Abstract
Background:
Bipolar patients are characterized by dysregulation across the full spectrum of mood, differentiating them from unipolar depression. The ability to switch neural resources between default mode network (DMN), salience network (SN), and executive control network (ECN) has been proposed as a key mechanism for adaptive mood regulation. The anterior insula is implicated in the modulation of functional network switching. Differential connectivity between anterior insula and functional networks may provide insights into pathophysiological differences between bipolar and unipolar mood disorders, with implications for diagnosis and treatment.
Methods:
Resting state fMRI data were collected from 98 subjects (35 unipolar, 24 bipolar and 39 healthy controls). Pearson correlations were computed between bilateral insula seed regions and a priori defined target regions from the DMN, SN, and ECN. After r-to-z transformation, a one-way MANCOVA was conducted to identify significant differences in connectivity between groups. Post-hoc pairwise comparisons were conducted and Bonferroni corrections were applied. Receiver-operator characteristics (ROC) were computed to assess diagnostic sensitivity.
Results:
Bipolar patients evidenced significantly altered right anterior insula functional connectivity with the inferior parietal lobule (IPL) of the ECN relative to unipolar patients and controls. Right anterior insula-IPL connectivity significantly discriminated bipolar patients.
Conclusions:
Impaired functional connectivity between the anterior insula and the IPL of the ECN distinguishes patients with bipolar depression from unipolar depression and healthy controls. This finding highlights a pathophysiological mechanism with potential as a therapeutic target and a clinical biomarker for bipolar disorder, exhibiting reasonable sensitivity and specificity.
Keywords: bipolar depression, unipolar depression, functional network connectivity, frontoparietal, salience, insula
Introduction
Unipolar and bipolar mood disorders are leading causes of disability, affecting approximately 300 million people worldwide (1, 2). Despite their public health impact and long-standing efforts to develop effective interventions, a strikingly high proportion of individuals struggling with mood disorders still do not fully benefit from currently available treatments (3, 4). Further, the clinical similarity between a unipolar or bipolar depressive episode presents a particular diagnostic challenge, with bipolar depression often misdiagnosed as unipolar depression, thus delaying appropriate treatment, or even worse, increasing the potential delivery of harmful therapies. Therefore, the field of psychiatry is faced with two important unmet challenges: first, better methods of differentiating unipolar versus bipolar depression are needed; and second, an improved understanding of pathophysiological mechanisms is necessary for the identification of therapeutic targets for intervention.
Advances in systems neuroscience have identified distinct and dissociable neural networks subserving adaptive functioning. Specifically, fronto-parietal executive control, salience, and default mode networks are linked to key adaptive processes, including emotion regulation, goal-directed attentional control, decision-making and self-monitoring (5, 6). A parallel body of research has identified deficits in these core adaptive capabilities underlying mood disorders, such as emotion and attention dysregulation across mood states (7-13); decreased reward and increased self- versus external-processing (i.e. rumination) in depression (13-16); and increased salience/reward processing and decreased behavioral inhibition in mania (17-20). Linking these core mood-related functional deficits to the integrity of underlying neural networks provides clues to the circuit-level etiology of mood disorders, leading to potential new avenues for diagnosis and treatment.
Adaptive Functioning and Neural Processing
Healthy, adaptive functioning relies upon the deployment of allostatic mechanisms to maintain homeostatic equilibrium(21-24). Achieving this goal in turn relies upon the continual balance between processing exogenous versus endogenous cues, which signal the need to either approach or withdraw in the service of optimal functioning(25-28). Healthy individuals are continually balancing these competing drives through the flexible, integrated regulation of emotion, cognition, and behavior in a complex feed forward-feedback system (24, 28). Too much reliance upon any one system threatens homeostatic balance; for example, overreliance upon salient exogenous cues at the expense of regulatory control can lead to over-approach, as seen in bipolar disorder (20). Overreliance upon endogenous, self-focused cues at the expense of external processing can lead to reduced motivation to interact with the external environment and thus reduced possibilities for reward, as seen in unipolar depression (29). From this perspective, a major dysfunction in mood disorders is the inability to flexibly and adaptively switch between competing processing modes and to adjust behavior accordingly. This dysfunction is linked to deficits in underlying circuit dynamics, and in particular to the rigid under- or over-reliance upon specific functional networks. Existing resting-state functional neuroimaging studies show increased self-focused attention (e.g. rumination) in unipolar depression is associated with increased default mode network recruitment and reduced salience network recruitment (30-36). Behavioral, emotional and cognitive dysregulation in bipolar disorder is associated with disrupted salience network recruitment and decreased fronto-parietal executive control network recruitment (37-43). Thus, dysfunction in mood disorders may be associated with inefficient or inflexible switching between processing modes.
Anterior Insula, Interoception and Network Switching
Several recent studies have linked the anterior insula to both the processing of interoceptive states and adaptive switching between functional networks, making this region an ideal candidate to explore potential neural circuit dysfunction in mood disorders (44, 45). Interoceptive awareness and accuracy is fundamental to the ability to selectively filter exogenous and endogenous stimuli for adaptive regulation (46). Changes in autonomic arousal function as prediction errors that reorient attention towards or away from salient cues, and in adaptive functioning, signal the need to consider and weigh response options and to regulate accordingly. Clinically speaking, both depressed and (hypo)manic mood states are characterized by deviations or extremes in levels of visceral autonomic arousal. Depressed individuals describe fatigue, heaviness, and lethargy that accompany an increase in negative self-focused thought and behavioral withdrawal. (Hypo)manic individuals describe a feeling of being full of energy or “keyed up” that accompanies expansive thinking, increased hedonic engagement, and risky behaviors. However, these amplified interoceptive cues fail to bring on needed regulatory adjustments that subserve optimal adaptive functioning, such as shifting from internally-focused to externally-focused attention and increasing behavioral approach in depression, or regulating away from salient or rewarding cues and increasing behavioral inhibition in mania. Understanding the relationship between the quality and intensity of interoceptive visceral states and the failure to adaptively and flexibly recruit functional neural networks in service of physiological and behavioral homeostasis may provide further clues to specific dysfunction in mood disorders.
The anterior insula has long been identified for its role in mapping the visceral, autonomic aspects of emotional experience to higher order cognitive processes (47, 48). More recently, the anterior insula has been identified as a major “rich club” hub within the salience network, with extensive functional inter-network connections to default mode, fronto-parietal executive control and cortico-striatal network hubs, positioning the AI as a key structure for integrating cognitive, behavioral and affective functional processes (45, 49, 50). “Rich club” hubs have been defined as regions in the brain with extensive global connections, and form the functional “backbone” of the brain (28, 49). From a neuronal network perspective, deficits in functioning of major rich club hub regions have far reaching consequences due to their global connectedness, such that dysfunction in these structures causes diffuse and widespread functional impairments (51). Recent research has provided evidence of the anterior insula’s role as a major hub implicated in predicting changing regulatory control demands and facilitating switching between default mode and executive control networks (45, 50, 52). Thus, the anterior insula is a region of particular interest in mood disorders both for its role in interoceptive awareness and for its role in flexibly switching between functional networks (53).
