, it is cured with UV light. The tray is then lowered, and the next layer is created in a similar fashion. This method requires support material to uphold any overhanging edges of the model, as these would break off if left unsupported during the manufacturing process (1). The supports are often made of gel or wax, and are removed after printing by melting or soaking the model in mild soap solution.
The advantages of this method are that a large variety of models can be created using various combinations of materials and colors, giving models with variable tensile strength and flexibility (1). Specifically, this is the only technology that allows for printing clear material with other colored materials embedded within it, which can be very useful in demonstrating anatomic details. Additionally, this technique allows for the creation of high-resolution models, with layer thicknesses that can be as low as 16 microns (8). The disadvantages are that the materials are expensive ($300/g) and the process can be more labor-intensive since support material must be removed after printing (1). Also, proper finishing of the models with sanding and polishing is often required.
Binder Jetting
In Binder Jetting, a bed of fine powder is exposed to a jet of liquid binding agent, which then bonds the powder. A new layer of loose powder is used in each step, and each layer fuses with the previous ones. After printing, any unbound powder is removed and recycled for further use (10). The model is also infiltrated with cyanoacrylate, wax, or resin to increase its strength (1).
The advantages are that the materials are less expensive ($150/kg), and several colors can be used, which is useful for creating color-coded anatomical models. The disadvantages are that the models are only printed with a single material, they are not translucent and they are often fragile prior to infiltration, making them prone to breakage and further adding to cost (8).
Sheet Lamination
Sheet Lamination involves the bonding of paper, metal, or plastic films layer by layer. This is not commonly used in medicine, but has shown promise in creating anatomically accurate models of bone (12).
The advantages are the low cost, non-toxic materials, and lack of stress deformation of the models (12). The disadvantages are that delicate parts can be easily damaged and model surfaces are often rough, making complex parts difficult to clean (1). Also, post-processing of the models requires peeling away of excess material manually, which can be labor-intensive.
Directed Energy Deposition
Directed Energy Deposition deposits material onto a location where an energy source is already directed to bond the material together (1). This method is not used in medicine.
Software Selection for 3D Printing
After printer selection, software selection is the most important step. There is a wide variety of software options for every budget. Choosing the appropriate software program is highly dependent on the intended use of the model. While lower cost options may be sufficient for beginners who are learning the 3D printing workflow, higher-end anatomic and surgical models to be used in the hospital setting require the use of regulated software programs. The FDA has been involved in reviewing 3D printed models for almost a decade (5). For 3D models that will be used for advanced surgical planning, operative templates, or surgical implants it is important to use FDA-cleared software programs in order to ensure the models created are both safe and effective.
To get started with 3D printing, specific types of image files are needed. Most radiological studies are saved as DICOM images on the picture archiving and communication system (PACS). These DICOM images need to be converted into a data set that is compatible with the 3D printer. The most commonly used format is STL. Many open source and low cost software products are available to convert DICOM files into STLs. 3D printing services also exist online and will process and print STL files for a fee.
Table 1 summarizes currently available 3D modeling software packages. In addition to these commonly used low-cost 3D software packages, there are commercial software packages such as Amira, Analyze, Mimics Innovation Suite, ScanIP, etc. that are expert level and are used for image segmentation beyond medical image analysis.
Creating a 3D Model for Printing
Prior to 3D printing, a 3D model must be designed, either physically or virtually. In addition to digital image based models, a physical object such as a pathological specimen or person’s face can also be captured digitally using laser scanning or 3D digitizer.
For medical image-derived models, serially sectioned volumetric images are most commonly used, with DICOM being the most common file format. The 3D printing software may dictate which image format is preferred. The following steps are necessary for the creation of an image-derived 3D model:
1. Selection of imaging sequence:
3D printing can use any volumetric set of DICOM or serial images. While CT and MRI images are the most commonly used, functional data from positron emission tomography (PET) images can also be fused.
Images need to be carefully selected to have high contrast ratio between the region of interest and the surrounding structures to have robust segmentation (Step 3) (e.g., CT with soft kernel for bone). The use of sharp kernel causes increased graininess of the edges, resulting in difficult extraction of the edges and potential roughness on the physical surface of model (13).
Typically, a 1 mm or less (preferably 0.625 mm) slice thickness and isotropic voxels of 1.25 mm or less are recommended to provide high contrast, signal-to-noise ratio and optimal spatial resolution (14, 15). These parameters can be manipulated depending on the region of interest (ROI), with the goal of minimizing partial volume effects and to prevent extensive post-processing. CT angiograms or MR angiograms are best suited for vessels. Dual Energy protocols are best for reduction of metal artifacts, particularly from implants or foreign bodies (16).
MRI has the best contrast for soft tissue and solid organs. MRI sequences without fat saturation are preferred to allow for better delineation between organs and fat (8). In general, MRI acquisition creates thicker slices, which are less amenable to high resolution models. To create a very detailed 3D model, it is imperative to have the thinnest slices possible for the data set. Thicker slices, like those of an MRI, will create a “stepped” or blocky appearance in the model in the segmentation process (15). MRI sequences with small isotropic voxels and smoothing of the model with post-processing software can allow for correction of this stepped appearance but with potential loss of anatomical accuracy.
