Abstract
Background.
There is growing concern about the health impact of heavy alcohol use in people infected with Human Immunodeficiency Virus (HIV+). Mixed findings of past studies regarding the cognitive impact of alcohol use in HIV+ adults have been mixed, with inconsistent evidence that alcohol consumption exacerbates HIV-associated brain dysfunction. This study examined contributions of current heavy drinking, lifetime AUD, and age to cognitive deficits in HIV+ adults, and relative to other HIV-associated clinical factors.
Methods.
Cognitive performance of HIV+ adults (n=104) was assessed and comparisons were made between heavy current to non-heavy drinkers (NIAAA criteria), lifetime AUD versus no-AUD, and older (> 50 years) vs. younger participants. Hierarchical regression analyses were conducted to examine the association between cognitive performance and current heavy drinking, lifetime AUD, and older age, while also correcting for HIV clinical factors and history of other substance use.
Results.
Individuals reporting current heavy drinking and meeting criteria for lifetime AUD demonstrated the greatest degree of deficits across multiple cognitive domains. Deficits were greatest among HIV+ adults with lifetime AUD, and older age was also associated with weaker cognitive performance. Lifetime AUD and older age independently exhibited stronger associations with cognitive performance than HIV clinical factors (e.g. viral load, current CD4, nadir CD4) or past opiate and cocaine use.
Conclusions.
Current heavy drinking and lifetime AUD adversely affect cognitive function in HIV+ adults. Greatest deficits existed when there was a history of AUD and continued current heavy drinking, indicating that past AUD continues to have an adverse impact and should not be ignored. That alcohol use was more strongly associated with cognitive performance than HIV clinical factors underscores clinical importance of targeting reduction of heavy alcohol consumption in HIV+ adults.
Keywords: Alcohol use disorder (AUD), current alcohol consumption, Cognition, HIV, Aging
INTRODUCTION
Human Immunodeficiency Virus (HIV) adversely affects cognitive and brain function in many infected adults. These negative effects occur despite marked reductions in mortality and morbidity, including HIV-associated dementia, since the advent of combination antiretroviral therapies (cART) (Cohen et al., 2001, Sacktor et al., 2002). Behavioral and psychosocial problems such as substance use remain common among HIV+ adults and may exacerbate brain and cognitive effects. Over the past decade there has been a growing awareness that alcohol consumption may adversely affect health, cognitive, and functional outcomes in HIV+ adults (Monnig et al., 2016, Jacob et al., 2013, Hanna et al., 2013, Korthuis et al., 2012, Justice et al., 2010, Conigliaro et al., 2004). Evidence supporting an interactive effect of alcohol and HIV on brain and cognitive function is supported by animal studies involving rodents (Griffin et al., 2004, Griffin et al., 2007, Jaeger and Nath, 2012, Walker and Koob, 2008, Potula et al., 2006, Persidsky et al., 2001, Loftis et al., 2016) and monkeys (Bladowska et al., 2013, Marcondes et al., 2008, Kumar et al., 2005).
Several potential mechanisms have been proposed by which alcohol may influence brain and cognitive function. Alcohol consumption is associated with gut microbial translocation, and alterations in the “gut-liver-brain” axis may play a role (Zakhari, 2009) (Vyboh et al., 2015). It is possible that harmful alcohol use contributes to organ and tissue injury, which has a systemic effect and contributes to neurocognitive dysfunction.(Marquine et al., 2016). Neuroimaging studies suggest that alcohol use disorder (AUD) co-occurring with HIV may exacerbate certain structural brain abnormalities which can affect cognitive functioning (Lewis-de Los Angeles et al., 2017, Bladowska et al., 2013, Sullivan et al., 2011, Rosenbloom et al., 2010, Nelson et al., 2005, Pfefferbaum et al., 2005, Chang et al., 2004, Pfefferbaum et al., 2002, Durazzo et al., 2007, Cardenas et al., 2005, Meyerhoff et al., 1995, Pfefferbaum et al., 2007) (Gullett et al., 2018).
