Abstract
Background
Quantitative assessment of optic nerve damage is important in the evaluation of optic neuritis (ON) and multiple sclerosis (MS).
Objective
To detect optic nerve damage using optical coherence tomography (OCT) and OCT angiography in MS.
Methods
Peripapillary retinal nerve fibre layer (NFL) thickness, macular ganglion cell complex (GCC) thickness and Optic Nerve Head Flow Index (ONH-FI) were measured. The ONH-FI was defined as flow signal averaged over the optic disc. Diagnostic accuracy was evaluated by the area under the receiver-operating characteristics curve (AROC).
Results
Sixty-eight eyes of 45 MS participants and 55 eyes of 32 healthy controls (HCs) were analysed. Of MS eyes, 25 had a history of ON (MS+ON) and 43 didn’t (MS−ON). MS−ON and MS+ON eyes had reductions in ONH-FI (p=0.031 and p=0.001, respectively), GCC thickness (p=0.245 and p<0.001, respectively), and NFL thickness (p=0.003 and p=0.024, respectively), compared with HCs. The highest AROC (0.940) was achieved by the logistic regression combination of all three variables, which was significantly higher than other variables (p=0.018).
Conclusion
MS produces both retinal structural loss and decreased ONH perfusion in MS eyes with and without history of ON. The combination of perfusion and structural measurements enhances detection of optic nerve damage in MS. OCT angiography may be a useful additional retinal marker in evaluation of ON in MS.
INTRODUCTION
Optic neuritis (ON) is the first symptom in a quarter of patients with multiple sclerosis (MS), and optic pathways are affected in up to 80% during the course of their disease.1 Effects of MS on optic nerve structure and function are traditionally measured by colour and contrast visual acuity, visual fields and funduscopic exam. Optical coherence tomography (OCT), an imaging technique developed to evaluate glaucoma and retinal disease, has been applied to study MS.2,3 OCT maps the thickness of the macular ganglion cell complex (GCC) and peripapillary retinal nerve fibre layer (NFL) reflecting the respective loss of retinal ganglion cells and axonal damage due to MS and ON.4,5
OCT angiography is a novel extension of OCT that non-invasively images blood flow down to the capillary level. An Optic Nerve Head Flow Index (ONH-FI) is derived from OCT angiography and measures vessel density and flow velocity in the capillary network and area (calibre) of large retinal vessels.6,7 ONH-FI has advantages over conventional fluorescein angiography for including deep ONH perfusion and laser Doppler for greater area of coverage.8,9 Our centre previously demonstrated reductions in ONH-FI in both glaucoma and MS.10,11 In this study, we explored relationships between ONH-FI, GCC and NFL for the detection of optic nerve damage in MS.
METHODS
study participants
A convenience sample of people with MS and age-matched and sex-matched healthy controls (HCs) which included family members and friends of patients with MS were recruited from the Oregon Health & Science University (OHSU) Multiple Sclerosis Center and by university advertisement. Inclusion criteria for all participants were ages 18–70 years and corrected visual acuity of at least 20/200 in either eye. A neurologist confirmed MS diagnosis (2010 McDonald criteria), with all MS subtypes included.12 Exclusion criteria was intravenous or oralsteroids in the prior 30 days, MS exacerbation in the prior 60 days, evidence that could confound OCT interpretation such as intraocular pressure >21 mm Hg, inability to maintain visual fixation, and refractive errors greater than +3 or −7 diopters in an ophthalmological exam within the last year. Institutional review board approval was obtained, the study carried out in accordance with the tenets of the Declaration of Helsinki, and written informed consent was obtained from each participant.
Experimental design
In this prospective cross-sectional study, consented subjects were examined at a single visit to the Casey Eye Institute at OHSU to obtain clinical data and OCT testing.
Clinical data
Clinical data included MS disease type and duration, and self-reported history of ON including typical symptoms and time course; both MS and ON histories were confirmed by medical record review. The physician-rated European Database for Multiple Sclerosis (EDMUS) based on neurological exam was used to rate MS disability.13 Vascular comorbidities (hypertension, hyperlipidaemia, diabetes, coronary artery disease, peripheral vascular disease, obesity and smoking history) were determined by self-report and medication review for all participants.
