Abstract
Background:
Intraductal oncocytic papillary neoplasm(IOPN) of the pancreas is a rare tumor. Recent molecular data indicate that it is distinct from other intraductal neoplasms; however, its clinicopathologic characteristics, especially the frequency/significance of an invasive carcinoma component, and biologic behavior remain to be fully defined.
Design:
Clinicopathologic characteristics and survival of 24 IOPNs were analyzed. By definition, all tumors exhibited intraductal growth and oncocytic morphology.
Results:
The female:male ratio was 1.7, mean age was 59. In 44% of the patients, the IOPN was discovered incidentally; however, the working diagnosis was “ductal adenocarcinoma” in 42%. Fourteen IOPNs occurred in the head of the pancreas. The median tumor size was 4.5 cm. The tumors often grew along adjacent benign ducts, mimicking invasion, but only 29% exhibited unequivocal invasive carcinoma, mostly in the form of microscopic foci(pT1a=4, pT1b=1, pT2=2) and only 6% had lymph node metastasis. Invasive carcinoma was predominantly composed of small tubular units lined by oncocytic cells, or individual oncocytic cells infiltrating the periductal stroma. Follow-up information was available for 18 patients(median=6.8 years). No patients died from the disease and the overall 10-year survival was 94%. Patients with invasive carcinoma trended towards a lower 5-year recurrence-free survival than those with non-invasive IOPNs(66% vs. 93%, p=0.066), but overall survival was not impacted by the presence of invasion(p=0.38).
Conclusion:
IOPN is a distinct tumor type in the pancreas. Despite its morphologic complexity and often extensive pagetoid spread to adjacent ducts, conventional invasive carcinoma is seen in only 29% and usually as microscopic foci. Thus, it is not surprising that IOPN exhibits indolent behavior even when invasion is present.
Keywords: Pancreas, intraductal neoplasm, oncocytic
INTRODUCTION
Intraductal oncocytic papillary neoplasm (IOPN) of the pancreas was first described as a distinct entity in a report of 11 cases in 1996.1 In 2010, the World Health Organization (WHO) classified IOPN as a subtype of intraductal papillary mucinous neoplasm (IPMN), since both IOPN and IPMN present similarly as a cystic pancreatic lesion due to dilation of the native ducts by the intraductal neoplasm.2
However, more recent studies have illustrated differences between these entities, warranting revisions in the way these neoplasms are viewed and classified. For example, mucin production is minimal in IOPNs and they typically form complex masses with both cystic and solid components, frequently leading to a radiological diagnosis of pancreatic ductal adenocarcinoma. Histologically, they are characterized by complex arborizing papillae lined by mitochondria-rich oncocytic cells1, 3–10 and they have distinguishing features in fine needle aspiration specimens as well9. More importantly, recent studies have demonstrated that IOPNs are genetically distinct as they generally lack the alterations commonly found in the other subtypes of IPMN, including mutations in KRAS and GNAS.6, 11–16
Still, the literature on IOPN, including the experience with the frequency and significance of associated invasive carcinoma components, is very limited due to the rarity of this entity. Evidence to date suggests that while IOPNs may exhibit invasion, their clinical behavior is relatively indolent, particularly when compared to conventional pancreatic ductal adenocarcinoma.1, 7 This study aims to more fully define the clinicopathologic characteristics and clinical behavior of IOPNs based on the largest cohort reported to date.
MATERIALS AND METHODS
This study was approved by the Institutional Ethics Review Boards of authors’ institutions. Cases with a diagnosis of IOPN or ‘oncocytic subtype’ of IPMN that had available histology slides or paraffin embedded tissue blocks and were included. Seven of the cases were included in our prior study on the genomic alterations in IOPNs.16 Available medical records, including imaging study reports, were reviewed to obtain clinical data including age, gender, symptoms, radiological findings, type of surgery, vital status, and follow-up time. Available gross photographs and descriptions as well as all histologic sections were reevaluated to assess pathological parameters, including tumor location, tumor size, gross appearance, histological appearance, presence and pattern of invasive carcinoma, lymphovascular invasion (LVI), perineural invasion (PNI), lymph node status, and margin status. Intraductal neoplasms with areas of non-oncocytic differentiation were excluded. (The diagnosis as well as presence and pattern of invasive carcinoma component was confirmed by OB, VA and DSK).
Mean, median, and ranges were used to describe quantitative variables. Kaplan-Meier survival curves and the log-rank test were used for survival analyses. Statistical comparisons made by log-rank test using Prism software (GraphPad Software Inc), and the threshold for statistical significance was set at p<0.05.