The Present Study
Given the role of the anterior insula in both interoceptive awareness and switching between functional networks, the regulatory dysfunction found across mood disorders, and the contributing role of visceral autonomic cues to mood disorder dysfunction, we sought to determine whether differential functional connectivity between the anterior insula (AI) and key nodes of the default mode (DMN), fronto-parietal executive control (ECN), and salience networks (SN) distinguishes unipolar depression, bipolar depression, and healthy controls at the syndromal level. We hypothesized bipolar patients would show weaker functional connectivity between AI and ECN than unipolar patients or healthy controls, suggesting deficits in the ability of the AI to instantiate the executive control of emotion in bipolar depression. By contrast, we hypothesized unipolar patients would show stronger functional connectivity between AI and DMN relative to bipolar patients or healthy controls, suggesting deficits in the ability to instantiate regulation away from internal, self-focused processing in unipolar depression. In addition, to explore the relationship between functional connectivity deficits and dimensional aspects of regulatory behavior, we examined the correlation of connectivity with psychological measures of perceived emotion control, behavioral approach and behavioral inhibition. We hypothesize dysfunction along the dimension of emotion regulation will be significantly related to the integrity of AI-functional network connectivity. Finally, to determine whether specific patterns of AI connectivity to functional networks might serve as a biomarker of unipolar versus bipolar depression, we examined the specificity and sensitivity of AI functional connectivity in classifying unipolar versus bipolar patients.
Methods
Participants
Participants for this study (N=98) included 35 unipolar depressed patients, 24 bipolar depressed patients, and 39 healthy controls (see Table 1 for complete demographics). Patients included for this study were initially recruited as part of three separate protocols investigating the neural mechanisms of three psychiatric interventions: electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and cognitive-behavioral therapy (CBT) at the Massachusetts General Hospital. Only baseline (pre-treatment) data were included in the present study. Data from a cohort of healthy subjects were included from a separate study (see Supplement for details on parent studies and inclusion criteria). All study procedures were approved by the Partners Healthcare Internal Review Board.
Table 1.
Study demographics.
| Measure | Bipolar N (%) |
Unipolar N (%) |
HC N (%) |
Sig. |
|---|---|---|---|---|
| Gender | 0.43a | |||
| Male | 12 (50.00) | 22 (62.9) | 19 (48.7) | |
| Female | 12 (50.00) | 13 (37.1) | 20 (51.3) | |
| Age | 45.58 ± 14.97c | 42.77 ± 14.73c | 25.90 ± 3.21 | <.001b |
| Race | ||||
| White | 22 (91.7) | 32 (91.3) | 29 (74.4) | 0.06a |
| Black | 1 (4.02) | 1 (2.9) | 3 (7.7) | |
| East Asian | 0 (0.0) | 1 (2.9) | 1 (2.6) | |
| Asian | 1 (4.2) | 0 (0.0) | 3 (7.7) | |
| Other or unreported | 0 (0.0) | 1 (2.9) | 3 (7.7) | |
| Other Diagnostic Variables | ||||
| Current Anxiety | 23 (95.83) | 29 (82.86) | 0 | 0.72c |
| Lifetime Mania (BP I) | 18 (75.00) | 0 | 0 | |
| Lifetime Hypomania (BP II) | 6 (25.00) | 0 | 0 |
Note. Significant differences between group demographics calculated using
Kruskal-Wallis H Test, or
one-way ANOVA.
Tukey HSD post-hoc test of pairwise comparison between patient groups non-significant at p = .64.
Clinical measures
Clinically relevant psychological dimensions were measured using the Affective Control Scale (ACS; measure of perceived emotion control), (54) and the Behavioral Inhibition System (BIS)/Behavioral Activation System (BAS) Scales (see Supplement) (55). Higher scores on the ACS indicate less perceived control of emotions. Higher scores on the BIS scale indicate greater behavioral avoidance and inhibition. Scores at the extreme ends of the BAS scales indicate either under-approach (low) or over-approach (high).
Medication Load
To control for the effects of medications, a standardized medication load metric was calculated for each individual by dividing the patient’s medication dosages by the World Health Organization (WHO) Defined Daily Dose for that medication (ATC/DDD; https://www.whocc.no/). Medications were then grouped into three classes (mood stabilizers, antidepressants, anxiolytics/hypnotics). Medication load values for each medication class were then entered separately into MANCOVA models.
MRI acquisition and preprocessing
All subjects included in this study were scanned using the same MR protocols in the same 3-Tesla Siemens Skyra MRI scanner (see Supplement for details). MRI preprocessing and first level analyses were carried out with a combination of tools from FSL v5.0.4 (FMRIB, Oxford, UK) and SPM2 (Wellcome Department of Cognitive Neurology, London, UK) using in-house scripts as previously described in Van Dijk et al. (56). See Supplement for complete details of preprocessing steps.
Anatomical Mask Definition
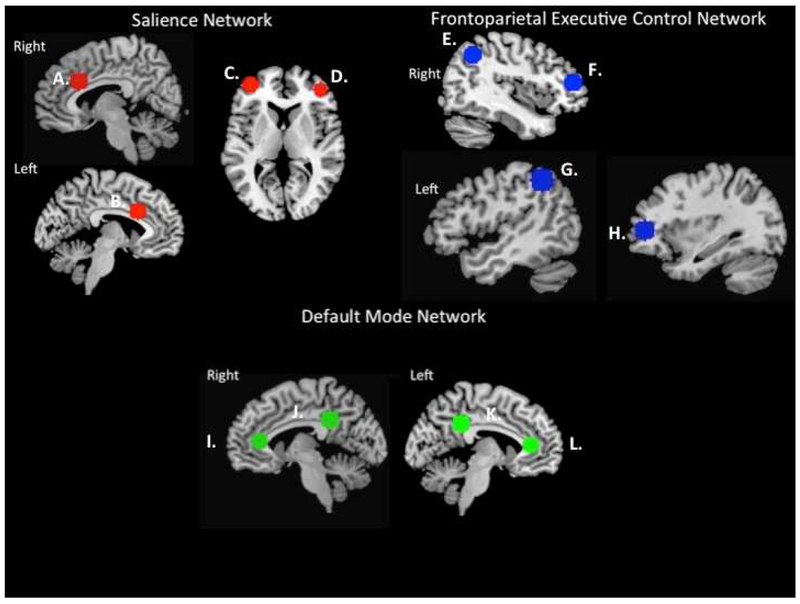
Left and right dorsal AI seed regions of interest (ROI) were defined using masks from the Kelly et al. (57) three-cluster solution (http://fcon_1000.projects.nitrc.org; see Supplement). Selection of target ROIs from the ECN, SN, and DMN were defined using max voxel locations as described in Seeley et al. (5). Target nodes for the ECN included bilateral posterior parietal cortex (in a region of inferior parietal lobule, IPL), and bilateral dorsolateral prefrontal cortex (DLPFC). Target nodes for the DMN included bilateral posterior cingulate cortex and bilateral vmPFC. Target nodes for the SN included bilateral VLPFC and bilateral dorsal anterior cingulate (see Figure 2, which includes MNI coordinates). Spherical ROI masks (10mm diameter) were created for each of the target ROIs using the MarsBar SPM toolbox, with max voxel locations as reported in Seeley et al. (5) specified as center of sphere.
Figure 2.
Functional network ROI locations. ROI masks derived from the Wake Forest University Pickatlas (WFU PickAtlas) Automatic Anatomical Labeling tool using 10mm spherical ROIs. Center of spheres selected from functional network max voxel locations specified in Seeley et al. (2007), listed as MNI coordinates (x, y, z). Salience network (SN): A) right dACC (6, 22, 30); B) left dACC (−6, 18, 30); C) left VLPFC/DLPFC (−38, 52, 10); D) right VLPFC (42, 46, 0); Frontoparietal executive control network (ECN): E) right lateral parietal (38, −56, 44), F) right DLPFC (46, 46, 14); G) left inferior parietal lobule (−48, −48, 48); H) left DLPFC (−34, 46, 10). Default mode network (DMN): I) right vmPFC (2, 36, 10); J) right posterior cingulate cortex (7, −43, 33); K) left posterior cingulate cortex (−7, −43, 33); L) left vmPFC (−2, 36, 10).