High contrast visual acuity was tested with a retro-illuminated ETDRS chart at 2 m. Low contrast visual acuity (LCVA) was tested with a 1.25% retro-illuminated SLOAN chart at 2 m ( precision-vision. com; 944 First Street, La Salle, Illinois, USA). If not done in the prior year, an ophthalmologist performed a dilated ophthalmic exam.
OCT testing
The high speed swept-source OCT prototype operated at an axial scan repetition rate of 100 kHz using a laser (Axsun Technologies, Billerica, Massachusetts. USA) with a 1050 nm central wavelength and a tuning range of 100 nm. Image resolution was 5.3 μm (full-width half-maximum) axially and 18 μm laterally (beam diameter at retinal focal plane). This system has been previously described.14
Structural OCT measurements
Two 8×8 mm images—one centred on the ONH and the other centred on the fovea—were acquired separately. Each image comprised four raster volume scans: two vertical priority and two horizontal priority, 640 lines × 640 axial scans. Each raster volumetric scan was obtained separately and took 4.3 s. An orthogonal registration algorithm removed motion error from the scans to merge them into a single three-dimensional (3D) OCT set for measurements. An automated algorithm was applied to detect NFL and GCC thicknesses from the 3D image. The NFL profile along the standard 3.4 mm diameter circle was then resampled from the NFL map, centred on the disc boundary manually decided by coauthor LL from the en face image. NFL thickness was averaged over the circumferential NFL profile. GCC thickness was averaged over a 6×6 mm square region centred on the fovea.
ONH-FI measurements
Quantification of ONH blood flow using ONH-FI has been described previously.10,11,15 Briefly, a 3×3×3 mm3 3D volumetric scan centred on the ONH is captured. Split-spectrum amplitude-decorrelation angiography14 is then used to compute flow signal (decorrelation value) on the 3D OCT data set. Next, an en face angiogram is formed by maximum flow projection of the whole depth. The optic disc boundary is manually delineated on the en face structural OCT images (grey-scale reflectance amplitude) generated from the same OCT angiography scan (figure 1, yellow circle). Finally, the ONH-FI is obtained by averaging the flow signal within the optic disc on the en face angiogram. Flow Index mainly measured the area (or calibre) of large vessels and both the area (or vessel density) and velocity of capillaries.6 The ONH-FI includes measurements on both the ONH microcirculation and the large retinal blood vessels within the disc.
Figure 1.
Colour fundus photos and en face optical coherence tomography (OCT) angiograms of the optic nerve head (ONH) in healthy control, multiple sclerosis without optic neuritis (MS−ON), and multiple sclerosis with optic neuritis groups (MS+ON). The ONH of MS+ON eye appears pale in the temporal half. In the ONH angiograms, the Flow Index was averaged over the whole ONH (yellow circles). In comparison to the healthy control and MS−ON eyes, the ONH microvascular network in MS+ON eye showed discernible attenuation, particularly in its temporal half, corresponding to the colour fundus photo.
Statistical analysis
All data analyses were performed using the SPSS software V.20.0 (SPSS, Chicago, Illinois, USA) and MedCalc V.10.1.3.0 (MedCalc Software, Ostend, Belgium, www.medcalc.be). Eyes were categorised as with history of ON (MS+ON), without history of ON (MS−ON) and HC eyes. The t test and χ2 test were used to analyse differences between each cohort. Spearman’s correlation was used for assessing associations between ONH-FI, structural and visual outcomes, and vascular risk factors. To adjust for age and the intereye correlation from the same participant, the generalised estimating equations (GEE) method was used throughout the analysis whenever applicable.16 The area under the receiver-operating-characteristic-curve (AROC) was used to calculate the diagnostic power of the diagnostic parameters. Logistic regression was employed to combine diagnostic parameters into composite diagnostic indices. The method of Delong et al was used to compare AROC of each parameter.17
RESULTS
study population
A total of 49 MS (82 eyes) and 31 HC participants (62 eyes) were enrolled. Twelve MS and seven HC eyes were excluded due to poor scan alignment, low signal strength or failed image registration for the GCC structure scan, NFL circular scan or ONH angiography scan. One patient with MS was excluded due to laterality of ON was not known and could not be determined by medical record review. Demographics of the analysed 68 MS and 55 HC eyes are in table 1. The HC and MS cohorts were overall matched for age, sex and presence of vascular risk factors. MS+ON participants were younger than MS−ON participants (t test, p=0.015) and less likely to have vascular risk factors (χ2 test, p=0.043), but otherwise matched. LCVA results were missing for seven MS+ON and six MS−ON eyes.