2. Segmentation of region of interest:
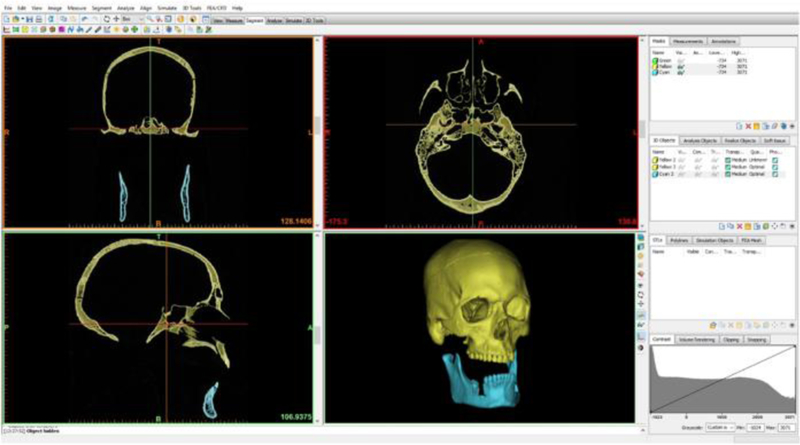
In general, the steps are: a) select volume of interest, b) mask and review volume of interest, c) subtract and extract masked volume from original volume, d) review subtracted image, and e) export DICOM volume file. An example of skull segmentation can be seen in Figure 2. Creating a mask simply means defining a collection of voxels that satisfy a specific set of criteria. This includes techniques such as thresholding where one can, for example, select a specific range of Hounsfield units (HU) on CT to segment out a structure of interest. Manual contouring techniques and edge detection methods can also be used to further enhance the quality of the segmentation process.
Figure 2.
Example demonstrating how the software package (Mimics Innovation Suite) can be used to segment the bones of the skull from source CT head images to generate a 3D model.
3. Fusion:
The use of multiple data sets or multiple modalities can allow for a more detailed model. For example, when image co-registration using both CT and MRI is required to create a 3D model, the images can be merged prior to segmentation or the CT can be segmented first and then merged with the MRI (8). The same process is followed if using different sequences from the same MRI or CT study to segment different structures (i.e. arterial and venous phases from the same study). Many different types of co-registration techniques exist, including rigid transformations, non-rigid transformations, and voxel-based technique (17). The technique used will depend on the clinical scenario and the software being used. Of note, not all software are capable of this process.
4. Surface generation:
The segmented DICOM volume file contains voxel data which needs to be converted into a series of triangle meshes representing a surface. This process converts a DICOM data set into STL format, which is compatible with 3D printers. The surface of the object is represented as set triangles of variable sizes and angles, and information about the object’s orientation in the 3D space is preserved. Smaller triangle size and increased number of triangles imparts greater detail to the surface characteristics of the desired object and hence the model. This also allows for a smoother model surface.
This basic STL format prevents inclusion of surface texture, color and part specific material properties that are readily acquired by current modes of imaging. Additive Manufacturing File (AMF) format is a newer technology that surpasses these limitations of STL format (15).
VRML (Virtual Reality Model Language) and ZPR (Zcorp proprietary) formats are less popular, but contain information about color. 3MF (3D Manufacturing Format) and Obj (Object) file formats can also be used. The file type chosen will depend on the type of printer being used and the desired properties of the model, with STL files being the most common type.
5. Image post processing of 3D Model:
Post-processing steps include:
Correcting the mesh model: This is the most important step to fail safe 3D printing. Small holes and self-intersecting triangle meshes can develop in the course of reconstruction to model or STL and need to be corrected. Most software has interactive triangle mesh processing to provide hole filling, mesh editing, smoothing, and self-intersecting inspection.
Removing mesh errors: Since the surface of a model has a defined thickness, it actually has two sides, inward facing and outward facing. These sides are composed of triangles facing outward and triangles facing inwards. All the boundaries of the triangles should be in contact with each other and none of the inward facing triangles should be in contact with outward facing triangles, and vice versa. This defines the positive volume of the model. Negative volume would result in the failure of printing.
Connecting parts: The unconnected desired parts of the virtual model need to be connected. A column or bar of triangles may be constructed for this purpose. The unconnected undesired parts of the virtual model should also be removed.
Closing ends: The ends of the tubular structures such as vessels need to be closed.
Correcting gaps: None of the surfaces should have gaps or holes. The gaps must be filled with mesh triangles. A remeshing process within the software should take care of any of these gaps.
Filling internal spaces: The internal spaces, particularly cancellous bone, may be filled in with honeycomb grid to save time and material for printing.
Creating supports and scaffolds: Care should be taken to create scaffolding and support for the anatomical structures, otherwise the model will collapse.
6. Saving the processed 3D dataset:
It is important to save every step of the process for the length of the project, from the DICOM to the segmentation masks to the processed models. That way, mistakes can be edited by going back to a specific step. DICOM files are already saved as part of the user’s imaging service. However, STL files should also be saved in a confidential manner as part of the standard patient record. This allows for the data to be easily referenced for problem solving or further post-processing at a later date. Saving the files in each stage also allows for the editing of mistakes by going back a specific step or refining the data-set for multistage reconstructions.
7. Sending the dataset to the 3D printer:
This step depends on several factors. You may be sending your data to a printing service. If you have your own printer, the type of printer may determine the efficiency of this step. Some printers require a computer to be attached to the printer, while others take files via SD card, USB device or even wifi, online/intranet networking. All printers require a final step of slicing the 3D surface model into a stack of 2D layers before printing. 3D printer Slicer software is needed to convert an STL file into G-code, a language that can be processed by a 3D printer to print a model layer by layer (18).
Most printers have their own software that prepares the model for printing. The software will identify any errors in the model and arrange the print in the virtual 3D space so that it has the most likelihood of printing without any issues. It will orient the model appropriately so that the resting surface has the most contact with the base of the printer. It might also add thin supports or beams that will hold up the print throughout the entire process. Additionally, multiple STL files can be inserted onto the print tray at the same time. The printer software can also assign different colors to the STL files so that a multi-colored 3D model can be created once the separate parts are assembled after printing. Once all of these steps are completed, the printer can fabricate a 3D object (Figure 3).
Figure 3.
