Abstract
Flagellar calcium binding proteins are expressed in a variety of trypanosomes and are potential drug targets for Chagas disease and African sleeping sickness. We report complete NMR chemical shift assignments of the flagellar calcium binding protein calflagin Tb24 of T. brucei. (BMRB no. 18011).
Keywords: FCaBP, T. brucei, Calcium, EF-hand, myristoyl switch, NMR
Biological Context
Flagellar calcium-binding proteins (FCaBPs) are immunogenic proteins found in the flagellum of protozoan parasites: Trypanosoma cruzi (Engman et al., 1989), Trypanosoma brucei (Haghighat and Ruben, 1992), and Trypanosoma rangeli (Porcel et al., 1996) (see Fig. 1). FCaBPs contain four EF-hand calcium-binding motifs (Ikura, 1996; Moncrief, 1990) (Fig. 1), the third and fourth (EF-3 and EF-4) of which bind calcium (Maldonado et al., 1999). The protein is modified at the N terminus by covalent attachment of myristate at Gly2 and palmitate at Cys4, both of which along with conserved lysine residues near the N-terminus (Maric et al., 2011) are required for association with the inner leaflet of the flagellar membrane (Godsel and Engman, 1999). Calcium is required for stable flagellar localization as well, since T. cruzi FCaBP can be washed out of detergent-permeabilized trypanosomes if calcium chelators are included in the wash solutions. The N-terminal acylation and calcium-dependent membrane localization of T. cruzi FCaBP suggested that the protein may possess a functional calcium-acyl switch, similar to the Ca2+-myristoyl switch observed previously for recoverin (Dizhoor et al., 1993; Zozulya and Stryer, 1992) and other members of the neuronal calcium sensor (NCS) family (Burgoyne, 2004).
Figure 1.
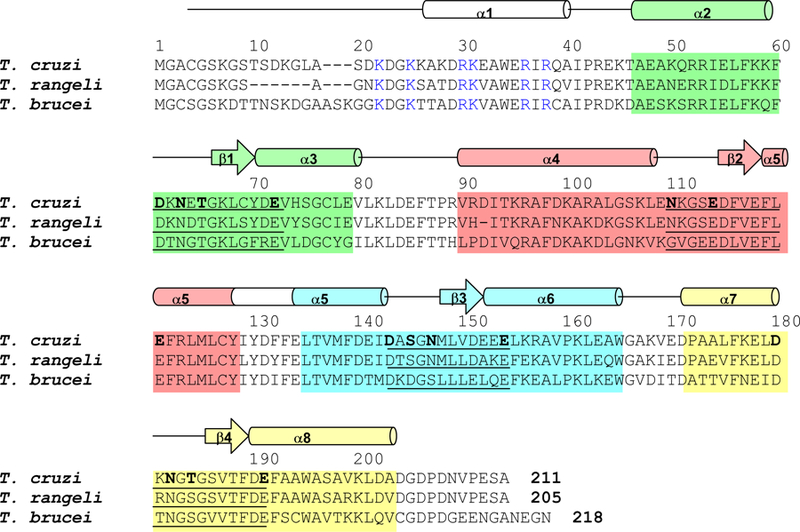
Alignment of the primary sequence of T. brucei calflagin Tb24 with those of T. rangeli FCaBP and T. cruzi FCaBP. Secondary structural elements indicated schematically were derived from analysis of NMR data (3Jhnhα, chemical shift index (Wishart et al., 1992) and sequential NOE patterns). The four EF-hands (EF1, EF2, EF3 and EF4) are highlighted green, salmon, cyan, and yellow, respectively. Residues in the 12-residue Ca2+-binding loops are underlined and chelating residues are highlighted bold. Invariant basic residues implicated in membrane binding are colored blue.