Table 1.
Participant demographics and clinical summary
| Cohorts (participants/eyes) |
||||
|---|---|---|---|---|
| All Ms (45/68) | MS+ON (20/25) | MS−ON (25/43) | HC (32/55) | |
| Age (years) | 45±11 | 41±10 | 49±12 | 41±12 |
| Female percentage | 69% | 75% | 64% | 84% |
| ≥1 Vascular risk factors percentage, (n) | 42% (19) | 25% (5) | 58% (14) | 44% (14) |
| MS type, percentage (n) | RR: 80%(36) | RR: 80% (16) | RR: 80% (20) | n/a |
| SP: 11% | P: 15% (3) | SP: 8% (2) | ||
| PP: 9% (4) | PP: 5% (1) | PP: 12% (3) | ||
| MS duration (years) | 14±10 | 15±10 | 13±9 | n/a |
| EDMUS Scale* | 3.0±2.2 | 3.0±2.2 | 3.1±2.3 | n/a |
| IOP (mm Hg) | 14±2.9 | 14±2.5 | 14±3.3 | 15±2.6 |
| High-contrast LogMAR BCVA |
0.04±0.13 | 0.03±0.16 | 0.04±0.11 | −0.05±0.10 |
| Low-contrast LogMAR BCVA |
0.67±0.17 | 0.71±0.22 | 0.63±0.14 | 0.61±0.21 |
EDMUS Scores 0–3 indicate absent to mild disease, 4–6.5 moderate disease with walking limitations and 7–10 severe disease up to death due to MS.
BCVA, best corrected visual acuity; EDMUS, European Database for Multiple Sclerosis; HC, healthy control; IOP, intraocular pressure; MS, multiple sclerosis; ON, optic neuritis, PP, primary progressive; RR, relapsing remitting; SP, secondary progressive.
Comparison of OCT diagnostic parameters
Figure 1 shows typical examples of OCT angiograms with discernible attenuation of ONH circulation in an MS−ON group and more so in the MS+ON group compared with HC. Using the GEE model to adjust for age and within-subject intereye correlations, both MS+ON and MS−ON groups demonstrated significant reductions in the OCT angiography parameter ONH-FI and OCT structural parameter NFL thickness compared with the HC group (figure 2). The reduction of GCC thickness in the MS−ON group was not significantly less than in the HC group. The greatest reductions of ONH-FI, NFL and GCC thicknesses were in the MS+ON group.
Figure 2.
Box plots of the optical coherence tomography diagnostic parameters to compare multiple sclerosis cohorts without (MS−ON) and with (MS+ON) history of optic neuritis against a healthy control (HC) cohort. P values were calculated by generalised estimating equation models accounting for age and within-subject intereye correlations. Shown are (2A) Optic Nerve Head (ONH) Flow Index, (2B) ganglion cell complex (GCC) thickness, and (2C) nerve fibre layer (NFL) thickness.
Correlations between OCT diagnostic parameters
There were no statistically significant correlations between ONH-FI and the structural OCT measures for any participant cohort. GCC and NFL thicknesses significantly correlated with each other in the HC cohort (Spearman’s ρ =0.477, p<0.001) and more so in the MS+ON (ρ =0.788, p<0.001) and MS−ON cohorts (ρ =0.719, p<0.001).
Correlations between OCT diagnostic parameters and clinical data
No statistically significant correlations were found between ONH-FI and demographic and clinical data within the all MS cohort, even when stratified for ON history or using the GEE model. Neurological (EDMUS) and visual (LCVA) disability scores both correlated with the GCC thickness (ρ =−0.394, p=0.001 for EDMUS; ρ =−0.329, p=0.013 for LCVA, respectively) and NFL thickness (ρ =−0.374, p=0.001 for EDMUS; ρ =−0.345, p=0.009 for LCVA, respectively) but not ONH-FI.