RESULTS
Clinical Findings
A total of 24 cases were collected; clinical and pathological characteristics are summarized in Table 1.
Table 1.
Clinicopathologic Features of the Cases Analyzed
| Features | N (%) |
|---|---|
| Mean Age (Range) | 59 (36–74) years |
| Female/Male | 15/9 |
| Preoperative Diagnosis | |
| Pancreatic ductal adenocarcinoma | 5/12 (42%) |
| Neuroendocrine tumor | 1/12 (8%) |
| Mucinous cystic neoplasm | 3/12 (25%) |
| Intraductal papillary mucinous neoplasm | 3/12 (25%) |
| Site | |
| Head | 14/21 (67%)* |
| Body/Tail | 6/21(28%) |
| Median tumor size (Range) | 4.5 (1–14) |
| Invasion (%) | 7/24 (29%) |
| LVI (%) | 2/15 (13%) |
| PNI (%) | 1/15 (7%) |
| Lymph node metastasis (%) | 1/17 (6%) |
| Positive margin (%) | 4/17 (24%) |
| Follow-up | |
| Median follow-up (range) | 6.8 years (0.1–18.5) |
| Died of post-operative complications or other causes | 5/18 (28%) |
| Died of disease | 0/18 (0%) |
| Alive with disease | 0/18 (0%) |
| No evidence of disease | 13/18 (72%) |
One of these IOPNs recurred in the tail and underwent completion pancreatectomy.
The patients included 15 females and 9 males. Their ages ranged from 36 to 74 years (mean=59). Almost half (44%) of the lesions were discovered incidentally during work-up for other intra-abdominal pathologies such as bladder neoplasm or polycystic ovarian cysts. For others, presenting symptoms included abdominal or back pain, nausea, hematochezia, and weight loss. None of the patients presented with jaundice. One patient had a history of acute pancreatitis.
The neoplasms were distributed throughout the gland, 67% in the head of the pancreas, 28% in the body/tail, and 5% diffusely involved the gland. The majority (80%) of IOPNs were radiologically described as a (multi)cystic or a complex/partially cystic pancreatic mass, while others were simply reported as a mass. The clinical differential diagnoses included pancreatic ductal adenocarcinoma (42%), pancreatic neuroendocrine tumor (8%), mucinous cystic neoplasm (25%) and intraductal papillary mucinous neoplasm (25%). Only one case was diagnosed as IOPN preoperatively on fine needle aspiration, which was reported previously9.
All patients were treated primarily by surgical resection, none received neoadjuvant chemotherapy. Only one patient with an associated invasive carcinoma component (resembling colloid carcinoma) and lymph node metastasis received 3 weeks adjuvant chemotherapy with Gemcitabin. This patient was then lost to follow-up.
Pathological Findings
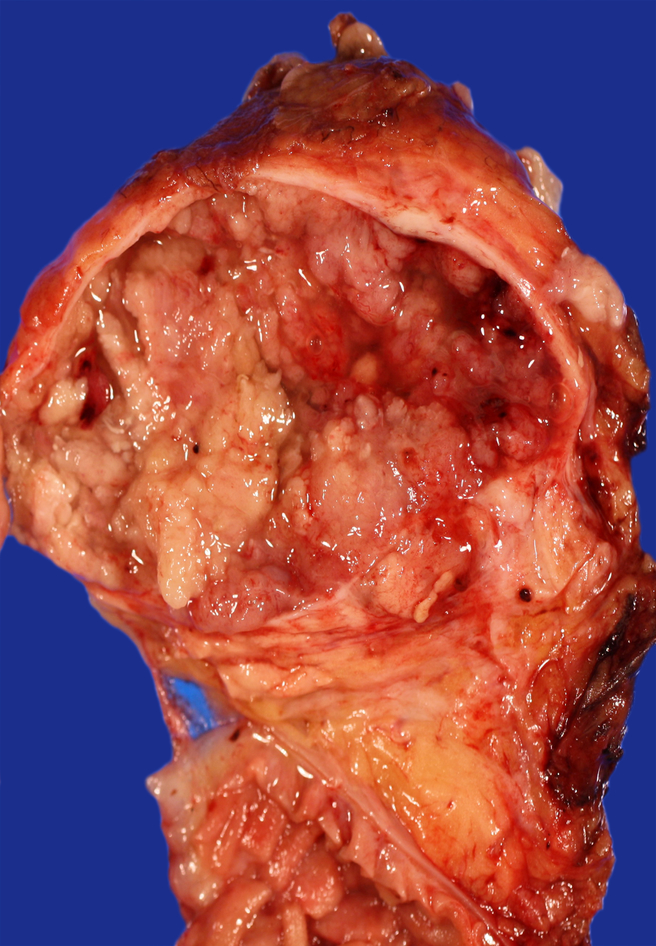
Grossly, the tumors ranged from 1 to 14 cm (median=4.5 cm) in greatest dimension. Detailed gross descriptions were available for 17 tumors, most of which were described as multilocular or unilocular cysts (n=16), some containing papillary projections or solid nodules (n=8) (Figure 1). One tumor was specifically described as an intraductal papillary tumor. Eight tumors (50%) had a grossly identified connection to the main pancreatic duct. In six tumors, the cysts contained clear viscous to mucoid fluid.