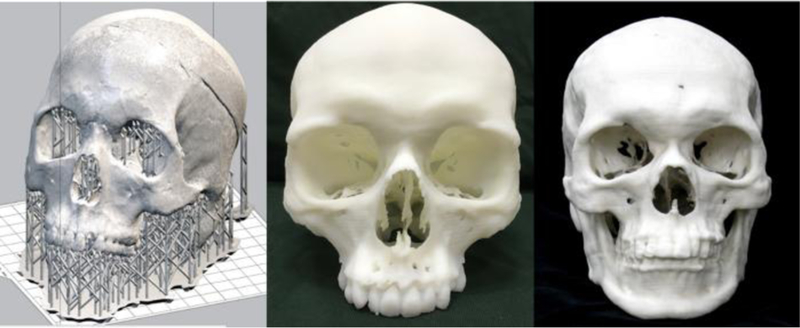
a) 3D printed model of a skull created after using postprocessing and 3D printing software, PreForm, to convert the model into an STL file and designing support structures for the model to be used during the printing process. The skulls pictured were printed on b) Stratasys and c) ZCorp printers respectively.
For clinical cases, it is recommended that you save the 3D model file and print file the same length as the standard patient records as the case data might be needed in the future. A unique identifying number should also be included on the model for easy identification at a later date.
Typically, print production ranges from a few hours to even days depending on the size of the model as well as type of the printer and materials to be used. The quality of the print is directly related to the acquired volumetric data, extent of post processing, and the printer (14). Printing time can vary widely, depending primarily on the size and complexity of the object printed. It also depends somewhat on print quality and density. Higher quality prints can be obtained at the cost of slower printing speed. Denser prints require more material and more printing time. Although small jobs can be completed within a few minutes, print times for more typical print jobs will run 1–15 hours. Errors between the 3D print and imaged anatomy are usually clinically negligible and due to suboptimal segmentation or STL conversion (13, 14).
Providing a 3D Printing Service
For radiologists interested in setting up a 3D printing service, different levels of use may be considered.
Entry level use
Educational use
Clinical use
Individuals often start at entry-level use prior to proceeding to other levels. In general, moving up each level will increase the printing costs by an order of magnitude and double the required staffing.
Entry Level Use
This level is for someone who will be printing a relatively small number of objects for personal use. This is an ideal level for becoming familiar with 3D printing. Other than oneself, no additional staffing is required.
The entry costs are relatively small and consist of a printer, printing stock and one’s time. A desktop 3D printer can be purchased for $500-$3000, depending on the features. Basic printing stock is about $30 per spool of material. 3D printing using these basic devices can be somewhat accurate, however discrepancies between image anatomy and the final printed models are more likely to occur, and these types of printers should not be used for clinical use (1).
Educational Use
3D printing technology is used increasingly for medical education (8, 19, 20). Educational printing needs vary widely, ranging from a single anatomical model to individual models for hundreds of students (Figure 4).
Figure 4.

3D printed model of a complex acetabular fracture. By providing greater anatomic detail and tactile information about the fracture type, these models can help to determine treatment options (surgical vs. nonsurgical) and to plan adequate fracture reduction preoperatively. The model pictured was printed on a Stratasys Objet500 Connex3 printer using jetting of VeroWhite material.
Inexpensive printers and printer supplies can sometimes suffice for educational purposes. However, if the need for printed models outstrips the output of a single printer, one must purchase either additional inexpensive printers or a faster and more expensive printer. If the need for printed models is large enough, it may also become necessary to hire part- or full-time staff to run the printers.
Clinical Use
One of the major uses of 3D-printed models is to improve the quality of preoperative planning (1, 21–27). Unlike 3D models used for educational purposes, those used for surgical planning must be available on a reliable schedule, and sometimes with relatively short turn-around times. Attaining an acceptable level of reliability may require more printers and service contracts on those printers, to hire multiple full-time staff, and to set up schedules so that the clinical needs can be met (5, 6). With sufficient clinical need, an alternative policy for outsourcing printing of these models may be needed, if in-house personnel and equipment cannot meet the need.
Clinical usage imposes additional constraints on a 3D printing center. These include the need for increased accuracy, decreased turn-around time and sometimes the ability to work with expensive materials to create custom implants. More advanced FDA cleared software programs are required to ensure model accuracy (5). Inexpensive clinical 3D-printing is possible in small lots (28, 29). However, printers used for clinical work will generally be much more expensive than those used for personal or educational purposes ($10,000−−100,000+) (30).
Cheng et al (31) described their experience with printing cardiac models for surgical planning. Although their surgeons found these models valuable, their models cost $1000-$1300, including the cost of the printer, materials, image segmentation and cleaning, and labor.
A clinical 3D printing lab must recoup its expenses through a reimbursement mechanism. A current barrier to this is the absence of a Current Procedural Terminology (CPT) code for 3D-printed models (9). This may eventually come to pass, but it will require a great deal of time to document the time saved and improved outcomes resulting from 3D-printed models.
It is inevitable that some 3D-printed implants will fail or lead to complications. The potential legal liability is an emerging area with few precedents (24, 32). In the U.S., the Food and Drug Administration (FDA) has issued several guidance documents for this growing field (33, 34). Currently, the FDA has been assessing cases based on their individual merit, resulting in various devices used for FDA-cleared interventions, such as a biodegradable airway splint described by Zopf et al (32). Morrison et al (35) gives an excellent overview of their experience navigating the regulatory hurdles for a 3D-printed implantable device.
Research Directions in 3D Printing:
Researchers have used 3D printers to fabricate various implantable prostheses from a wide variety of materials such as silicone, polymethyl methacrylate, polyamide mesh, titanium (36–38) and others (19, 39, 40). Some investigators have modified 3D printers to fabricate surgical instruments (41).
General Directions
Increased availability and wider clinical acceptance of medical 3D printing has created opportunities for radiologists to become more involved in research related to 3D printing. This section reviews some of these opportunities.
Research applications of 3D printing have been broadly categorized as follows: Medical models, medical aids (guides, splints, and prosthesis), inert implants, instruments and tools, and biomanufacturing.