Diagnostic accuracy of OCT parameters
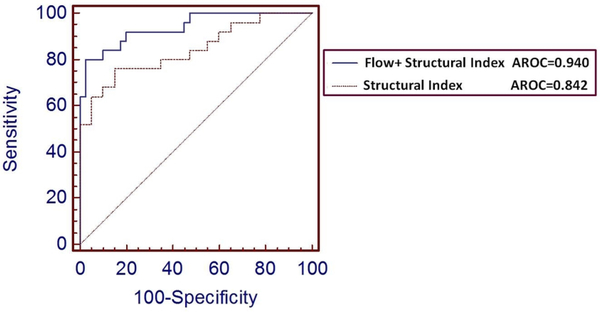
There were no significant differences between the diagnostic accuracies of the ONH-FI, GCC and NFL parameters applied individually in identifying MS+ON eyes based on a comparison of AROC (table 2). A calculated Structural Index combining the structural OCT parameters (GCC and NFL thicknesses) did not significantly improve the diagnostic accuracy over any single parameter. However, the Flow+Structural Index, a combination of ONH-FI with Structural Index demonstrated a significantly better AROC than the Structural Index (p=0.018, figure 3 and table 2).The Flow+Structural Index detected optic nerve damage in 84.0% of MS+ON eyes and 37.2% of MS−ON eyes, both at 90% specificity (table 2).
Table 2.
Diagnostic accuracy of OCT parameters
| Diagnostic parameter | ONH-FI | GCC | NFL | Structural Index (GCC+NFL) | Flow+Structural Index (ONH-FI+GCC+ NFL) |
|---|---|---|---|---|---|
| AROC (MS+ON) | 0.862 0.754~0.935 | 0.834 0.722~0.915 | 0.795 0.677~0.885 | 0.842 0.730~0.921 | 0.940 0.852~0.983 |
| Sensitivity (MS+ON) | 68.0% 46.5%~85.0% | 68.0% 46.5%~85.0% | 60.0% 38.7%~78.8% | 68.0% 46.5%~85.0% | 84% 63.9%~95.4% |
| AROC (MS−ON) | 0.619 0.515~0.715 | 0.637 0.534~0.732 | 0.717 0.617~0.803 | 0.690 0.589~0.780 | 0.727 0.628~0.812 |
| Sensitivity (MS−ON) | 27.9% 15.3%~43.7% | 20.9% 10.1~36.0% | 34.9% 21.0%~50.9% | 30.2% 17.2%~46.1% | 37.2% 23.0%~53.3% |
Sensitivities at 90% specificity and the cut-off point for distinguishing MS+ON and HC were evaluated. 95% CI was shown below. The Flow+Structural Index of the three OCT diagnostic measures to differentiate MS+ON and HC eyes was derived from logistic regression using the following formula: Flow+Structural Index=1/(1+exp(−(27.702–0.107*GCC-81.536*ONH-FI-0.05*NFL))). The Structural Index=1/(1+exp(−(15.037–0.110*GCC−0.046*NFL))).
AROC, area under the receiver-operating characteristics curve; GCC, ganglion cell complex; HC. healthy control; MS, multiple sclerosis; NFL, nerve fibre layer; OCT, optical coherence tomography; ON, optic neuritis; ONH-FI, Optic Nerve Head Flow Index.
Figure 3.
The Flow+Structural Index, a combination of the OCT angiography parameter, Optic Nerve Head Flow Index, with the structural OCT parameters of nerve fibre layer and ganglion cell complex (Structural Index) demonstrated significantly better area under the receiver-operating characteristics curve (AROC) than the Structural Index (p=0.018).
DISCUSSION
This study demonstrates reduced ONH perfusion by OCT angiography in patients with MS, particularly those with a history of ON. The specificity and sensitivity of ONH-FI in detecting optic nerve abnormalities in the patients with MS+ON were comparable to those of the structural OCT parameters, GCC and NFL, found in our cohort and in the literature.4,18 In addition to analysing OCT angiographic parameters as in our previous paper,11 we combined both OCT angiographic and structural parameters. Importantly, the combination of the OCT angiography and structural parameters into a Flow+Structural Index significantly improved the diagnostic accuracy of detecting MS eyes affected by ON.