Three FCaBP homologs in T. brucei have been characterized and named the calflagins: Tb24, Tb17 and Tb44 (Wu et al., 1994). The calflagins are similar in sequence to T. cruzi FCaBP (Fig. 1) and exhibit Ca2+-induced localization to the flagellar membrane during cell motility, chemotaxis, and cell signaling. T. brucei calflagin Tb24 possesses different membrane targeting properties compared to that of T. cruzi FCaBP. Tb24 requires both myristoylation and palmitoylation for binding to flagellar membranes (Emmer et al., 2010; Pinto et al., 2003), whereas the unpalmitoylated Tb24 is trafficked to the pellicular (cell body) membrane (Emmer et al., 2009). This is in stark contrast to T. cruzi FCaBP, whose unpalmitoylated form is mislocalized in the cytoplasm (Godsel and Engman, 1999). The different membrane targeting properties of modified forms of these similar proteins is perhaps due to some protein structural difference between the two, or due to different lipid composition of various trypanosome membrane domains (Tyler et al., 2009). T. brucei Tb24 and T. cruzi FCaBP may also differ by interacting with distinct membrane-bound protein targets (Buchanan et al., 2005; Wingard et al., 2008).
Atomic resolution structures of T. brucei FCaBPs in both the Ca2+-free and Ca2+-bound states are needed to elucidate their Ca2+-induced conformational changes that control membrane-targeting, and to understand their structural differences with T. cruzi FCaBP. We report here detailed NMR resonance assignments for Ca2+-free calflagin Tb24 (henceforth referred to simply as Tb24) as a first step toward this end.
Methods and Experiments
Expression and Purification of Tb24.
Recombinant and uniformly 15N- or 15N/13C-labeled Tb24 was expressed in E. coli strain, BL21(DE3) grown on M9 medium supplemented with 15N-NH4Cl and/or 13C6-glucose. Recombinant protein expression was induced by exogenously adding 0.5 mM isopropyl β-D-l-thiogalactopyranoside (IPTG) to cells grown overnight at 25 °C. Typically, a 1-L culture yields about 20 mg of purified protein. Detailed procedures for purifying Tb24 are described elsewhere (Emmer et al., 2010).
NMR spectroscopy.
Samples of recombinant Ca2+-free Tb24 (0.7 mM) were prepared in 90%/10% H2O/D2O or 100% D2O with 10 mM sodium phosphate (pH 7.0), 4 mM DTT-d11 and 0.3 mM EDTA-d12. NMR experiments were conducted using Bruker Advance 600 MHz spectrometer equipped with a triple resonance cryogenic probe. All experiments were performed at 298 K. Backbone and side-chain chemical shift assignments were obtained using 15N-HSQC, HNCO, HNCACB, CBCACONH, HBHACONH and 15N-HSQC-TOCSY (mixing time of 60 ms) spectra (Ikura et al., 1990). Methyl group side-chain resonances were assigned using 13C-CT-HSQC and 13C-HCCH-TOCSY. For aromatic side-chain chemical shift assignments, HBCBCGCDHD, HBCBCGCDCEHE, 13C-CT-HSQC-TOCSY spectra (Yamazaki et al., 1993) along with 13C-HSQC-NOESY, recorded with a mixing time of 120 ms, were used. NMR data were processed using NMRPipe (Delaglio et al., 1995) software package and analyzed using SPARKY.
Assignments and Data Deposition
Figure 2 presents HSQC spectra of Ca2+-free Tb24 at pH 7.0 to illustrate representative backbone resonance assignments. NMR assignments were based on 3D heteronuclear NMR experiments performed on 13C/15N-labeled Tb24 (residues 2–218). The protein sample in this study consists of 217 native residues with an unmyristoylated N-terminus (Gly 2) and lacking palmitoylation at Cys 3. All non-proline residues exhibited strong backbone amide resonances with uniform intensities, indicative of a well-defined three-dimensional protein structure. More than 95% of the backbone resonances (1HN, 15N, 13Cα, 13Cβ, and 13CO) and ~82% of aliphatic side chain resonances were assigned. The chemical shift assignments (1H, 15N, 13C) of Ca2+-free Tb24 have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under accession number 18011.
Figure 2.