While past studies of the cognitive impact of alcohol use in HIV+ adults provide some evidence that alcohol exacerbates HIV-associated brain dysfunction, findings have been mixed, making it difficult to reach definitive conclusions. Relatively few studies have systematically examined the neurocognitive effects of alcohol in HIV+ adults treated with cART. Differences in findings across studies likely related to whether heavy drinking occurred in the past or present, and also whether clinical factors that could affect cognition (e.g. age, HIV clinical status, other drug use) were accounted for. Early studies tended to examine alcohol use effects in HIV+ adults in the broader context of substance use, making interpretation of the specific contributions of alcohol difficult (Concha et al., 1992, Hinkin et al., 2004, Justice et al., 2004). Conclusions vary depending on how alcohol was classified; any versus no use, current mild-moderate drinking versus heavy drinking, or whether there was a history of AUD, binge drinking, or other alcohol use (Monnig et al., 2016).
Past studies have also varied with respect to which cognitive domains were examined and how they were assessed. For example, Green et al. found that HIV+ participants with a history of past AUD had verbal IQ, verbal reasoning, and reaction time deficits suggesting additive and interactive effects of AUD and HIV on cognition (Green et al., 2004). Other studies found AUD history to be associated with higher rates of HIV-associated neurocognitive deficits (Becker et al., 2004), but with effects ranging from a single cognitive domain such as semantic memory (Fama et al., 2011) to global cognitive function (Gongvatana et al., 2014b). The cognitive effects of alcohol use among HIV+ adults may also be exacerbated by advanced age (Downer et al., 2014; Durazzo et al., 2013; Gongvatana et al., 2014; Houston et al., 2014; Kist et al., 2014). We previously found an interaction between lifetime AUD and age in a study of otherwise healthy adults without HIV (Woods et al., 2016), in which adverse effects associated with both current and lifetime alcohol consumption were observed in the older adults. However, it is not clear whether adverse neurocognitive effects stem from the cumulative effects of chronic heavy alcohol consumption or current consumption in the HIV+ population.
This study was conducted to clarify the cognitive effects of chronic heavy alcohol consumption versus current consumption among HIV+ persons. We sought to determine: (1) The relative contributions of current heavy drinking (high vs. moderate-low), lifetime AUD, and age, to deficits in cognitive performance among HIV+ adults, (2) Whether these associations remain when examined relative to clinical measures including current HIV status, prior history of HIV, and prior cocaine and opiate use, (3) The relationship between binge drinking and cognition. We hypothesized that cognitive deficits would exist among HIV+ adults who currently drink heavily and among those with lifetime AUD, with greatest deficits among those with both. We also hypothesized that these alcohol effects would be exacerbated in older HIV+ adults, who were hypothesized to have greater cognitive deficits even after age-adjustment of neuropsychological measures. We hypothesized that current heavy drinkers and individuals with a history of lifetime AUD would continue to demonstrate deficits above and beyond groups without alcohol use histories, particularly in domains most affected by alcohol such as learning and memory. Lastly, we hypothesized that binge drinkers would have greater cognitive deficits relative to non-binge drinkers.
METHODS
Participants.
Participants (N=104) were studied as part of a National Institute on Alcohol Abuse and Alcoholism (NIAAA) sponsored project designed to examine the contribution of heavy alcohol use to neurocognitive and brain dysfunction in HIV+ adults. The sample consisted of HIV-infected adults recruited from the Brown University Center for AIDS Research (CFAR), and HIV infection was confirmed by enzyme-linked immunosorbent assay (ELISA) and Western blot, and the level of detectable plasma HIV RNA utilized was <75 copies/mL. Sample demographics are provided in Table 1.
Table 1.
Clinical and Demographic Characteristics of the Study Cohort
| HIV+ (n=104) |
EtOH-Heavy (n=30) |
EtOH-Low (n = 74) |
AUD (n =50) |
No-AUD (n =50) |
|
|---|---|---|---|---|---|
| Age (years) | 47.9 (9.0) | 47.7 (9.2) | 48.0 (9.0) | 47.9 (7.1) | 48.1 (10.7) |
| Education (years) | 13.3 (2.8) | 12.6 (2.9) | 13.5 (2.7) | 13.1 (2.4) | 14.0 (2.9) |
| Male | 68% | 71% | 62% | 72% | 66% |
| Caucasian | 77% | 73% | 78% | 74% | 84% |
| Clinical HIV | |||||
| Duration (years) | 17.5 (8.5) | 16.5 (9.0) | 17.9 (8.4) | 18.2 (8.4) | 16.7 (8.7) |
| CD4 nadir (cells/ml) | 221.5 (175.6) | 215.2 (214.4) | 224.2 (157.7) | 223.9 (159.7) | 223.3 (191.3) |
| Current CD4 (cells/ml) | 567.6 (287.8) | 562.8 (273.9) | 606.2 (290.0) | 564.6 (286.7) | 578.1 (284.3) |
| Undetectable Viral Load (%) | 75% | 75% | 75% | 73% | 80% |
| cART (%) | 97% | 96% | 97% | 96% | 98% |
| AIDS (%) | 49% | 53% | 47% | 51% | 47% |
| HCV-Ever | 32% | 33% | 32% | 37% | 27% |
| Drug Dependence A | |||||
| Cocaine Use Disorder | 52% | 56% | 41% | 64%* | 30% |
| Narcotic Use Disorder | 22% | 22% | 18% | 28% | 16% |
Note: Cocaine and Narcotic Dependence were measures determined by KMSK lifetime use reporting; AUD grouping determined by KMSK alcohol sum score; EtOH grouping determined by Timeline Follow Back.