Resting-State fMRI Analyses
Connectivity maps were obtained at the individual subject level for bilateral dorsal AI seed regions by averaging the signal across all voxels in the ROI and calculating Pearson’s product moment correlation between the mean ROI time-series and the time-series from each whole brain acquired voxel. Correlation maps were converted to z-maps using Fisher’s r-to-z transformation. Mean Fisher’s z-transformed values were extracted from target ROI masks using MarsBar and imported into SPSS (IBM, version 24.0) for analysis.
Group-Level Analyses
To control for risk of Type 1 error resulting from testing multiple dependent variables, group differences in AI seed region functional connectivity with functional network target ROIs were calculated using a conservative multivariate analysis of covariance (MANCOVA) in SPSS (IBM, version 24.0), with subject group (unipolar, bipolar, healthy control) as a fixed factor, AI ROI-to-target ROI z-transformed values as the dependent variables, and age, gender and medication load entered as covariates. Following evaluation of model assumptions and multivariate results, post-hoc pairwise comparisons of resulting estimated marginal means were conducted. To control for multiple comparisons, Bonferroni corrections were applied to the estimated marginal means of each of these pairwise comparisons.
Exploratory Analysis: Relationship to Clinically Relevant Variables
To explore the dimensional relationship between self-reported emotion dysregulation and AI-functional network ROI connectivity patterns, a series of Pearson’s partial correlations (2-tailed) were conducted between AI ROI- target ROI functional connectivity and scores on the HAM-D, ACS, BIS and BAS-Reward scales, controlling for age, gender and medication load. To correct for multiple comparisons, bias corrected and accelerated (BCa) 95% confidence intervals for the results of correlational analyses were calculated using an iterative bootstrap method (resample and replace, 1000 samples) in SPSS. Because these measures were not administered to the healthy control sample, correlation analyses are limited to patient samples only.
Receiver-Operating Characteristics (ROC)
To explore the sensitivity and specificity of AI functional connectivity patterns in classifying bipolar versus unipolar depression, ROC analyses were conducted on significant results from group level analyses. Specifically, significant AI-network connectivity results were entered as the test variable, with diagnostic group entered as the state variable, assuming a non-parametric distribution. Resulting cutoff scores representing the greatest balance between highest sensitivity and specificity were used to calculate sample-based sensitivity/specificity proportions and the predictive value of connectivity strength.
Results
Baseline Sample Differences
Healthy control subjects were significantly younger than patients (Table 1); therefore, age was included as a covariate in all analyses. Table 2 shows baseline clinical characteristics of unipolar and bipolar depressed patients. Patient groups did not significantly differ in depression severity scores (HAM-D-17), and endorsed moderate levels of depression. Bipolar patients showed significantly greater impairment in perceived affective control (ACS), less behavioral inhibition (BIS) and greater behavioral approach (BAS-Reward) than unipolar depressed patients (Table 2).
Table 2.
Baseline clinical characteristics.
| Bipolar Means (SD) |
Unipolar Means (SD) |
t | Sig. | |
|---|---|---|---|---|
| Medication Load | ||||
| Mood stabilizers | 1.55 (1.21) | 0.50 (0.48) | 4.14 | <.001 |
| Antidepressants | 1.14 (1.06) | 1.98 (1.66) | −2.2 | 0.03 |
| Anxiolytics | 0.34 (0.32) | 0.24 (0.32) | 1.35 | 0.18 |
| Baseline Clinical Characteristics | ||||
| HAM-D-17 | 13.63 (7.61) | 16.82 (6.38) | 1.68 | 0.10 |
| ACS | 4.35 (0.62) | 3.94 (0.69) | 2.31 | 0.03 |
| BIS | 23.5 (6.14) | 16.82 (3.41) | 5.25 | <.000 |
| BAS - Drive | 7.17 (3.03) | 4.33 (2.90) | 3.57 | 0.001 |
| BAS-Reward | 11.75 (2.33) | 8.58 (3.75) | 3.66 | 0.001 |
| BAS-Fun Seeking | 6.21 (3.35) | 4.93 (2.82) | 1.51 | 0.14 |
Note. Standardized medication load metric was calculated for each individual patient by dividing each of the patient’s prescribed medication dosages by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology Anatomical Therapeutic Chemical Defined Daily Dose for that medication (ATC/DDD; https://www.whocc.no/). HAM-D-17 = Hamilton Depression Rating Scale, 17-items; ACS = Affective Control Scale. BIS = Behavioral Inhibition Scale. BAS-Drive = Behavioral Activation, Drive Subscale; BAS-Reward = Behavioral Activation, Reward Subscale; BAS-Fun Seeking = Behavioral Activation, Fun Seeking Subscale.
Insula to Functional Network Connectivity
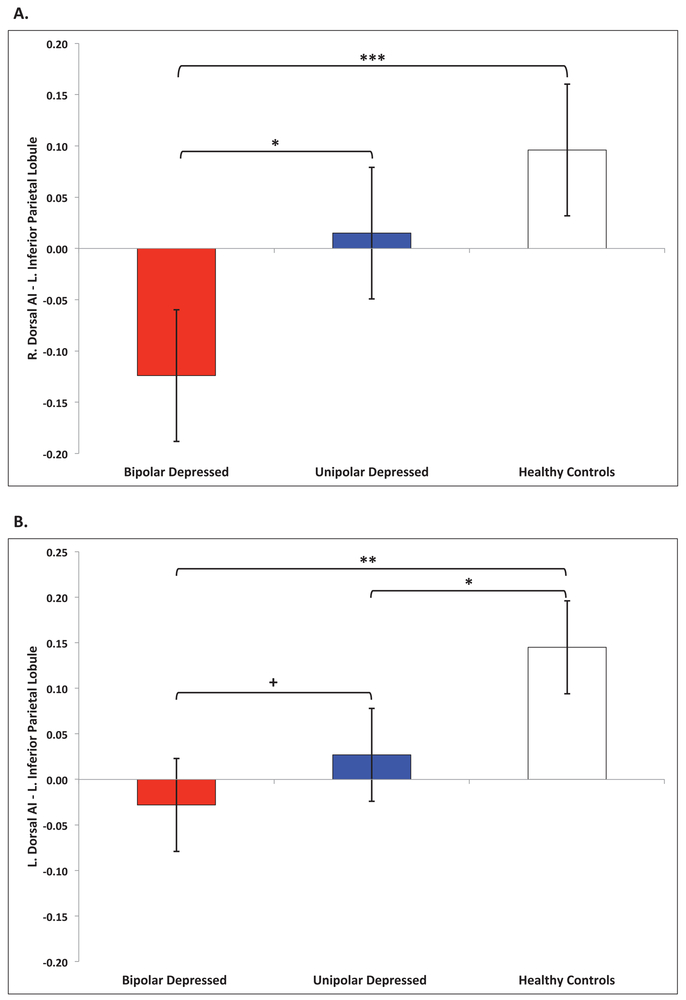
No significant multivariate effects were found for age, gender or medication load (all p ’s >.05) A significant multivariate effect was found for group (bipolar, unipolar, healthy controls) on functional connectivity, Pillai’s Trace=. 12, F=1.74, df=(24, 160), p =.02. Tests for equality of covariance and error variance were non-significant (Box’s test, p=.56; Levene’s test p ’s>. 05) indicating model assumptions were not violated. The full results of follow-up pairwise comparisons of resulting estimated marginal means are summarized in Table 3. Significantly altered functional connectivity between bilateral dorsal AI and left IPL (ECN) was found in bipolar patients relative to healthy controls (right AI: Cohen’s d=1.02; left AI: d =0.94), and in bipolar patients relative to unipolar patients (right AI: d=0.70), such that bipolar patients demonstrated reversed polarity (anticorrelated connectivity) relative to unipolar patients and healthy controls (Figure 3a). Significantly weaker left dorsal AI-left IPL (ECN) functional connectivity (d=0.67), but not right dorsal AI-left IPL (ECN), was found in unipolar patients relative to healthy controls (Figure 3b). Weaker functional connectivity between right dorsal AI and right VLPFC (SN) was found in bipolar patients relative to healthy controls (d=0.64), however this difference did not survive correction for multiple comparisons (Supplement, Figure S1a). No significant differences in right dorsal AI-right VLPFC (SN) functional connectivity were found between unipolar patients and bipolar patients or unipolar patients and healthy controls. Stronger functional connectivity between left dorsal AI and bilateral vmPFC (DMN) was found in unipolar patients relative to healthy controls (r. vmPFC: d=0.52; left vmPFC: d=0.58), but this difference did not survive correction for multiple comparisons (Supplement, Figure S1b). No significant differences in left dorsal AI-vmPFC (DMN) functional connectivity were found between bipolar and unipolar patients or bipolar patients and healthy controls (Table 3).