As discussed in our companion paper on clinical applications of 3D printing, some of the research has already transformed clinical practice. There have been many case reports showing that 3D models have helped with complex cases, including applications in cardiovascular surgery, orthopedic surgery, neurosurgery, dental and craniofacial surgery, abdominal surgery, and thoracic surgery (42–45). 3D printing is gaining attention in medical care, and hospitals are starting to create 3D printing labs. For example, Figure 5 demonstrates how 3D printing has been used in the creation of custom tracheal stents in patients with tracheobronchomalacia.
Figure 5.
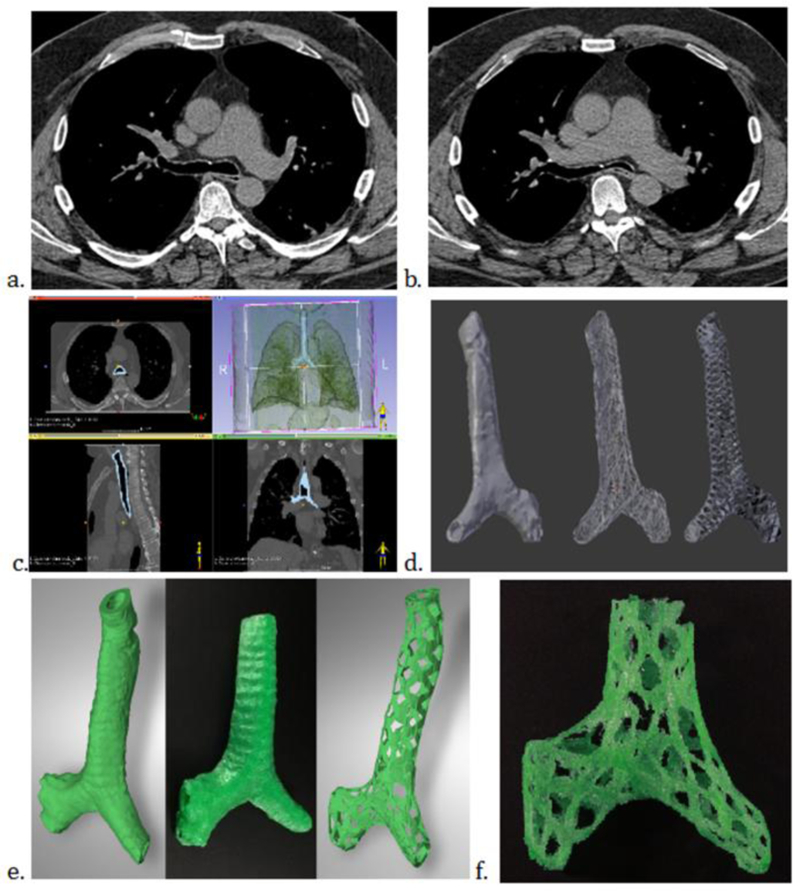
3D printed tracheal model with fabricated tracheal stents. 58-year-old man with longstanding respiratory symptoms due to tracheobronchomalacia. a and b. Computed tomography (CT) of the chest with inspiration (a.) and expiration (b.) revealing greater than 50% collapse in the carina and left mainstem bronchus. c. CT DICOM data used to segment the trachea and create a STL file (3D Slicer version 4.6, www.slicer.org). e and f. 3D rendered models (e) and 3D printed stent (f). The final model was used in the treatment of this patient.
3D printed models are increasingly used in physician and patient education, simulation, preoperative planning, intra operative templates, customized cutting guides, and virtual surgical techniques (46, 47).
Patient specific cutting guides and patient specific implants have shown added value (i.e., cranial, lumbar, tracheal, chest wall) (42). Movrin et al used custom designed implants for reconstruction and treatment for blow out orbital fractures (48). Others have shown utility of 3D printing for bone graft harvesting; such as in the design of customized mandibular tray, repair of facial clefts, and the creation of facial or extremity implants in dental and orthopedic military blast injury settings (42, 49).
Perhaps the most ambitious application of 3D printing is bioprinting, where living cells, bone, cartilage skin and vessels are grown on 3D cell scaffolds (50, 51). Mannoor et al successfully created a living bionic ear composed of living cells and electronic nanoparticles ear organ (52). The creation of bioartificial livers is another promising application of bioprinting (53). The merging of bone and cartilage tissue in implants using regenerated tissue is another potential research area in bioprinting (45). Cardiovascular bioprinting and molecular 3D printing in the research setting has also shown promise (54). Printers that can create these tissues are far more expensive than those used to print plastic models (30). Although radiologists have not typically been involved in engineering phases of tissue and organ printing, they should be familiar with these techniques.
Materials Research
Research into materials used for 3D printing has been especially fruitful. Much of this research aims to develop materials that more closely reflect the dynamic capabilities of tissues (flexibility, conductivity, and biocompatibility) in the clinical setting. Research on flow physiology (i.e., printed vascular models, appropriate acquisition of imaging information and design of studies) has benefited from new materials.
In a recent study of PAR post procedure evaluation of the aortic wall and root by Ripley et al, while flexible material was utilized to print the aortic root, the material did not match the true elastic modulus of the aortic wall, defined as the amount that a material will stretch or deform in response to force or stress (55). Though past studies have attempted to print the heart in the different phases, improved ability to create models during dynamic states of motion that better simulate the innate motion in the human body (i.e., cardiac systole and diastole) are needed (56). This new area of 3D printing is called dynamic 3D or “4D printing” and is quickly expanding in industry (57, 58).
Potential solutions to current design challenges include: printers with multi-material capability, materials with improved temporal resolution, use of CAD software which either mirrors normal anatomy on the unaffected side (or designs a “wall” to enclose a segmented blood pool (often termed “hollowing”)) or subtracts the STL of the enhanced blood pool from the STL of the surrounding tissues (55, 59). The wall should be printed using a high-resolution technology (material jetting or vat photo polymerization) to achieve a smooth lumen. These models can be used to test the effect of interventions on local hemodynamics. The models can help optimize in vivo techniques (coronary contrast opacification gradients), respiratory dynamics (H3 MR imaging), and flow encoded MR imaging (60–65).