In our previous study, the ONH-FI of the MS+ON group was reduced by 12% compared with HC eyes (p=0.004), but there’s no reduction of ONH-FI in the MS−ON group (p=0.924).11 With larger samples in this study, we found the ONH-FI in the MS−ON group was 5.5% lower than HC eyes (p=0.031). The reduction of the ONH-FI (14.7%) in the MS+ON group was in agreement with the previous result.
Interestingly, while ONH-FI reduction is greatest in MS+ON eyes, similar to structural measures, there is low correlation between the angiography and structural measures. The result is consistent with a previous study in glaucoma in which the ONH-FI in glaucomatous eyes didn’t correlate with NFL thickness (Pearson’s R2=0.004, p=0.853).10 The low correlation in the present study could be due to several factors, an important one being population variation in baseline values. In the HC cohort, the NFL coefficient of variation was 8% for ONH-FI and GCC, and 9%, consistent with previously reported ranges.2,11,19 Just as it is easier to detect abnormally thin GCC or NFL in those born with fewer optic nerve fibres, and harder to detect thinning in those with more fibres, it is likely to be similarly easier to detect reduced ONH-FI values in those starting with lower vessel density. Measurement variability could also play a role, although likely a small one as the repeatability of ONF-FI, GCC and NFL is excellent. The coefficients of variation for intravisit repeatability and intervisit reproducibility of ONH-FI have been shown to be 1.03% and 4.53%, respectively,11 and the NFL coefficients of variation of repeatability were 1.1% for GCC and 1.3%.20 Another reason for lack of correlation relates to the different natures of the angiography and structural OCT parameters captured. As expected, GCC and NFL were highly correlated as they both reflect the number of nerve fibres prone to the same injury in MS. However as it relates to vascular or other underlying pathophysiological mechanisms in MS, ONH-FI could be expected to behave independently from the structural parameters. Finally, the effects of timing of OCT acquisition on the outcomes themselves may be a factor. In early damage, dysfunctional nerve fibres may not yet demonstrate thickness loss, but could already demonstrate reduced blood flow due to metabolic abnormalities. In later stages, death of ganglion cells may trigger glial replacement (scarring) that potentially limits GCC and NFL thinning. Evolution of the ONH-FI in acute or chronic ON is unknown. Therefore, although patients with acute ON were excluded from this study, subacute structural and angiographic changes related to the timing of injury may be at play.
ONH-FI did not correlate significantly with disease severity measures although in this study, as with others, both structural OCT parameters significantly correlated with neurological (EDMUS Scale) and visual (LCVA) disabilities.19,21,22 The clinical significance of ONH-FI is unclear. The cross-sectional study design, relatively small sample size and limited number of clinical evaluations conducted may have influenced the lack of correlation and can be optimised for future studies.
Presence of vascular risk factors did not correlate with ONH-FI which supports an interpretation that reduction of optic nerve perfusion in MS is primarily a sequela of MS pathophysiological mechanisms and not systemic vascular disease. Accelerated atherogenesis has been suspected in MS due to shared pathophysiological mechanisms of chronic inflammatory responses and oxidative stress. While larger epidemiological studies23,24 indicate greater than normal numbers of vascular (cardiac, cerebrovascular, peripheral vascular) comorbidities, in MS populations than the general population, small cross-sectional studies have not been able to directly demonstrate dysfunction using peripheral arterial tonometry or Doppler ultrasound.25,26 Our data do not support the presence of a primary vasculopathy in MS.
Improved diagnostic accuracy with Flow+Structural Index has scientific and clinical implications. First, it appears that ONH-FI measures an effect of MS and ON independent of structural damage which may uncover additional pathophysiological mechanisms in MS and potential therapeutic targets. Second, the significantly improved diagnostic accuracy may serve to reduce sample size calculations for clinical trials. Larger and longitudinal studies will determine the utility of the Flow+Structural Index to detect and quantify optic nerve damage in MS, monitor disease progression and study the effects of MS therapeutics as well as disease progression.
Study limitations include use of participant self-reports and medication review for vascular risk factors. MS subjects didn’t undergo visual field testing. While ONH-FI demonstrated good correlation with visual fields in a glaucoma study,10 the correlation needs confirmation in MS. A sizeable minority of eyes (15%) was not analysed due to quality issues from both angiography and structural OCT scans. Our prototype device does not have automated focus. Motion artefact was another reason for quality issues in some angiography scans. OCT angiography is a new technology that is undergoing rapid development to improve both device and software. A more refined commercial device with more mature software that can remove motion error may improve the yield of image acquisition and reliability of image processing in the future.