Table 3.
Summary of pairwise group differences in anterior insula to target network ROI functional connectivity.
| Bipolar vs HC |
Unipolar vs HC |
Bipolar vs Unipolar |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insula Seed Region |
Fx Network |
Target ROI |
MNI Coordinates |
Mean Δ (SE)a |
pb | 95% C.I. Lower, Upper |
Mean Δ (SE) |
pb | 95% C.I. Lower, Upper |
Mean Δ (SE) |
pb | 95% C.I. Lower, Upper |
| R. Dorsal Anterior | ||||||||||||
| ECN | L. IPL | −48 −48 48 | −0.22 (.07) | <.001*** | −0.35, −0.09 | −0.08 (.05) | 0.13 | −0.19, 0.02 | −0.14 (.05) | 0.01* | −0.25, −0.03 | |
| R. IPL | 38 −56 44 | −0.04 (.07) | 0.56 | −0.17, 0.09 | 0.04 (.05) | 0.49 | −0.07, 0.14 | −0.08 (.05) | 0.17 | −0.18, 0.03 | ||
| L. DLPFC | −34 46 6 | −0.09 (.08) | 0.29 | −0.25, 0.07 | −0.05 (.06) | 0.48 | −0.17, 0.08 | −0.04 (.07) | 0.54 | −0.17, 0.09 | ||
| R. DLPFC | 46 46 14 | −0.07 (.06) | 0.28 | −0.19, 0.06 | 0.02 (.05) | 0.65 | −0.08, 0.12 | −0.09 (.05) | 0.08 | −0.19, 0.01 | ||
| SN | L. VLPFC | −38 52 10 | −0.06 (0.07) | 0.40 | −0.20, 0.08 | −0.06 (.06) | 0.31 | −0.17, 0.05 | 0.003 (.06) | 0.96 | −0.12, 0.11 | |
| R. VLPFC | 42 46 0 | −0.17 (.08) | 0.04 | −0.32, −0.01 | −0.11 (.06) | 0.09 | −0.23, 0.02 | −0.06 (.06) | 0.36 | −0.19, 0.07 | ||
| L. dACC | −6 18 30 | 0.07 (.06) | 0.27 | −0.06, 0.19 | 0.01 (.05) | 0.91 | −0.09, 0.10 | 0.06 (.05) | 0.23 | −0.04, 0.17 | ||
| R. dACC | 6 22 30 | 0.10 (.07) | 0.19 | −0.05, 0.24 | 0.05 (.06) | 0.40 | −0.07, 0.17 | 0.05 (.06) | 0.45 | −0.07, 0.17 | ||
| DMN | L. PCC | −7 −43 33 | 0.07 (.09) | 0.45 | −0.11, 0.25 | −0.04 (.07) | 0.59 | −0.18, 0.10 | 0.11 (.07) | 0.15 | −0.04, 0.25 | |
| R.PCC | 7 −43 33 | 0.07 (.90) | 0.44 | −0.11, 0.26 | 0.03 (.07) | 0.73 | −0.12, 0.17 | 0.05 (.08) | 0.55 | −0.11, 0.20 | ||
| L. vmPFC | −2 36 10 | 0.10 (.08) | 0.22 | −0.06, 0.25 | 0.06 (.06) | 0.31 | −0.06, 0.19 | 0.03 (.06) | 0.61 | −0.10, 0.16 | ||
| R. vmPFC | 2 36 10 | 0.06 (.08) | 0.42 | −0.09, 0.22 | 0.05 (.06) | 0.40 | −0.07, 0.17 | 0.01 (.06) | 0.87 | −0.12, 0.14 | ||
| L. Dorsal Anterior | ||||||||||||
| ECN | L. IPL | −48 −48 48 | −.17 (.06) | 0.003** | −0.29, −0.06 | −0.12 (.05) | 0.01* | −0.21, −0.03 | −0.05 (.05) | 0.25 | −0.15, 0.04 | |
| R. IPL | 38 −56 44 | −0.01 (.06) | 0.92 | −0.13, 0.12 | 0.003 (.05) | 0.95 | −0.10, 0.10 | −0.01 (.05) | 0.85 | −0.11, 0.09 | ||
| L. DLPFC | −34 46 6 | −0.04 (0.8) | 0.59 | −0.19, 0.11 | −0.04 (.06) | 0.48 | −0.16, 0.08 | 0.003 (.06) | 0.97 | −0.12, 0.13 | ||
| R. DLPFC | 46 46 14 | −0.06 (.06) | 0.34 | −0.18, 0.06 | −0.01 (.05) | 0.82 | −0.11, 0.09 | −0.05 (.05) | 0.34 | −0.15, 0.05 | ||
| SN | L. VLPFC | −38 52 10 | −04 (.07) | 0.56 | −0.17, 0.09 | −0.07 (0.06) | 0.22 | −0.17, 0.04 | 0.03 (.06) | 0.63 | −0.08, 0.14 | |
| R. VLPFC | 42 46 0 | −0.11 (.07) | 0.14 | −0.24, 0.03 | −0.08 (.06) | 0.15 | −0.19, 0.03 | −0.02 (.06) | 0.69 | −0.14, 0.09 | ||
| L. dACC | −6 18 30 | 0.02 (.06) | 0.75 | −0.11, 0.14 | −0.01 (.05) | 0.77 | −0.11, 0.08 | 0.03 (.05) | 0.50 | −0.07, 0.13 | ||
| R. dACC | 6 22 30 | −0.06 (.07) | 0.40 | −0.08, 0.20 | 0.01 (.06) | 0.84 | −0.10, 0.12 | 0.05 (.06) | 0.41 | −0.07, 0.16 | ||
| DMN | L. PCC | −7 −43 33 | −0.002 (.09) | 0.99 | −0.18, 0.19 | 0.01 (.07) | 0.86 | −0.13, 0.16 | −0.01 (.08) | 0.89 | −0.16, 0.14 | |
| R.PCC | 7 −43 33 | 0.03 (.09) | 0.75 | −0.15, 0.21 | 0.06 (.07) | 0.43 | −0.08, 0.20 | −0.03 (.07) | 0.71 | −0.17, 0.12 | ||
| L. vmPFC | −2 36 10 | 0.06 (.07) | 0.41 | −0.09, 0.21 | 0.13 (.06) | 0.03 | 0.02, 0.25 | −0.07 (.06) | 0.25 | −0.19, 0.05 | ||
| R. vmPFC | 2 36 10 | 0.04 (.07) | 0.57 | −0.10, 0.19 | 0.12 (.06) | 0.05 | 0.00, 0.23 | −0.07 (.06) | 0.22 | −0.19, 0.05 | ||
Note: Results of MANCOVA model-adjusted pairwise comparisons between groups.