Figure 1.
Macroscopic picture of IOPN with cyst lined by papillary excrescences.
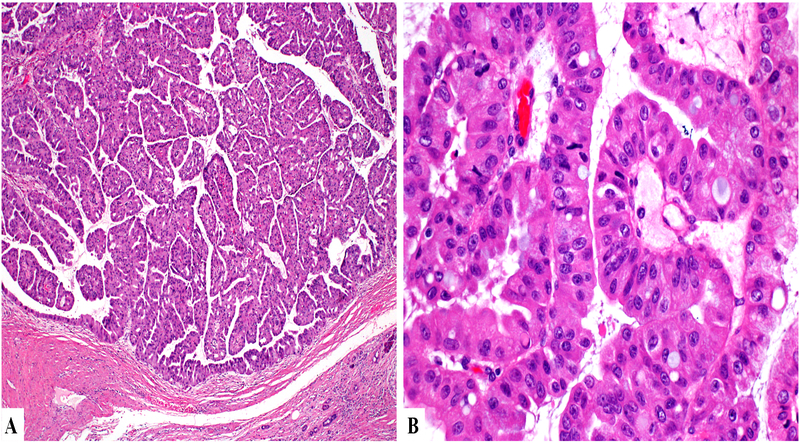
Microscopically, the tumors appeared as multilocular or unilocular cysts containing complex and arborizing papillae with delicate fibrovascular cores (Figure 2A). The multilocular and heterogeneous gross appearance corresponds to ducts, which were variably distended by intraductal tumor, while other unilocular cysts represented ducts dilated due to downstream tumoral obstruction. The papillae were lined by multiple layers of neoplastic cells with abundant granular eosinophilic cytoplasm and large, fairly uniform nuclei containing single, prominent nucleoli. Intraepithelial luminal formations were common (Figure 2B). Scattered goblet cells were also identified. In one case, the epithelium of adjacent papillae was fused, producing a more solid growth pattern within the dilated ducts. In most cases, the intervening pancreas showed a very significant degree of atrophy.
Figure 2.
On microscopy, the lesion exhibits papillary architecture (A) with distinct oncocytic cytology and intracytoplasmic lumens (B).
Invasive Component
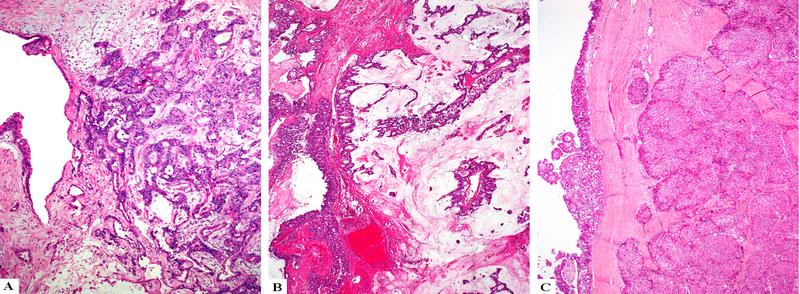
Seven IOPNs (29% of the cohort) had an associated invasive carcinoma component (Figure 3). Five of these were pT1 carcinomas (pT1a=4, pT1b=1) based on this invasive component and in these cases, invasion was typically limited to the peri-ductal/peri-cystic tissues (i.e. tumor cells extending away from the edges of the ducts into surrounding stroma only). The remaining two cases had pT2 carcinomas and these showed invasion into peripancreatic soft tissue and/or the duodenal wall. Only one of these IOPNs with widespread invasion had a lymph node metastasis.