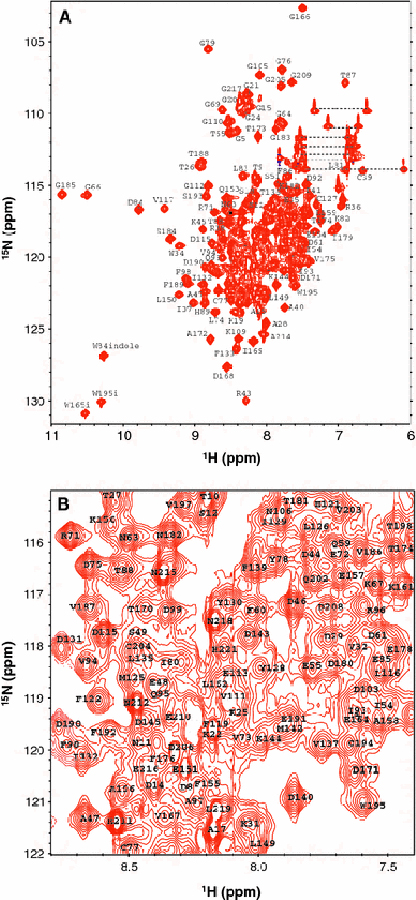
Two-dimensional 15N-HSQC NMR spectra of Ca2+-free Tb24 recorded at 600 MHz proton frequency (A) and expanded view of spectrally crowded central region (B). Side chain amide resonances of Asn and Gln are connected with dotted lines. Representative resonance assignments are indicated and the complete list of assignments can be found at the BMRB repository (accession no. 18011).
The chemical shift index of each amino acid residue reveals a protein secondary structure in Tb24 similar to that observed previously for T. cruzi FCaBP (Wingard et al., 2008) (Fig. 1). Tb24 contains 8 α-helices and two antiparallel β-sheets (αl: 26–38; α2: 47–60; β1: 67–69; α3: 70–79; α4: 90–105; β2: 116–118; α5: 119–139; β3: 149–151; α6: 152–164; α7: 172–182; β4: 186–188; α8: 189–204). The NMR assignments reported here for Ca2+-free Tb24 are compared to those reported previously for T. cruzi FCaBP (Wingard et al., 2008) (Fig. 3). Similar chemical shifts are seen for conserved residues in the N-terminal region that might interact with membrane targets (highlighted blue in Fig. 1), suggesting that Tb24 may use dual acyl groups for membrane anchoring similar to that described previously for T. cruzi FCaBP (Godsel and Engman, 1999). The largest chemical shift differences are observed for residues in the second EF-hand (highlighted red in Fig. 3), suggesting that these residues may play a structural role in explaining the different membrane-targeting properties of Tb24 versus T. cruzi FCaBP (Emmer et al., 2010; Emmer et al., 2009; Godsel and Engman, 1999).
Figure 3.
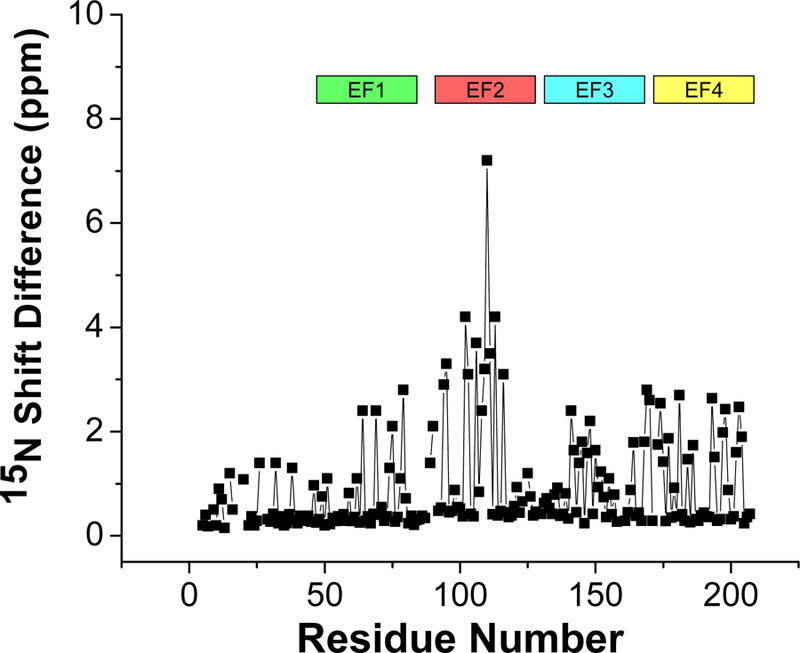
Backbone amide 15N chemical shift difference (between Tb24 and T. cruzi FCaBP) vs. residue number. EF-hand residues are highlighted using the same color scheme in Fig. 1.