Difference in estimated marginal means.
Uncorrected significance probabilities. Uncorrected significant findings denoted in bold font. Bonferroni corrected p-values denoted at
p<.001,
p<.01,
p<.05. ECN = frontoparietal executive control network. SN = salience network. DMN = default mode network. dACC = dorsal anterior cingulate cortex. DLPFC = dorsolateral prefrontal cortex. IPL = inferior parietal lobule. PCC = posterior cingulate cortex. VLPFC = ventrolateral prefrontal cortex. vmPFC = ventromedial prefrontal cortex.
Figure 3.
ECN functional connectivity. Functional connectivity differences between (A) right dorsal anterior insula (B) left dorsal anterior insula and left inferior parietal lobule ROI of the ECN. ***p <.001 Bonferroni corrected. **p<.01 Bonferroni corrected. *p<.05 Bonferroni corrected. +p<.05 uncorrected.
Correlation with Dimensional Psychological Variables
Complete results for both dimensional (across diagnoses) and categorical (within diagnoses) correlational analyses can be found in the Supplement (Table S1).
Perceived emotion control.
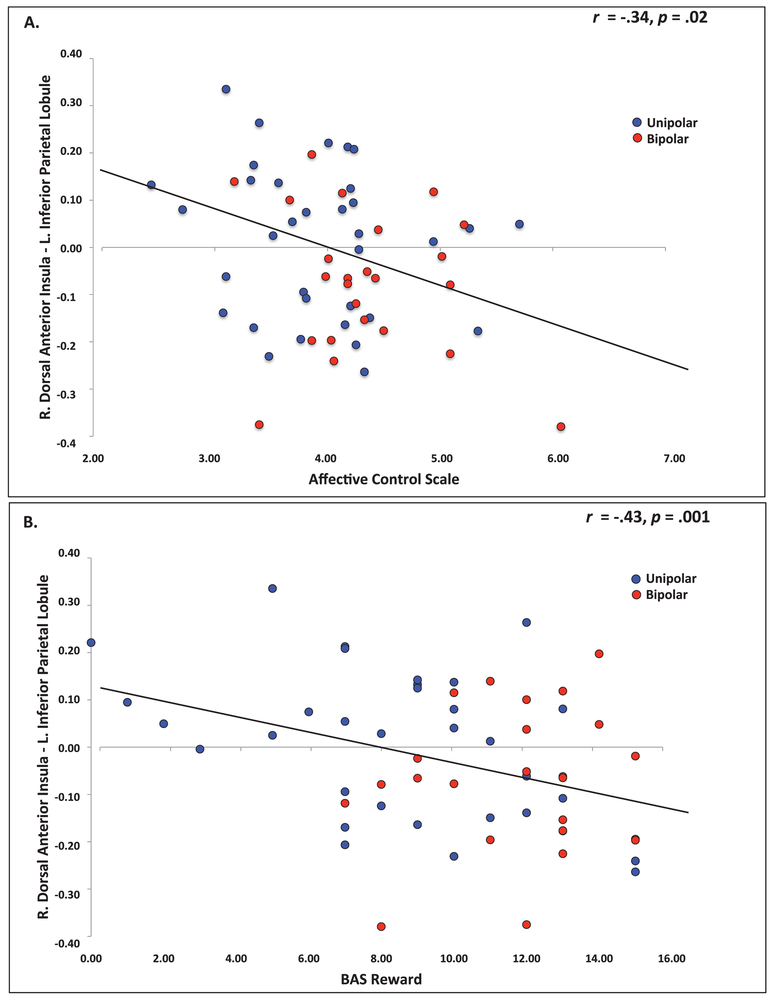
Weaker right dorsal AI – left IPL (ECN) functional connectivity was significantly associated with greater impairment in perceived emotion control (ACS scores) across the spectrum of depression (Figure 4a). Stronger right dorsal AI – right VLPFC (SN) functional connectivity was significantly associated with greater impairment in perceived control in unipolar depression (Supplement, Figure S2).
Figure 4.
Partial correlation between clinical dimensions and functional connectivity. (A) Partial correlation between perceived emotion control (ACS) and right dorsal AI – left IPL functional connectivity strength. Higher scores on the ACS reflect greater impairment in emotion control. (B) Partial correlation between behavioral responsivity to reward (BAS-Reward) and right dorsal anterior insula – left inferior parietal lobule (ECN) functional connectivity strength.
Behavioral inhibition and reward.
Weaker right dorsal AI - left IPL (ECN) functional connectivity was significantly associated with greater responsivity to reward across patient groups (Figure 4b). Weaker right dorsal AI functional connectivity with right dACC was significantly associated with greater behavioral inhibition in bipolar patients. Weaker right dorsal AI functional connectivity with right VLPFC (SN) was significantly associated with greater behavioral inhibition/avoidance in bipolar patients, and lesser behavioral inhibition/avoidance in unipolar patients (Figure S3).
Receiver-Operating Characteristics (ROC)
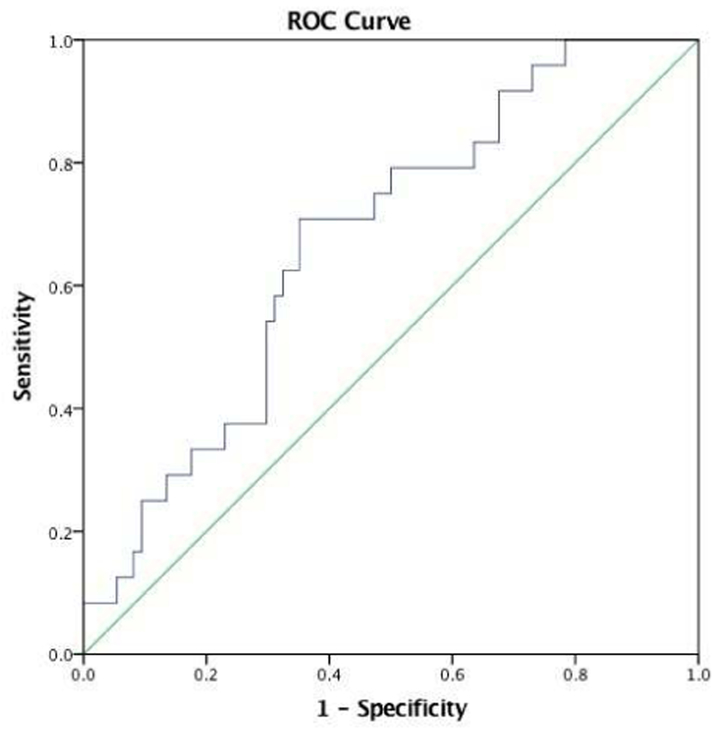
Right dorsal AI – left IPL (ECN) functional connectivity significantly classified bipolar patients relative to unipolar and healthy controls combined (AUC=.67, SE=.059, p=.01, 95% CI: .57, .79) with 71% sensitivity, 65% specificity and a negative predictive value of 87% and positive predictive value of 40% (Figure 6). It did not classify unipolar patients from the other two groups combined (AUC=.50, SE=.06, p=.99, 95% CI: .38, .62). When looking at the two patient groups alone, right dorsal AI-left IPL functional connectivity classified bipolar from unipolar patients with a moderate effect size, approaching statistical significance (AUC=.64, SE=.07, p=.07, 95% CI: .50, .78), with 71% sensitivity, 60% specificity and a negative predictive value of 75% and positive predictive value of 55%. No other anterior insula functional connectivity differences emerged as significant classifiers of diagnosis.