Figure 3.
IOPNs can invade as small infiltrative tubules of oncocytic cells (A), as mucinous tumors with free-floating oncocytic cells (B), or as large pushing nodules (C).
The invasive component was predominantly characterized by small tubular units, lined by oncocytic cells, or individual oncocytic cells infiltrating the stroma (Figure 3A). In three cases, there was minimal stromal mucin accumulation in which the neoplastic cells were suspended, resembling the pattern of colloid carcinoma (Figure 3B) and in remaining two cases the invasive component revealed a nodular growth pattern with pushing borders (Figure 3C).
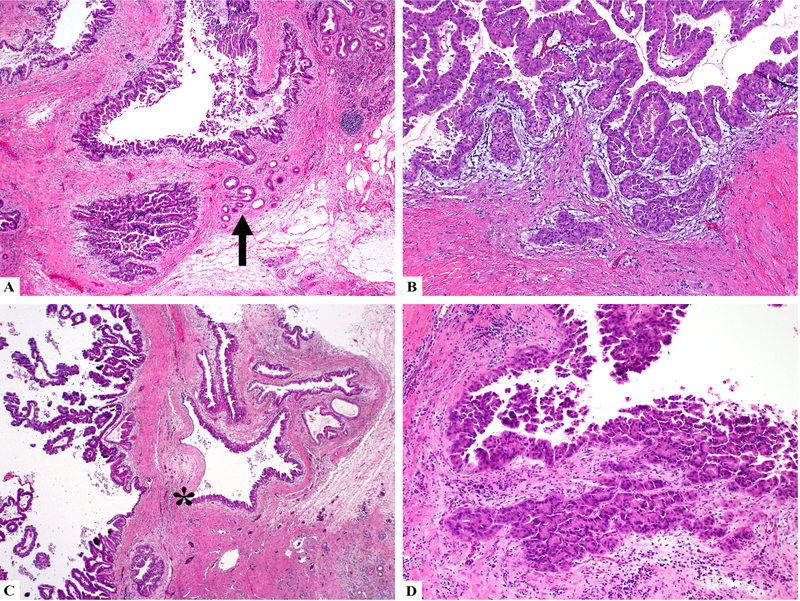
Of note, many of the remaining IOPNs revealed morphologic features mimicking invasive carcinoma (n=11). The neoplasms often grew along adjacent benign ducts, which mimicked invasion especially because of the more dispersed appearance of the atrophic glands (Figure 4A). Myxoid or edematous stroma surrounding the massively dilated ducts involved by IOPN also sometimes simulated desmoplastic stroma, which could be misinterpreted as invasion (Figure 4B) when it was associated with involved smaller ductules. If present, abrupt transition from a morphologically normal epithelium to oncocytic epithelium within the same duct was helpful in recognizing intraductal extension into small ducts (Figure 4C); however, in most ducts this feature was lacking. Additionally, the prominent nucleoli created a highly atypical appearance when seen in the smaller ductular units. However, the overall lobulated architecture, presence of the same stroma surrounding adjacent ducts, as well as maintained luminal connections on deeper sections favored pseudo-invasion. Tangential sectioning also mimics invasion by creating a complex appearance (Figure 4D).
Figure 4.
IOPNs may exhibit pseudo-invasive patterns including growing along adjacent benign ducts (arrow head) (A), or myxoid stroma changes that simulate desmoplasia (B). If present, abrupt transition (star) from a morphologically normal epithelium to oncocytic epithelium within the same duct is helpful in recognizing pseudoinvasion (C). Tangential sectioning also mimics invasion by creating a complex appearance (D).
Long-Term Outcomes
Clinical follow-up data were available for 18 patients, with median follow-up of 6.8 years (range, 0.1–18.5 years). None of the patients died of disease. One patient with no invasive carcinoma died of post-operative complications 3 weeks after surgery. Four patients died of other causes at 13, 15, 16, and 18.5 years (only one had invasive disease). One patient with invasive disease (pT1b) in the head of the pancreas had recurrence 3.4 years after the original surgery and underwent completion pancreatectomy, which also revealed invasive disease (pT1a). At the time of last follow-up, the remaining twelve patients were alive with no evidence of disease.
The 10-year overall survival of the whole cohort was 94% and there was no difference between the invasive (n=4) and non-invasive (n=14) IOPN cohorts (p = 0.38). When we considered recurrence-free survival, the invasive IOPN cohort exhibited 66% 5-year recurrence-free survival, compared to 93% in the non-invasive cohort; however, this difference also fell short of statistical significance (p = 0.066).