Acknowledgements
We thank Jerry Dallas for technical support and help with NMR experiments. Work supported by NIH grants (EY012347) to J.B.A and (RR11973) to the UC Davis NMR facility.
References
- Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araujo AP and Engman DM (2005) A flagellum-specific calcium sensor. J Biol Chem, 280, 40104–40111. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD (2004) The neuronal calcium-sensor proteins. Biochim. Biophys. Acta, 1742, 59–68. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, J, P. and Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR, 6, 277–293. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Chen CK, Olshevskaya E, Sinelnikova VV, Phillipov P and Hurley JB (1993) Role of the acylated amino terminus of recoverin in Ca(2+)-dependent membrane interaction. Science, 259, 829–832. [DOI] [PubMed] [Google Scholar]
- Emmer BT, Daniels MD, Taylor JM, Epting CL and Engman DM (2010) Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot. Cell, 9, 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL and Engman DM (2009) Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J. Cell Sci, 122, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman DM, Krause KH, Blumin JH, Kim KS, Kirchhoff LV and Donelson JE (1989) A novel flagellar Ca2+-binding protein in trypanosomes. J. Biol. Chem, 264, 18627–18631. [PubMed] [Google Scholar]
- Godsel LM and Engman DM (1999) Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J, 18, 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat NG and Ruben L (1992) Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol. Biochem. Parasitol, 51, 99–110. [DOI] [PubMed] [Google Scholar]
- Ikura M (1996) Calcium binding and conformational response in EF-hand proteins. Trends Biochem. Sci, 21, 14–17. [PubMed] [Google Scholar]
- Ikura M, Kay LE and Bax A (1990) A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonanc three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry, 29, 4659–4667. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, Mirzoeva S, Godsel LM, Lukas TJ, Goldenberg S, Watterson DM and Engman DM (1999) Identification of calcium binding sites in the trypanosome flagellar calcium-acyl switch protein. Mol. Biochem. Parasitol, 101, 61–70. [DOI] [PubMed] [Google Scholar]
- Maric D, McGwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL and Engman DM (2011) Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J. Biol. Chem, 286, 33109–33117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrief ND, Kretsinger RH, and Goodman M (1990) Evolution of EF-hand calcium-modulated proteins. J. Mol. Evol, 30, 522–562. [DOI] [PubMed] [Google Scholar]
- Pinto AP, Campana PT, Beltramini LM, Silber AM and Araujo AP (2003) Structural characterization of a recombinant flagellar calcium-binding protein from Trypanosoma cruzi. Biochim. Biophys. Acta, 1652, 107–114. [DOI] [PubMed] [Google Scholar]
- Porcel BM, Bontempi EJ, Henriksson J, Rydaker M, Aslund L, Segura EL, Pettersson U and Ruiz AM (1996) Trypanosoma rangeli and Trypanosoma cruzi: molecular characterization of genes encoding putative calcium-binding proteins, highly conserved in trypanosomatids. Exp. Parasitol, 84, 387–399. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL and Engman DM (2009) Flagellar membrane localization via association with lipid rafts. J. Cell. Sci, 122, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard JN, Ladner J, Vanarotti M, Fisher AJ, Robinson H, Buchanan KT, Engman DM and Ames JB (2008) Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J. Biol. Chem, 283, 23388–23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD and Richards FM (1992) The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry, 31, 1647–1651. [DOI] [PubMed] [Google Scholar]
- Wu Y, Deford J, Benjamin R, Lee MG and Ruben L (1994) The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem. J, 304, 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Forman-Kay JD and Kay LE (1993) Two-dimensional NMR experiments for correlating 13C and 1H / chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. J. Am. Chem. Soc, 115, 11054–11055. [Google Scholar]
- Zozulya S and Stryer L (1992) Calcium-myristoyl protein switch. Proc. Natl. Acad. Sci. USA, 89, 11569–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]