Discussion
Dysregulation lies at the heart of both unipolar and bipolar mood disorders, contributing to the profound impairments caused by these disorders. In the current study, we sought to better understand disrupted regulatory control across mood disorders by investigating inter-network functional connectivity of the AI (an integral region for both interoceptive awareness and regulatory control) and key nodes of the ECN, DMN and SN, using both a syndromal and dimensional approach. At the categorical, syndromal level, bipolar depression was distinguished from both unipolar depression and healthy controls by significantly altered bilateral dorsal AI functional connectivity to the left IPL, a key node of the frontoparietal ECN. At the dimensional level, weaker right AI-left IPL functional connectivity was significantly associated with impaired perceived emotion control and increased behavioral drive towards rewarding experiences across patient groups, independent of diagnosis. Last, functional connectivity between the right anterior insula and left IPL was a significant classifier of bipolar disorder patients. These results suggest altered functional connectivity of the right AI and the left IPL of the ECN may be both a dimensional and syndromal marker of bipolar disease.
Syndromal categorical differences
The left IPL has been identified as an important node in an integrative multi-network system and plays a major role in emotion-cognition integration (58-60). A recent meta-analysis identified functional roles in executive control, reasoning, working memory, and behavioral response inhibition for a left IPL region overlapping with the a priori ROI in our study (58). The left IPL has also been identified as part of a dorsal attention network responsible for voluntarily re-orienting attention and behavior in response to relevant interoceptive stimuli in order to align with ongoing goal-directed needs (61, 62). It is strongly activated in tasks requiring simultaneous processing of interoceptive or affective cues and cognitive demands. For example, Gu et al. (63) report strong left IPL activation when subjects performed a cognitive task in the context of an interoceptive distractor. Similarly, Crichtley et al. (64) found significantly greater engagement of the left IPL when participants simultaneously monitored their own heartbeat (interoception) and judged the asynchronous timing of an audio tone (a cognitive task). In healthy subjects, this left IPL region shares strong functional connections to AI, VLPFC, and dmPFC regions of the SN, and plays an important role in regulating the endogenous stimuli-driven ventral attention network (58, 62). Our data highlight the connectivity of the left IPL with the AI, a primary node for interoceptive information processing. Thus, evidence suggests the left IPL is of particular importance in integrating both bottom-up and top-down processing of interoceptive, affective and cognitive functions in the service of guiding behavior, and communication between the anterior insula and the left IPL facilitates this adaptive regulatory control. Evidence in the current study suggest deviations in the correlated activation of right AI and left IPL to facilitate cognitive-affective integration and regulation may be a key indicator of dysfunction in bipolar depression.
The altered right AI-left IPL functional connectivity found in bipolar patients in this study represents a reverse in correlation polarity rather than a difference in correlation strength as originally hypothesized. The interpretation of negative functional connectivity remains controversial. However, one recent study investigated the relationship between negative functional connectivity values and network functional efficiency, or how efficiently information is exchanged within a network of neural regions (65). Specifically, significant relationships were found between ROI-ROI functional correlations and a network-level measure related to the functional efficiency across the distributed network of neural regions that connects two functional nodes (shortest path length; SPL). Higher SPL values, representing less global efficiency, were related to stronger negative functional correlations between ROIs. Chen et al. (65) suggest this is due to an accumulation of oscillatory phase lags in correlated time series fluctuations along a multi-nodal functional pathway between regions, representing inefficient network organization and resulting in large phase differences manifest as negative cross correlations. Their results held at multiple oscillatory bandwidths and did not change with global signal regression. Although speculative, and in need of further study, this interpretation suggests the altered AI-IPL functional connectivity found in the current study may represent inefficient synchrony across a broader functional network subserving cognitive control in bipolar depression, in which the right AI and left IPL function as key network hubs.
Unipolar depressed individuals also showed weaker AI-left IPL functional connectivity compared to healthy subjects, however this deficit was limited to the left dorsal AI. Previous studies have suggested valence-specific functional laterality for the dorsal AI (66, 67). Specifically, the right AI is implicated primarily in the regulation of negatively valenced or avoidance related stimuli, as is evident by greater afferent projections from the sympathetic nervous system. By contrast, the left AI is implicated in the regulation of positively valenced or approach-related stimuli, with greater parasympathetic nervous system projections. (67) Thus, the left lateralized finding in unipolar depression is intriguing and may be related to deficient regulation of positive or rewarding stimuli. It is interesting that bipolar patients show a deficit in both left and right functional connectivity of the anterior insula to the IPL, while unipolar patients show only left-sided deficits, suggesting a disruption in regulation of positive, rewarding or approach-related interoceptive stimuli in unipolar depression and disruption in regulation of both positive and negative approach- and avoidance-related stimuli in bipolar depression.
Dimensional findings: affective control, behavioral inhibition and approach sensitivity
Weaker right AI-left IPL functional connectivity in the current study was significantly associated with impaired perceived emotion control and increased behavioral drive towards rewarding experiences across patient groups, independent of diagnosis. These findings highlight the potential role of this biological signature as a dimensional marker of shared psychopathology and pathophysiology in mood disorders, although the robustness and utility of this finding will need to be confirmed through replication in a new sample. Altered right AI-left IPL connectivity also separated bipolar disorder from both unipolar depression and healthy controls, and unipolar depression from healthy controls, suggesting a neurobiological continuum that links dimensional deficits in affective control and approach behavior with syndromal categorization in mood disorders. Altered integration of the right AI and the left IPL in the current study is therefore both a dimensional and syndromal marker of disease, and emerges as a potential target for future therapeutic strategies.
Clinical biomarkers
Our ROC analysis found that the functional connectivity between the right AI and left IPL was a significant classifier of bipolar disorder patients (Figure 7). This finding highlights the importance of this biological signature in the pathophysiology of bipolar disorder. Practically, it suggests the potential value of this anatomically constrained functional signal in supporting medical decision-making both in clinical practice or patient selection in clinical trials. The development of biomarkers to classify bipolar disorder from unipolar depression is an unresolved goal of critical importance in psychiatry, as the clinical presentation is often similar and therefore difficult to tease apart based solely on the anamnesis. Appropriate diagnosis cannot only shorten the time to effective treatment selection but it can also prevent iatrogenic switch to mania from antidepressants in bipolar patients. Replication of the current findings in separate larger samples would certainly be necessary to fully evaluate the utility of AI-IPL functional connectivity as a clinically useful biomarker, as is the integration of this with other signals to improve the sensitivity and specificity.
Limitations
There are several limitations in the current study. First, as a sample of convenience, the healthy control subjects included in this study were not sufficiently age matched to patient participants. However, the healthy control cohort represents an age range of optimal functioning free of age-related deficits in affective and cognitive processing, thus representing a “gold standard” of functioning against which to compare dysfunction in mood disorders. Additionally, age was controlled for in all statistical analyses as a covariate of no interest, and was a non-significant factor in our omnibus test. However, results should be replicated in an age-matched sample of healthy individuals to confirm the results found here. Second, although the distribution of gender was not significantly different across samples, this does not reflect the increased prevalence of depression found in women in epidemiological studies, potentially limiting the generalizability of these findings (68, 69). Third, the current sample included a small proportion of bipolar II patients (n=6). Although removing these subjects did not alter the significance of the findings reported here, future studies with balanced samples are needed to examine differential effects of bipolar diagnosis. Fourth, we opted for a more conservative ROI-ROI analysis approach; however, this may mask important differences elsewhere in the brain that are missed by the selection of specific ROIs. Therefore, future studies examining whole-brain functional connectivity across depression are needed. Fifth, all included patients were on a stabilized medication regimen. Although effects of medication were controlled for in all analyses, they cannot be completely ruled out. Replication in unmedicated samples would help to clarify these effects. Sixth, in recognition that taking a reductionist approach to understanding biomarkers of dysfunction of mood disorders by implying one brain region is the root of all dysfunction is not only highly unlikely, but also highly limiting, we caution against overextension of the findings here, and encourage future studies that examine more broadly the role of AI in bipolar and unipolar depression in the context of distributed neural networks.