In conclusion, reductions in ONH-FI were detected in MS eyes using OCT angiography, particularly in eyes with a history of ON. Combining ONH-FI with structural OCT parameters increased the diagnostic accuracy such that optic nerve damage was detected in almost all MS+ON eyes. Further studies are needed to determine the value of OCT angiographic measurement in the prognostic evaluation and monitoring of MS and ON.
Acknowledgements
The authors thank Melissa Tee, Janice Ladwig and Denzil Romfh for their work as clinical coordinators in this study.
Funding This study was supported by the following NIH grants: UL1TR000128, UL1 RR024140, R01-EY013516, R01-EY023285, DP3 DK104397 and P30 EY010572 (Bethesda, MD). Additional support includes a grant from the Medical Research Foundation of Oregon (GNEUR0728A) and a Career Development Award from the Department of Veterans Affairs (B7493-W, Rebecca Spain). Supported by unrestricted departmental funding from Research to Prevent Blindness (New York, USA). Oregon Health & Science University (OHSU) foundation, NSFC (Grant No. 61471226) and the Champalimaud Foundation (Lisbon, Portugal).
Footnotes
Competing interests RIS, LL, XZ and DB have nothing to disclose. Oregon Health & Science University (OHSU) and DH, YJ and OT have a significant financial interest in Optovue, a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Ethics approval OHSU IRB.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014;13:83–99. [DOI] [PubMed] [Google Scholar]
- 2.Pulicken M, Gordon-Lipkin E, Balcer LJ, et al. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007;69:2085–92. [DOI] [PubMed] [Google Scholar]
- 3.Spain RI, Maltenfort M, Sergott RC, et al. Thickness of retinal nerve fiber layer correlates with disease duration in parallel with corticospinal tract dysfunction in untreated multiple sclerosis. J Rehabil Res Dev 2009;46:633–42. [DOI] [PubMed] [Google Scholar]
- 4.Costello F, Hodge W, Pan YI, et al. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 2008;14:893–905. [DOI] [PubMed] [Google Scholar]
- 5.Sriram P, Wang C, Yiannikas C, et al. Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS One 2014;9:e102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokayer J, Jia Y, Dhalla AH, et al. Blood flow velocity quantification using splitspectrum amplitude-decorrelation angiography with optical coherence tomography. Biomed Opt Express 2013;4:1909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 2015;133:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayreh SS. Colour and fluorescence of the optic disc. Ophthalmologica 1972;165:100–8. [DOI] [PubMed] [Google Scholar]
- 9.Petrig BL, Riva CE, Hayreh SS. Laser Doppler flowmetry and optic nerve head blood flow. Am J Ophthalmol 1999;127:413–25. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121:1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Jia Y, Spain R, et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 2014;98:1368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Confavreux C, Compston DA, Hommes OR, et al. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 1992;55:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20:4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3:3127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 18.Balcer LJ, Miller DH, Reingold SC, et al. Vision and vision-related outcome measures in multiple sclerosis. Brain 2015;138:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012;119:1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le PV, Zhang X, Francis BA, et al. Advanced imaging for glaucoma study: design, baseline characteristics, and inter-site comparison. Am J Ophthalmol 2015;159:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abalo-Lojo JM, Limeres CC, Gómez MA, et al. Retinal nerve fiber layer thickness, brain atrophy, and disability in multiple sclerosis patients. J Neuroophthalmol 2014;34:23–8. [DOI] [PubMed] [Google Scholar]
- 22.Baier ML, Cutter GR, Rudick RA, et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology 2005;64:992–5. [DOI] [PubMed] [Google Scholar]
- 23.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler 2015;21:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;74:1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keményová P, Siarnik P, Sutovský S, et al. Impairment of endothelial function in patients with multiple sclerosis. Neuro Endocrinol Lett 2015;36:67–71. [PubMed] [Google Scholar]
- 26.Fjeldstad AS, McDaniel J, Witman MA, et al. Vascular function and multiple sclerosis. J Neurol 2011;258:2036–42. [DOI] [PubMed] [Google Scholar]