Participants were excluded for history of (1) head injury with loss of consciousness > 10 minutes; (2) severe anxiety, depression, or neurological disorders, including dementia, seizure disorder, stroke, and opportunistic brain infection; (3) severe psychiatric illness that might impact brain function (e.g., schizophrenia, bipolar illness); and (4) current (past six months) substance dependence or positive urine toxicology screen for cocaine, opiates, or illicit stimulants or sedatives. Inclusion/exclusion criteria were assessed using structured clinical interview by the study physician and self-reported history. The study was approved by the institutional review boards, and informed consent was obtained before enrollment.
Alcohol Consumption.
Participants were recruited with the goal of obtaining relatively equal samples of nondrinkers, moderate drinkers, and heavy alcohol users (ethanol [EtOH]-none; EtOH-moderate, EtOH-heavy) based on NIAAA criteria (http://rethinkingdrinking.niaaa.nih.gov.asp) derived from Timeline follow-back (TLFB; (Fals-Stewart et al., 2000)) and by clinical assessment. The TLFB involves a self-report of drinking over the past 90 days and was used to calculate the average number of drinks per week over the past 3 months. The EtOH-heavy group consisted of people who reported drinking 5 or more drinks in a single day for men (or average more than 14 per week), and 4 or more in a single day (or average more than 7 in a week) for women. The EtOH-moderate group consisted of people who reported consuming less than EtOH-heavy quantities, while EtOH-none reported never drinking. About 31.8% (n = 21, 8 women) were heavy alcohol consumers compared to 68.2% (n = 45, 27 women) who were not. There were no significant differences by EtOH level or age between EtOH-none (n = 11) versus EtOH-moderate (n = 34) participants on any cognitive domain examined (F (1, 45) = 1.4, p > 0.05). Given the study hypotheses of adverse neurocognitive effects among heavy drinkers and also the lack of differences from these preliminary analyses, the EtOH-none and EtOH-mild/moderate groups were pooled into a single group of HIV+ adults who reported currently drinking below the NIAAA threshold for “at-risk” consumption (EtOH-low).
Drug and Alcohol Use Disorder.
No participants were currently using cocaine or opiates based on self-report and urinalysis, and none met criteria for current cocaine or opiate use disorders based on the Kreek–McHugh–Schluger–Kellogg scale (KMSK; (Kellogg et al., 2003). The KMSK quantifies self-reported exposure to opiates, cocaine, alcohol, and/or tobacco. Each section of the KMSK assesses the frequency, amount, and duration of use during the person’s period of highest consumption. The KMSK scale was also used to assess life-time history of AUD, confirmed with data from the medical history and clinical interview. To receive a diagnosis of AUD a cutoff score of 11 was used, which provides 90% sensitivity and 90% specificity for diagnosis of AUD with the SCID-I (Kellogg et al., 2003). Six participants were excluded from this analysis because of incomplete KMSK scores. There was no statistical difference between any of the mean cognitive performance scores for the excluded participants when compared to those who were retained.
Neurocognitive Assessment.