DISCUSSION
The family of intraductal neoplasms of the pancreas includes a variety of entities.17 Some of these are simply regarded as subtypes of IPMN.5, 18–22 Although IOPN was first described as a distinct intraductal neoplasm,1 in 2010, the WHO placed it under the heading of IPMN, since it was relatively ill characterized.2 During the last few decades, much has been learned about the nature and behavior of other IPMNs. However, experience with IOPN is still limited due to its rarity.1, 3, 5, 9, 10
In this study, we analyzed 24 IOPNs. These tumors typically occur in late adulthood and there is a female predominance. While most IOPNs are located in the pancreatic head or uncinate process, it is not known whether this represents a true predilection or is a reflection of the tendency of pancreatic head lesions to present with symptoms. The tumors appear to be at least partially cystic and often multilocular. Papillary nodules are so exuberant that they often appear as solid areas radiologically, which leads to the impression of pancreatic ductal adenocarcinoma (almost half of the cases were given this clinical, and the papillary nodules also often are not noted on gross examination, although they are typically abundant microscopically. Along with the distinctive cytological features of oncocytic cells, often with single large nucleoli, arborizing papillary architecture is a hallmark of IOPNs. Intracytoplasmic lumen formation, which can focally lead to a cribriform architecture, is also characteristic. Compared to IPMNs, IOPNs have much less mucin production.
Like other intraductal neoplasms of the pancreas, IOPNs have the potential for the development of invasive carcinoma. Within our cohort, 29% of the cases exhibited invasion but most of these were small invasive carcinomas. This figure is significantly lower than that in the study of 18 cases by Marchegiani et al., in which 61% of the cases were reported to have invasion.7 However, the pathological criteria for invasive carcinoma were not clearly defined in their study. Furthermore, as we found in the current study, IOPNs often have very complex intraductal spread into the atrophic lobules of the adjacent pancreas, which, combined with characteristic myxoid changes in the periductal stroma, can simulate invasive carcinoma. Historically, this infiltrative appearance contributed to prior labeling of many IOPNs as “papillary adenocarcinoma” or as pancreatic ductal adenocarcinoma. Of note, a pseudo-invasive appearance is not unique to IOPNs and is also seen frequently in other intraductal neoplasms, especially in intraductal tubulopapillary neoplasm.23, 24 It should also be kept in mind that the criteria for invasion in cystic and intraductal neoplasms have changed significantly over the years. Our results show that true invasion in IOPNs may appear in different patterns including small infiltrative tubules and carcinoma with mucinous and oncocytic features. Similar to colloid carcinoma arising in association with intestinal type IPMNs, the carcinomas with mucinous and oncocytic features arising in association with IOPNs consist of clusters of oncocytic epithelium “floating” in nodular pools of stromal mucin. However, unlike the IPMNs, IOPNs seldom give rise to ordinary PDAC.25
The most important finding in this study is the clinical behavior of IOPN. The long-term follow-up of patients with IOPN reveals that despite the presence of invasive carcinoma in 29%, survival outcomes are very favorable. In our series, of 18 patients with available follow-up information, no patients died of the disease, median overall survival was 15.3 years and only one (6%) suffered from a recurrence. Marchegiani et al.7 also found very similar results with a median overall survival of 130 months, despite reporting higher invasion and recurrence rates. Even after a second resection, none of their patients died of the disease, either.7
In addition, IOPNs lack the striking intestinal differentiation of many main duct IPMNs and have different mucin expression patterns. Diffuse CDX2 and MUC2 expression, which is uniformly present in intestinal type IPMNs, is virtually non-existent in IOPNs. Instead, there is often diffuse and strong MUC6 labeling.4, 6 Our experience on expression profile of mucins and differentiation markers [MUC1/MUC2/MUC5AC/MUC6/CDX2/hepatocyte paraffin-1] was reported as part of other studies including both IOPNs and IPMNs (Table 2).4, 6 Also, IOPNs do not harbor the majority of previously reported IPMN- or pancreatic ductal adenocarcinoma-related mutations.11–13, 26 A striking molecular aspect of IOPN is the distinct paucity of KRAS mutations, which is otherwise ubiquitous in most pancreatic ductal neoplasms, including a very high percentage of IPMNs and the majority of PDACs.14 In a recent study analyzing eleven IOPNs by targeted next-generation sequencing for a panel of 300 key cancer-associated genes, our group has confirmed that IOPNs are genetically distinct: None of our typical IOPNs had KRAS or GNAS mutations and only one had RNF43, PIK3R1, and PIK3R3 mutations. Instead, ARHGAP26, ASXL1, EPHA8, and ERBB4 genes were each found to be mutated in more than one IOPN.16 Excluding reports of potential involvement of the EPHA8 gene, these genes have not been previously associated with other types of pancreatic neoplasms.27–30
Table 2.