Finally, although multiple studies have shown dysfunctional recruitment of the VLPFC during emotion regulation in bipolar disorder, and increased recruitment of DMN in unipolar disorder, significant findings from the current study related to VLPFC-AI and DMN-AI functional connectivity did not survive correction for multiple comparisons (see Supplement for further discussion). Future replications in larger sample sizes would help to clarify whether the uncorrected results found here represent a true signal or false positives.
Conclusion
Bipolar depression in the present study was distinguished from unipolar depression and healthy controls by significantly altered dorsal AI functional connectivity to the IPL, a key node in the ECN, and this altered AI-IPL functional connectivity was related to greater impairments in the behavioral dimensions of perceived emotion control and reward sensitivity. This may be tied to the inability to engage regulatory control in response to physiological shifts in autonomic arousal occurring as reduced arousal in depression and increased arousal in mania. Thus, bipolar patients may not adaptively respond to shifts in physiological states due to a disruption in the coordination between interoceptive awareness and regulatory control. Impaired AI-IPL functional connectivity may be a biomarker of this dysfunction in bipolar depression, making the IPL a potential classifier of bipolar versus unipolar depression, and a viable candidate target for focal interventions, e.g. neuromodulation. Replication of these results in a separate, independent sample would allow for the evaluation of the clinical utility of this potential biomarker of bipolar depression.
Supplementary Material
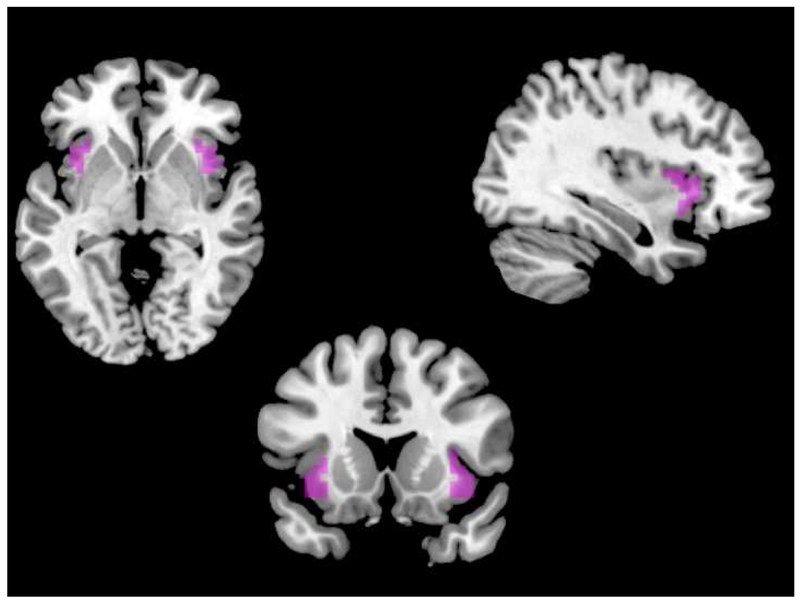
Figure 1.
Anterior insula ROIs. Bilateral masked dorsal anterior insula clusters derived from Kelly et al. (2012) 3-cluster parcellation solution. Masks available for download at http://fcon_1000.projects.nitrc.org.
Figure 5.
ROC analysis. Right dorsal AI – left IPL (ECN) functional connectivity significantly moderately classified bipolar patients relative to unipolar or healthy controls. AUC = .67, SE = .059, p = .01, 95% CI: .57, .79; 71% sensitivity, 65% specificity; 87% negative predictive value.
Acknowledgments
Dr. Ellard gratefully acknowledges the support of a NIH Ruth L. Kirschstein National Research Service Award (F32 MH098490) and the NIH National Institute of Neurological Disorders and Stroke (NIH NINDS) Training Program in Recovery and Restoration of CNS Health and Function award (T32 NS100663). Dr. Camprodon acknowledges support from the Brain and Behavior Foundation and the NIH (RO1 MH112737, R21 DA042271, R21 AG056958, R21 MH113018). Drs. Ellard, Dougherty and Deckersbach acknowledge additional support from contract (W911NF-14-2-0045) from the Defense Advanced Research Projects Agency. Dr. Dougherty further acknowledges support from NIH grants (R01 MH045573-24) and (1R01AT008563-01A1) and Medtronic. Dr. Roffman acknowledges support from the NIH (R01 MH101425).
Footnotes
Financial Disclosures
No commercial funding to any contributing authors directly supported the preparation of this work. Additional funding disclosures: Dr. Nierenberg reports grants and personal fees from Takeda/Lundbeck and AlfaSigma (formerly known as PamLabs). He has received research funding from GlaxoSmithKlein, NeuroRx Pharma, Marriott Foundation, National Institute of Health, Brain & Behavior Research Foundation, Janssen, Intracellular Therapies, and Patient Centered Outcomes Research Institute (PCORI). Dr. Nierenberg also receives personal or consulting fees from Alkermes, PAREXEL, Sunovian, Naurex, Hoffman La Roche/Genentech, Eli Lilly & Company, Pfizer, SLACK Publishing, and Physician's Postgraduate Press, Inc. Dr. Van Dijk is currently employed by Pfizer Inc. Pfizer Inc. had no input or influence on the study design, data collection, data analyses, or interpretation of results. Dr. Dougherty receives device donations and has received consulting income from Medtronic. Dr Dougherty has pending patent applications related to deep brain stimulation for mental illness. Dr. Dougherty further reports consulting income from Insys, speaking fees from Johnson & Johnson, and research support from Cyberonics and Roche. Dr. Deckersbach reports research support from Agency for Healthcare Research and Quality, National Institutes of Health, Defense Advanced Research Projects Agency, Patient Centered Outcomes Research Institute, Depression and Bipolar Alternative Treatment Foundation, International OCD Foundation, Otsuka Pharmaceuticals, Brain & Behavior Research Foundation, Tourette Syndrome Association, National Institute on Aging, Janssen Pharmaceuticals, the Forest Research Institute, Shire Development, Inc., Medtronic, Cyberonics, Northstar, and Takeda. He has received personal/consulting fees from BrainCells, Inc., Clintara, Inc., Systems Research and Applications Corporation, Catalan Agency for Health Technology Assessment and Research, National Association of Social Workers Massachusetts, Massachusetts Medical Society, National Institute on Drug Abuse, and Oxford University Press. He has received both grants and personal fees from Sunovion Pharmaceuticals, Inc., Tufts University, National Institute of Mental Health, and Cogito, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. (2012): Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. (2009): The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 18:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M (2003): Diagnosis and definition of treatment-resistant depression. Biological psychiatry. 53:649–659. [DOI] [PubMed] [Google Scholar]
- 4.Gitlin M (2006): Treatment-resistant bipolar disorder. Mol Psychiatry. 11:227–240. [DOI] [PubMed] [Google Scholar]
- 5.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldao A, Nolen-Hoeksema S, Schweizer S (2010): Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical psychology review. 30:217–237. [DOI] [PubMed] [Google Scholar]
- 8.Sheppes G, Suri G, Gross JJ (2015): Emotion regulation and psychopathology. Annu Rev Clin Psychol. 11:379–405. [DOI] [PubMed] [Google Scholar]
- 9.Mennin DS, Holaway RM, Fresco DM, Moore MT, Heimberg RG (2007): Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behav Ther. 38:284–302. [DOI] [PubMed] [Google Scholar]
- 10.Townsend J, Altshuler LL (2012): Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 14:326–339. [DOI] [PubMed] [Google Scholar]
- 11.Peckham AD, Johnson SL, Tharp JA (2016): Eye Tracking of Attention to Emotion in Bipolar I Disorder: Links to Emotion Regulation and Anxiety Comorbidity. Int J Cogn Ther. 9:295–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLeod C, Mathews A, Tata P (1986): Attentional bias in emotional disorders. J Abnorm Psychol. 95:15–20. [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M (2008): Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 43:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papageorgiou C, Wells A (2003): An empirical test of a clinical metacognitive model of rumination and depression. Cognitive therapy and research. 27:261–273. [Google Scholar]
- 15.Henriques JB, Davidson RJ (2000): Decreased responsiveness to reward in depression. Cognition & Emotion. 14:711–724. [Google Scholar]
- 16.Eshel N, Roiser JP (2010): Reward and punishment processing in depression. Biol Psychiatry. 68:118–124. [DOI] [PubMed] [Google Scholar]
- 17.Whitton AE, Treadway MT, Pizzagalli DA (2015): Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current opinion in psychiatry. 28:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessa M, Linke J (2009): Emotional processing in bipolar disorder: behavioural and neuroimaging findings. International review of psychiatry. 21:357–367. [DOI] [PubMed] [Google Scholar]