All participants completed a battery of standardized neuropsychological tests widely used by our group and others to assess the following cognitive domains: speed of information processing, attention/executive functioning, learning, recall memory, verbal fluency, and psychomotor speed. The battery was comprised of the following tests chosen for their sensitivity to HIV-associated neurocognitive deficit: Hopkins Verbal Learning Test—Revised (HVLT-R; verbal learning and memory; Benedict et al., 1998; Brandt and Benedict, 1991); Brief Visuospatial Memory Test—Revised (BVMT-R; visuospatial learning memory;(Patel et al., 2014) Benedict, 1997); Controlled Oral Word Association Test (COWAT–FAS; verbal fluency; Benton et al., 1994 (Patel et al., 2014)); semantic fluency (animals); Stroop Color and Word Test (attention/executive function; Golden, 1978)(Patel et al., 2014); Trails Making Test, Parts A and B (executive function; Reitan, 1992(Patel et al., 2014)); Letter–Number Sequencing (working memory) from the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III; Wechsler, 1997); Grooved Pegboard Test fine motor speed; (Klove, 1963); and the Digit Symbol–Coding and Symbol Search (speed of processing measures) tests from the WAIS-III.
T-scores are defined as a standard score derived from the normative comparison group such that the partipants T-score is the deviation from the mean, where the mean is equal to 50 and the standard deviation is 15. T-scores from delayed recall on the HVLT-R and BVMT-R were averaged to calculate the delayed recall domain. Learning trial performance (T-scores) on these two tasks was averaged to create the learning domain. COWAT and semantic fluency T-scores were averaged for the verbal fluency domain. Stroop, Letter–Number Sequencing, and Trails A and B T-scores were averaged to compute the attention/working memory/executive functioning domain. Digit Symbol–Coding and Symbol Search T-scores were averaged to calculate the speed of processing domain. The Grooved Pegboard Test T-score was used for the psychomotor speed domain. Test scores were adjusted using established norms for age, education, gender, and race (Heaton, 2004). A global cognitive index (GCI) was calculated by averaging domain composite T-scores.
Statistical Analyses.
All analyses were conducted using SPSS-24 software (IBM, Armonk, NY). Demographic and clinical characteristics of the overall sample were determined, and differences between EtOH-Low and EtOH-Heavy were examined by independent t-tests. Cross-tabulation and chi-square procedures were used to determine whether the proportion of men and women and racial groups differed as a function of EtOH group. Subsequent analyses employed general linear modeling for both between-group comparisons and hierarchical regression analysis of clinical factors most strongly associated with neurocognitive performance and deficits. Alcohol use was examined in two different ways: 1) Current alcohol use based on clinical interview and TLFB; and 2) AUD history based on the KMSK indices using the validated cut-point of 11. For between-group comparisons, age was stratified (young: age < 50 years; old: age ≥ 50 years). As age was corrected for using T-scores based on standardized normative data for the neurocognitive dependent measures, age was included in the models to specifically assess for abnormal change in the normal age-related neurocognitive function. Thus, the presence of an age effect denotes exacerbation of normal age-related performance. Differences in neurocognitive performance were examined as a function age and alcohol grouping using general linear modeling.
Initial analyses were performed with standardized composite domain T-scores as the dependent measures, with current alcohol use (EtOH-Heavy vs. EtOH-Low) and age group (young vs. old) treated as independent between-group factors in a 2 × 2 multivariate analysis of variance (MANOVA). Another MANOVA was conducted comparing age group by AUD group (lifetime AUD vs. no-AUD). A final 2 ×2 MANOVA was performed to compare cognitive performance as a function of current EtOH use and lifetime AUD treated as the independent factor. For each MANOVA, omnibus effects for overall cognitive functioning were tested based on all composite domain scores. Interactions were examined using tests of simple effects. Planned univariate comparisons were then conducted with each cognitive domain T-score serving as dependent variables for each of the above described 2 ×2 between-group comparisons.
Hierarchical stepwise regression analyses were then conducted to determine the relative contribution of current alcohol use vs. lifetime AUD to neurocognitive performance. Separate analyses were conducted using Global Cognitive T-Score and each of the cognitive domain T-scores as the dependent measure. For these analyses, KMSK component indices of lifetime alcohol use history (quantity, frequency, and duration) and current EtOH grouping was entered, along with age (treated as a continuous variable), sex, racial group, and HIV clinical variables (current CD4, nadir CD4, time since HIV diagnosis, HCV status, and cART use). Given previous literature suggesting the use of an index score representing several critical clinically-related factors, we repeated these analyses replacing the respective clinical and demographic factors with the VACS index score (Tate et al., 2013). In a final set of analyses, KMSK total scores for past alcohol, opiate, and cocaine use were entered into a model to determine whether lifetime AUD and age were associated with neurocognitive performance after accounting for past cocaine and opiate use.