Other recent materials developments include the following: bioresorbable stents for treatment of trachea-bronchomalacia in children, liquid latex molding and 3D combination for free flap soft tissue reconstruction planning, as well as creation of MR visible or compatible materials for interventional procedures (laryngoscope using biocompatible polymer and individualized vaginal template for guided brachytherapy for cervical cancer) (32, 66–70). Radiologists can work in tandem with the engineering teams to help determine best practices depending on which imaging modality is used for 3D printing.
Other Research Opportunities
Other applications of 3D printing include medical forensics, imaging optimization/quality control, and patient physician communication improvements (71, 72). 3D printing may also be of use in the advancement of drug delivery mechanisms via production of advanced blood brain barrier modelling for in vitro platforms, enhancing the current understanding of the cerebrovascular mechanisms for central nervous system (CNS) drug delivery (73).
Radiology driven, organized prospective research studies in 3D printing are needed to formally support improved outcomes, assess clinical benefits, provide a documented cost effectiveness analysis, and substantiate expected improvements in communication. Research data are needed to establish evidence based guidelines that could ultimately lead to reimbursement for 3D printing in the clinical setting (9). One of the current barriers to wider acceptance of 3D printing in Radiology departments is the approval of formal current procedural terminology (CPT) codes. While some research in this area has been performed, standard protocols for assessment are needed for example in assessment for surgical planning beyond surgeon satisfaction and self-assessment (74, 75). Several groups are actively researching the impact that 3D models can have in medicine, including decreasing operative and treatment costs and improving patient outcomes. This research will continue to be of utmost importance to eventually obtain reimbursement for 3D models.
Conclusion
Radiologists play an important role in the future of 3D printing. There is currently a need for radiologists to become familiar with 3D printing in order to explore the unlimited potential of this technology. Their involvement is critical for future clinical applications. This paper has attempted to provide readers with a “how to get started” guide, from software selection, creating a 3D model, to the many ways of creating a 3D print. Never before has 3D printing been more accessible to radiologists. It is important for the field of radiology to see the potential of this emerging technology and help guide its usage in future medical practice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Taryn Hodgdon, University of Ottawa, Department of Radiology The Ottawa Hospital 501 Smyth Road Ottawa, Ontario, Canada K1H 8L6.
Raman Danrad, LSU Health Sciences Center-New Orleans Department of Radiology, 3rd Floor 1542 Tulane Avenue New Orleans, LA 70112, Phone: 504-568-5523, rdanra@lsuhsc.edu.
Midhir J. Patel, University of South Florida Health - Department of Radiology 2 Tampa General Circle, STC 7028 Tampa, FL 33606, Phone: (813) 259-8515, mpatel3@health.usf.edu
Stacy E. Smith, Department of Radiology Brigham and Women’s Hospital Harvard Medical School 75 Francis Street, Boston, MA 02115, Phone: (617) 732-7537, Ssmith70@partners.org.
Michael L. Richardson, University of Washington School of Medicine Department of Radiology 4245 Roosevelt Way NE Seattle, WA 98105, Phone: 206-734-6186, mrich@uw.edu
David H. Ballard, Mallinckrodt Institute of Radiology Washington University School of Medicine 510 S. Kingshighway Blvd Campus Box 8131 St. Louis, Missouri, 63110, Phone: (314) 226-5464, Fax: (314) 747-4671, davidballard@wustl.edu.
Sayed Ali, Temple University Hospital Department of Radiology 3401 North Broad Street Philadelphia, PA 19140, Phone: 215-707-6847 (O), alisayan@tuhs.temple.edu.
Anthony Paul Trace, Eastern Virginia Medical School, Department of Radiology, PO Box 1980, Norfolk, VA 23501, Phone: 757-388-1141, Fax: 757-388-1145, AnthonyPaulTrace@gmail.com, TraceAP@evms.edu.
Carolynn M. DeBenedectis, University of Massachusetts Medical School, Department of Radiology, 55 Lake Ave, North, Worcester, MA 01655, Phone: 508-856-6316, Carolynn.debenedectis2@umassmemorial.org.
Matthew E. Zygmont, Department of Radiology and Imaging Sciences, Emory University Hospital Midtown, 550 Peachtree Street, Atlanta, GA 30308, mzygmon@emory.edu.
Leon Lenchik, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, Phone: 336-716-4316, Fax: 336-716-1278, llenchik@wakehealth.edu.