- 19.Townsend J, Altshuler LL (2012): Emotion processing and regulation in bipolar disorder: a review. Bipolar disorders. 14:326–339. [DOI] [PubMed] [Google Scholar]
- 20.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. (2008): Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar disorders. 10:310–322. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS (1998): Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 840:33–44. [DOI] [PubMed] [Google Scholar]
- 22.Esch T (2003): Stress, adaptation, and self-organization: balancing processes facilitate health and survival. Forschende Komplementarmedizin und klassische Naturheilkunde= Research in complementary and natural classical medicine. 10:330–341. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS (2007): Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 87:873–904. [DOI] [PubMed] [Google Scholar]
- 24.Barrett LF, Quigley KS, Hamilton P (2016): An active inference theory of allostasis and interoception in depression. Philos Trans R Soc Lond B Biol Sci. 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carver CS (2006): Approach, avoidance, and the self-regulation of affect and action. Motivation and emotion. 30:105–110. [Google Scholar]
- 26.Elliot AJ (2006): The hierarchical model of approach-avoidance motivation. Motivation and emotion. 30:111–116. [Google Scholar]
- 27.Harshaw C (2015): Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull. 141:311–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanes L, Barrett LF (2016): Redefining the Role of Limbic Areas in Cortical Processing. Trends Cogn Sci. 20:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S (2008): Rethinking rumination. Perspectives on psychological science. 3:400–424. [DOI] [PubMed] [Google Scholar]
- 30.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. (2013): Insular dysfunction within the salience network is associated with severity of symptoms and aberrant internetwork connectivity in major depressive disorder. Front Hum Neurosci. 7:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. (2016): Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. 46:1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs RH, Watkins ER, Peters AT, Feldhaus CG, Barba A, Carbray J, et al. (2016): Targeting Ruminative Thinking in Adolescents at Risk for Depressive Relapse: Rumination-Focused Cognitive Behavior Therapy in a Pilot Randomized Controlled Trial with Resting State fMRI. PLoS One. 11:e0163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton JP, Farmer M, Fogelman P, Gotlib IH (2015): Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 78:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belleau EL, Taubitz LE, Larson CL (2015): Imbalance of default mode and regulatory networks during externally focused processing in depression. Soc Cogn Affect Neurosci. 10:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG (2013): Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 37:2529–2553. [DOI] [PubMed] [Google Scholar]
- 37.Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G (2011): Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brady RO Jr., Masters GA, Mathew IT, Margolis A, Cohen BM, Ongur D, et al. (2016): State dependent cortico-amygdala circuit dysfunction in bipolar disorder. J Affect Disord. 201:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bebko G, Bertocci M, Chase H, Dwojak A, Bonar L, Almeida J, et al. (2015): Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res. 231:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. (2013): Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spielberg JM, Beall EB, Hulvershorn LA, Altinay M, Karne H, Anand A (2016): Resting State Brain Network Disturbances Related to Hypomania and Depression in Medication-Free Bipolar Disorder. Neuropsychopharmacology. 41:3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoddard J, Gotts SJ, Brotman MA, Lever S, Hsu D, Zarate C, et al. (2016): Aberrant intrinsic functional connectivity within and between corticostriatal and temporal-parietal networks in adults and youth with bipolar disorder. Psychol Med. 46:1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goya-Maldonado R, Brodmann K, Keil M, Trost S, Dechent P, Gruber O (2016): Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum Brain Mapp. 37:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terasawa Y, Fukushima H, Umeda S (2013): How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Hum Brain Mapp. 34:598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman DJ, Hamilton JP, Gotlib IH (2011): Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig AD (2003): Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 13:500–505. [DOI] [PubMed] [Google Scholar]
- 48.Craig AD (2009): How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- 49.Van Den Heuvel MP, Mandl RC, Kahn RS, Pol H, Hilleke E (2009): Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human brain mapping. 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, et al. (2014): The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 99:180–190. [DOI] [PubMed] [Google Scholar]
- 51.Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, et al. (2014): The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang J, Beck J, Heller K, Egner T (2015): An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nat Commun. 6:8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. (2015): Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams KE, Chambless DL, Ahrens A (1997): Are emotions frightening? An extension of the fear of fear construct. Behaviour research and therapy. 35:239–248. [DOI] [PubMed] [Google Scholar]
- 55.Carver CS, White TL (1994): Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of personality and social psychology. 67:319. [Google Scholar]
- 56.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, et al. (2012): A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 61:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Xie S, Guo X, Becker B, Fox PT, Eickhoff SB, et al. (2017): Correspondent Functional Topography of the Human Left Inferior Parietal Lobule at Rest and Under Task Revealed Using Resting-State fMRI and Coactivation Based Parcellation. Hum Brain Mapp. 38:1659–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cromheeke S, Mueller SC (2014): Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Structure and Function. 219:995–1008. [DOI] [PubMed] [Google Scholar]
- 61.Seghier ML (2013): The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu X, Liu X, Van Dam NT, Hof PR, Fan J (2013): Cognition-emotion integration in the anterior insular cortex. Cereb Cortex. 23:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci. 7:189–195. [DOI] [PubMed] [Google Scholar]
- 65.Chen G, Chen G, Xie C, Li SJ (2011): Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 1:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duerden EG, Arsalidou M, Lee M, Taylor MJ (2013): Lateralization of affective processing in the insula. Neuroimage. 78:159–175. [DOI] [PubMed] [Google Scholar]
- 67.Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, et al. (2017): Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex. 88:124–142. [DOI] [PubMed] [Google Scholar]
- 68.Nivoli AM, Pacchiarotti I, Rosa AR, Popovic D, Murru A, Valenti M, et al. (2011): Gender differences in a cohort study of 604 bipolar patients: the role of predominant polarity. Journal of affective disorders. 133:443–449. [DOI] [PubMed] [Google Scholar]
- 69.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993): Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of affective disorders. 29:85–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.