Immunoprofile of the IOPNs Analyzed
MUC2 and CDX2 labeling was observed in goblet cells.
Note: These results were previously published in part in Basturk O et al. Virchows Arch. 2016 Nov;469(5):523–532 (PMID: 27591765).
In summary, our results are in concordance with the original report of IOPN1 that it has distinct morphologic, clinical, molecular and behavioral characteristics that warrant its recognition as a distinct entity. Despite being relatively large and highly complex tumors that often receive a pre-operative diagnosis of pancreatic ductal adenocarcinoma, the vast majority of patients with this tumor are cured with complete resection, including those with invasive disease, which is typically limited in extent.
ACKNOWLEDGMENTS
The authors thank Ms. Allyne Manzo and Ms. Lorraine Corsale for their assistance with the figures.
Footnotes
This study was presented in part at the 105th annual meeting of the United States and Canadian Academy of Pathology in Seattle, WA, in March 2016.
DISCLOSURE
Supported in part by the Cancer Center Support Grant (CCSG)/Core Grant/P30 CA008748 and by the Melamed Family Foundation. The remaining authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–994. [DOI] [PubMed] [Google Scholar]
- 2.Adsay NV, Kloppel G, Fukushima N, et al. Intraductal neoplasms of the pancreas In: Bosman FT, Carneiro F, Hruban RH, eds. WHO Classification of Tumors. Lyon, France: WHO Press; 2010:pp 304–313. [Google Scholar]
- 3.Adsay NV. Cystic lesions of the pancreas. Mod Pathol. 2007;20 Suppl 1:S71–93. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloppel G, Basturk O, Schlitter AM, et al. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–466. [DOI] [PubMed] [Google Scholar]
- 6.Basturk O, Chung SM, Hruban RH, et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2016;469:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg. 2015;220:839–844. [DOI] [PubMed] [Google Scholar]
- 8.D’Onofrio M, De Robertis R, Tinazzi Martini P, et al. Oncocytic Intraductal Papillary Mucinous Neoplasms of the Pancreas: Imaging and Histopathological Findings. Pancreas. 2016;45:1233–1242. [DOI] [PubMed] [Google Scholar]
- 9.Reid MD, Stallworth CR, Lewis MM, et al. Cytopathologic diagnosis of oncocytic type intraductal papillary mucinous neoplasm: Criteria and clinical implications of accurate diagnosis. Cancer Cytopathol. 2016;124:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid MD, Lewis MM, Willingham FF, et al. The Evolving Role of Pathology in New Developments, Classification, Terminology, and Diagnosis of Pancreatobiliary Neoplasms. Arch Pathol Lab Med. 2017;141:366–380. [DOI] [PubMed] [Google Scholar]
- 11.Xiao HD, Yamaguchi H, Dias-Santagata D, et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J Pathol. 2011;224:508–516. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohri D, Asaoka Y, Ijichi H, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47:203–213. [DOI] [PubMed] [Google Scholar]
- 14.Patel SA, Adams R, Goldstein M, et al. Genetic analysis of invasive carcinoma arising in intraductal oncocytic papillary neoplasm of the pancreas. Am J Surg Pathol. 2002;26:1071–1077. [DOI] [PubMed] [Google Scholar]
- 15.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basturk O, Tan M, Bhanot U, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod Pathol. 2016;29:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hruban R, Pitman MB, Klimstra DS. Tumors of the Pancreas Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 18.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. [DOI] [PubMed] [Google Scholar]
- 19.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. [DOI] [PubMed] [Google Scholar]
- 22.Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basturk O, Hong S-M, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basturk O, Adsay V, Askan G, et al. Intraductal Tubulopapillary Neoplasm of the Pancreas: A Clinicopathologic and Immunohistochemical Analysis of 33 Cases. Am J Surg Pathol. 2017;41:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg. 2015;220:845–854.e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terris B, Dubois S, Buisine MP, et al. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–637. [DOI] [PubMed] [Google Scholar]
- 27.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098–1109 e1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molin MD, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]