Summer J. Decker, USF Health Morsani College of Medicine, Department of Radiology, 2 Tampa General Circle, STC 7033, Tampa, FL 33606, Phone:813-259-8782, sdecker@health.usf.edu
References
- 1.Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, George E, Wake N, Caterson EJ, Pomahac B, Ho VB, Grant GT, Rybicki FJ. Medical 3D Printing for the Radiologist. Radiographics : a review publication of the Radiological Society of North America, Inc 2015;35(7):1965–88. doi: 10.1148/rg.2015140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marro A, Bandukwala T, Mak W. Three-Dimensional Printing and Medical Imaging: A Review of the Methods and Applications. Current problems in diagnostic radiology 2016;45(1):2–9. doi: 10.1067/j.cpradiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Mishra S Application of 3D printing in medicine. Indian heart journal 2016;68(1):108–9. doi: 10.1016/j.ihj.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, Ersoy H, Mather RT, Judy PF, Cai T, Coyner K, Schultz K, Whitmore AG, Di Carli MF. Initial evaluation of coronary images from 320-detector row computed tomography. The international journal of cardiovascular imaging 2008;24(5):535–46. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 5.Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3D printing in medicine. 3D Printing in Medicine 2017;3(1):1. doi: 10.1186/s41205-016-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prima M Additively manufactured medical products–the FDA perspective. 3D Print Med 2015;2. doi: 10.1186/s41205-016-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GB, Lee S, Kim H, Yang DH, Kim YH, Kyung YS, Kim CS, Choi SH, Kim BJ, Ha H, Kwon SU, Kim N. Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology. Korean journal of radiology 2016;17(2):182–97. doi: 10.3348/kjr.2016.17.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman T, Michalski M, Goodman TR, Brown JE. 3D printing from diagnostic images: a radiologist’s primer with an emphasis on musculoskeletal imaging-putting the 3D printing of pathology into the hands of every physician. Skeletal radiology 2016;45(3):307–21. doi: 10.1007/s00256-015-2282-6. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto JS, Morris JM, Foley TA, Williamson EE, Leng S, McGee KP, Kuhlmann JL, Nesberg LE, Vrtiska TJ. Three-dimensional Physical Modeling: Applications and Experience at Mayo Clinic. Radiographics : a review publication of the Radiological Society of North America, Inc 2015;35(7):1989–2006. doi: 10.1148/rg.2015140260. [DOI] [PubMed] [Google Scholar]
- 10.Anastasiou A, Tsirmpas C, Rompas A, Giokas K, Koutsouris D, editors. 3D printing: Basic concepts mathematics and technologies. 13th IEEE International Conference on BioInformatics and BioEngineering; 2013 10–13 Nov. 2013. [Google Scholar]
- 11.Wojtyla S, Klama P, Baran T. Is 3D printing safe? Analysis of the thermal treatment of thermoplastics: ABS, PLA, PET, and nylon. Journal of occupational and environmental hygiene 2017;14(6):D80–d5. Epub 2017/02/07. doi: 10.1080/15459624.2017.1285489. [DOI] [PubMed] [Google Scholar]
- 12.Kakizawa H, Toyota N, Akiyama Y, Kijima Y, Ishida O, Ito K. A three-dimensional laminated paper model of the scaphoid from computed tomography. Acta radiologica (Stockholm, Sweden : 1987) 2007;48(1):80–8. doi: 10.1080/02841850601026419. [DOI] [PubMed] [Google Scholar]
- 13.Leng S, McGee K, Morris J, Alexander A, Kuhlmann J, Vrieze T, McCollough CH, Matsumoto J. Anatomic modeling using 3D printing: quality assurance and optimization. 3D Printing in Medicine 2017;3(1):6. doi: 10.1186/s41205-017-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortolotto C, Eshja E, Peroni C, Orlandi MA, Bizzotto N, Poggi P. 3D Printing of CT Dataset: Validation of an Open Source and Consumer-Available Workflow. Journal of digital imaging 2016;29(1):14–21. doi: 10.1007/s10278-015-9810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford JM, Decker SJ. Computed tomography slice thickness and its effects on three-dimensional reconstruction of anatomical structures. Journal of Forensic Radiology and Imaging 4:43–6. doi: 10.1016/j.jofri.2015.10.004. [DOI] [Google Scholar]
- 16.Marcus RP, Morris JM, Matsumoto JM, Alexander AE, Halaweish AF, Kelly JA, Fletcher JG, McCollough CH, Leng S. Implementation of iterative metal artifact reduction in the pre-planning-procedure of three-dimensional physical modeling. 3D Printing in Medicine 2017;3(1):5. doi: 10.1186/s41205-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poornima B AS. Image Registration Techniques for Satellite and Medical Images: A Survey. International Journal of Scientific & Engineering Research 2013;4(12):2090–101. [Google Scholar]
- 18.Oberg EJ, Franklin D; Horton Holbrook L.; Ryffel Henry H.; Green Robert E.; McCauley Christopher J. Machinery’s Handbook (25th ed.). New York, NY, USA: Industrial Press; 1996. [Google Scholar]
- 19.AlAli AB, Griffin MF, Butler PE. Three-Dimensional Printing Surgical Applications. Eplasty 2015;15:e37. [PMC free article] [PubMed] [Google Scholar]
- 20.Preece D, Williams SB, Lam R, Weller R. “Let’s get physical”: advantages of a physical model over 3D computer models and textbookxs in learning imaging anatomy. Anatomical sciences education 2013;6(4):216–24. doi: 10.1002/ase.1345. [DOI] [PubMed] [Google Scholar]
- 21.Brown GA, Firoozbakhsh K, DeCoster TA, Reyna JR Jr., Moneim M. Rapid prototyping: the future of trauma surgery? The Journal of bone and joint surgery American volume 2003;85-A Suppl 4:49–55. [PubMed] [Google Scholar]
- 22.Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR American journal of roentgenology 2011;196(6):W683–8. doi: 10.2214/ajr.10.5681. [DOI] [PubMed] [Google Scholar]
- 23.Frame M, Huntley JS. Rapid prototyping in orthopaedic surgery: a user’s guide. TheScientificWorldJournal 2012;2012:838575. doi: 10.1100/2012/838575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik HH, Darwood AR, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, Baskaradas A. Three-dimensional printing in surgery: a review of current surgical applications. The Journal of surgical research 2015;199(2):512–22. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Martelli N, Serrano C, van den Brink H, Pineau J, Prognon P, Borget I, El Batti S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159(6):1485–500. doi: 10.1016/j.surg.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Murray DJ, Edwards G, Mainprize JG, Antonyshyn O. Advanced technology in the management of fibrous dysplasia. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2008;61(8):906–16. doi: 10.1016/j.bjps.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Zheng YX, Yu DF, Zhao JG, Wu YL, Zheng B. 3D Printout Models vs. 3D-Rendered Images: Which Is Better for Preoperative Planning? Journal of surgical education 2016;73(3):518–23. doi: 10.1016/j.jsurg.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Xue GH, Fu JZ. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Scientific reports 2014;4:6973. doi: 10.1038/srep06973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim AM, Jose RR, Rabie AN, Gerstle TL, Lee BT, Lin SJ. Three-dimensional Printing in Developing Countries. Plastic and reconstructive surgery Global open 2015;3(7):e443. doi: 10.1097/gox.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naftulin JS, Kimchi EY, Cash SS. Streamlined, Inexpensive 3D Printing of the Brain and Skull. PloS one 2015;10(8):e0136198. doi: 10.1371/journal.pone.0136198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng YLC, S.J. Manufacturing of cardiac models through rapid prototyping technology for surgery planning. Materials science forum 2006;505–507: 10.4028/www.scientific.net/MSF.505-507.1063. [DOI]
- 32.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. The New England journal of medicine 2013;368(21):2043–5. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration. CDRH May 10, 2016, Document no. 1400002. Technical Considerations for Additive Manufactured Devices. Draft Guidance for Industry and Food and Drug Administration Staff 2016.
- 34.US Food and Drug Administration. CDRH fiscal year 2015 (FY 2015) proposed guidance development and focused retrospective review of final guidance 2015.
- 35.Morrison RJ, Kashlan KN, Flanangan CL, Wright JK, Green GE, Hollister SJ, Weatherwax KJ. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clinical and translational science 2015;8(5):594–600. doi: 10.1111/cts.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan H, Fu J, Li X, Pei Y, Li X, Pei G, Guo Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature. World journal of surgical oncology 2015;13:308. doi: 10.1186/s12957-015-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong SB, Eliaz N, Leisk GG, Sach EM, Latanision RM, Allen SM. A new Ti-5Ag alloy for customized prostheses by three-dimensional printing (3DP). Journal of dental research 2001;80(3):860–3. doi: 10.1177/00220345010800030301. [DOI] [PubMed] [Google Scholar]
- 38.Ng CS. Recent and Future Developments in Chest Wall Reconstruction. Seminars in thoracic and cardiovascular surgery 2015;27(2):234–9. doi: 10.1053/j.semtcvs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Banks J Adding value in additive manufacturing: researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE pulse 2013;4(6):22–6. doi: 10.1109/mpul.2013.2279617. [DOI] [PubMed] [Google Scholar]
- 40.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. Journal of biological engineering 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rankin TM, Giovinco NA, Cucher DJ, Watts G, Hurwitz B, Armstrong DG. Three-dimensional printing surgical instruments: are we there yet? The Journal of surgical research 2014;189(2):193–7. doi: 10.1016/j.jss.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arora A, Datarkar AN, Borle RM, Rai A, Adwani DG. Custom-made implant for maxillofacial defects using rapid prototype models. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2013;71(2):e104–10. doi: 10.1016/j.joms.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Grant GT, Taft RM, Wheeler ST. Practical application of polyurethane and Velcro in maxillofacial prosthetics. The Journal of prosthetic dentistry 2001;85(3):281–3. doi: 10.1067/mpr.2001.114089. [DOI] [PubMed] [Google Scholar]
- 44.Harrysson OL, Hosni YA, Nayfeh JF. Custom-designed orthopedic implants evaluated using finite element analysis of patient-specific computed tomography data: femoral-component case study. BMC musculoskeletal disorders 2007;8:91. doi: 10.1186/1471-2474-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodfield TB, Guggenheim M, von Rechenberg B, Riesle J, van Blitterswijk CA, Wedler V. Rapid prototyping of anatomically shaped, tissue-engineered implants for restoring congruent articulating surfaces in small joints. Cell proliferation 2009;42(4):485–97. doi: 10.1111/j.1365-2184.2009.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo S-J, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A, Seed M, Grosse-Wortmann L, van Arsdell G. 3D printing in medicine of congenital heart diseases. 3D Printing in Medicine 2016;2(1):3. doi: 10.1186/s41205-016-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bizzotto N, Tami I, Santucci A, Adani R, Poggi P, Romani D, Carpeggiani G, Ferraro F, Festa S, Magnan B. 3D Printed replica of articular fractures for surgical planning and patient consent: a two years multi-centric experience. 3D Printing in Medicine 2016;2(1):2. doi: 10.1186/s41205-016-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabakovic SZ, Konstantinovic VS, Radosavljevic R, Movrin D, Hadzistevic M, Hatab N. Application of Computer-Aided Designing and Rapid Prototyping Technologies in Reconstruction of Blowout Fractures of the Orbital Floor. The Journal of craniofacial surgery 2015;26(5):1558–63. doi: 10.1097/scs.0000000000001883. [DOI] [PubMed] [Google Scholar]
- 49.Zhou LB, Shang HT, He LS, Bo B, Liu GC, Liu YP, Zhao JL. Accurate reconstruction of discontinuous mandible using a reverse engineering/computer-aided design/rapid prototyping technique: a preliminary clinical study. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2010;68(9):2115–21. doi: 10.1016/j.joms.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Lee JY, Choi B, Wu B, Lee M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013;5(4): 10.1088/1758-5082/5/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makitie AA, Korpela J, Elomaa L, Reivonen M, Kokkari A, Malin M, Korhonen H, Wang X, Salo J, Sihvo E, Salmi M, Partanen J, Paloheimo KS, Tuomi J, Narhi T, Seppala J. Novel additive manufactured scaffolds for tissue engineered trachea research. Acta oto-laryngologica 2013;133(4):412–7. doi: 10.3109/00016489.2012.761725. [DOI] [PubMed] [Google Scholar]
- 52.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC. 3D printed bionic ears. Nano letters 2013;13(6):2634–9. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Yan Y, Zhang R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends in biotechnology 2007;25(11):505–13. doi: 10.1016/j.tibtech.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Giannopoulos AA, Mitsouras D, Yoo SJ, Liu PP, Chatzizisis YS, Rybicki FJ. Applications of 3D printing in cardiovascular diseases. Nature reviews Cardiology 2016;13(12):701–18. doi: 10.1038/nrcardio.2016.170. [DOI] [PubMed] [Google Scholar]
- 55.Ripley B, Kelil T, Cheezum MK, Goncalves A, Di Carli MF, Rybicki FJ, Steigner M, Mitsouras D, Blankstein R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. Journal of cardiovascular computed tomography 2016;10(1):28–36. doi: 10.1016/j.jcct.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markert M, Weber S, Lueth TC. A beating heart model 3D printed from specific patient data. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2007;2007:4472–5. doi: 10.1109/iembs.2007.4353332. [DOI] [PubMed] [Google Scholar]
- 57.Gao B, Yang Q, Zhao X, Jin G, Ma Y, Xu F. 4D Bioprinting for Biomedical Applications. Trends in biotechnology 2016;34(9):746–56. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Li YC, Zhang YS, Akpek A, Shin SR, Khademhosseini A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016;9(1):012001. doi: 10.1088/1758-5090/9/1/012001. [DOI] [PubMed] [Google Scholar]
- 59.Kunz M, Ma B, Rudan JF, Ellis RE, Pichora DR. Image-guided distal radius osteotomy using patient-specific instrument guides. The Journal of hand surgery 2013;38(8):1618–24. doi: 10.1016/j.jhsa.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Biglino G, Verschueren P, Zegels R, Taylor AM, Schievano S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2013;15:2. doi: 10.1186/1532-429x-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canstein C, Cachot P, Faust A, Stalder AF, Bock J, Frydrychowicz A, Kuffer J, Hennig J, Markl M. 3D MR flow analysis in realistic rapid-prototyping model systems of the thoracic aorta: comparison with in vivo data and computational fluid dynamics in identical vessel geometries. Magnetic resonance in medicine 2008;59(3):535–46. doi: 10.1002/mrm.21331. [DOI] [PubMed] [Google Scholar]
- 62.Cao P, Duhamel Y, Olympe G, Ramond B, Langevin F. A new production method of elastic silicone carotid phantom based on MRI acquisition using rapid prototyping technique. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2013;2013:5331–4. doi: 10.1109/embc.2013.6610753. [DOI] [PubMed] [Google Scholar]
- 63.Ionita CN, Mokin M, Varble N, Bednarek DR, Xiang J, Snyder KV, Siddiqui AH, Levy EI, Meng H, Rudin S. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. Proceedings of SPIE--the International Society for Optical Engineering 2014;9038:90380m. doi: 10.1117/12.2042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markl M, Schumacher R, Kuffer J, Bley TA, Hennig J. Rapid vessel prototyping: vascular modeling using 3t magnetic resonance angiography and rapid prototyping technology. Magma (New York, NY) 2005;18(6):288–92. doi: 10.1007/s10334-005-0019-6. [DOI] [PubMed] [Google Scholar]
- 65.Steigner ML, Mitsouras D, Whitmore AG, Otero HJ, Wang C, Buckley O, Levit NA, Hussain AZ, Cai T, Mather RT, Smedby O, DiCarli MF, Rybicki FJ. Iodinated contrast opacification gradients in normal coronary arteries imaged with prospectively ECG-gated single heart beat 320-detector row computed tomography. Circulation Cardiovascular imaging 2010;3(2):179–86. doi: 10.1161/circimaging.109.854307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindegaard JC, Madsen ML, Traberg A, Meisner B, Nielsen SK, Tanderup K, Spejlborg H, Fokdal LU, Norrevang O. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2016;118(1):173–5. doi: 10.1016/j.radonc.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Mitsouras D, Lee TC, Liacouras P, Ionita CN, Pietilla T, Maier SE, Mulkern RV. Three-dimensional printing of MRI-visible phantoms and MR image-guided therapy simulation. Magnetic resonance in medicine 2017;77(2):613–22. doi: 10.1002/mrm.26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrison RJ, Hollister SJ, Niedner MF, Mahani MG, Park AH, Mehta DK, Ohye RG, Green GE. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Science translational medicine 2015;7(285):285ra64. doi: 10.1126/scitranslmed.3010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paydarfar JA, Wu X, Halter RJ. MRI- and CT-Compatible Polymer Laryngoscope: A Step toward Image-Guided Transoral Surgery. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016;155(2):364–6. doi: 10.1177/0194599816650176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ripley B, Levin D, Kelil T, Hermsen JL, Kim S, Maki JH, Wilson GJ. 3D printing from MRI Data: Harnessing strengths and minimizing weaknesses. Journal of magnetic resonance imaging : JMRI 2017;45(3):635–45. doi: 10.1002/jmri.25526. [DOI] [PubMed] [Google Scholar]
- 71.Ebert LC, Thali MJ, Ross S. Getting in touch−−3D printing in forensic imaging. Forensic science international 2011;211(1–3):e1–6. doi: 10.1016/j.forsciint.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 72.Schievano S, Sebire NJ, Robertson NJ, Taylor AM, Thayyil S. Reconstruction of fetal and infant anatomy using rapid prototyping of post-mortem MR images. Insights into imaging 2010;1(4):281–6. doi: 10.1007/s13244-010-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, Cucullo L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert opinion on drug discovery 2017;12(1):89–103. doi: 10.1080/17460441.2017.1253676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D’Urso PS, Barker TM, Earwaker WJ, Bruce LJ, Atkinson RL, Lanigan MW, Arvier JF, Effeney DJ. Stereolithographic biomodelling in cranio-maxillofacial surgery: a prospective trial. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery 1999;27(1):30–7. [DOI] [PubMed] [Google Scholar]
- 75.Muller A, Krishnan KG, Uhl E, Mast G. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. The Journal of craniofacial surgery 2003;14(6):899–914. [DOI] [PubMed] [Google Scholar]