RESULTS
Clinical and Demographic Characteristics.
Age, years of education, sex and racial composition were not significantly different between the EtOH-Heavy and EtOH-Low groups (p > 0.05). Current alcohol use varied as a function of lifetime AUD history (Chi2 = 12.6 (df =1, p < .01), and age was not significantly different between lifetime AUD groups (p > 0.05). Clinical and demographic characteristics of the sample are grouped by current alcohol use and provided in Table 1.
Neurocognitive Performance.
Overall performance was within the normal range when all domains were averaged for the full sample, as indicated by the global cognitive index (Global T = 48.7 ± 9.1). However, performance in domains of learning and memory for the entire HIV sample was approximately one standard deviation below the normative mean (Learning T-Score = 39.8 ± 13.4; Memory T-Score = 40.1 ± 13.9). When examining the AUD group separately, performance on learning and memory domains fell at the borderline impaired and low average normative range (Learning T-Score = 35.8 ± 1.9; Memory T-score = 37.1 ± 1.9).
Current Alcohol Use.
A between-group difference in overall neurocognitive performance was observed between the EtOH-Heavy and EtOH-Low for the main effect of current alcohol use on the MANOVA (F (6, 91) = 2.29, p < .05). There was no difference in overall performance between age groups, or for the EtOH by age-group interaction (p > .05). Univariate comparisons indicated significant differences in performance between EtOH-Heavy and EtOH-Low for domains of attention-executive functioning (F (1, 96) = 4.46, p < .05) and learning (F (1, 96) = 4.38, p < .05). Groups did not differ with respect to memory, processing speed, motor, or verbal functioning (p > .05). Means and standard deviations for each domain for the entire HIV sample and for the two groups (EtOH-Heavy vs. EtOH-Low) are displayed in Table 2.
Table 2.
Cognitive Performance by EtOH Group (T-scores)
| Composite Cognitive Measure | HIV Cohort (n = 104) |
AUD (n = 50) |
No-AUD (n = 50) |
|---|---|---|---|
| Global Cognition | 48.7 (0.9) | 49.3 (1.5) | 51.5 (1.1) |
| Speed of Processing | 51.7 (1.0) | 49.3 (1.5) | 54.1 (1.2) |
| Attention/Executive | 52.3 (0.9) | 49.2 (1.2) | 55.4 (1.2) |
| Learning | 39.8 (1.3) | 35.8 (1.9) | 44.0 (1.7) |
| Memory | 41.0 (1.4) | 37.1 (2.0) | 45.1 (1.7) |
| Verbal | 52.0 (1.2) | 54.3 (1.3) | 56.7 (1.2) |
| Motor | 48.7 (0.9) | 52.0 (1.8) | 51.8 (1.5) |
Note: T-scores have a mean of 50 and a standard deviation of 10, such that higher scores indicate better performance. T-scores for overall cognitive function and by cognitive domain; EtOH-Heavy and EtOH-Low group performances were identical to AUD and No-AUD groups and are not displayed for simplification; Means (Standard Deviation).
p <.05. AUD = Lifetime Alcohol Use Disorder.
Lifetime AUD.
The MANOVA examining between-group effects for lifetime AUD and age on overall cognitive performance (GCI) indicated a significant main effect for lifetime AUD (F [1, 90] = 2.66, p < .05) and an interaction between lifetime AUD and age that approached statistical significance (F [1, 90] = 2.04, p = .06). Participants with lifetime AUD had weaker performance than those without lifetime AUD (Table 3).
Table 3.
Cognitive Performance by EtOH and Age Group (T-scores)
| EtOH-Heavy AUD (n = 20) |
EtOH-Heavy No-AUD (n = 10) |
EtOH-Low AUD (n = 49) |
EtOH-Low No-AUD (n = 25) |
HIV-Older >50 yrs. (n = 47) |
HIV-Younger < 50 yrs. (n = 57) |
|
|---|---|---|---|---|---|---|
| GCI | 45.5 (7.1)* | 52.3 (2.6) | 48.4 (8.4) | 52.2 (6.7) | 45.9 (1.4)** | 51.0 (1.1) |
| Speed of Processing | 48.8 (8.5)* | 54.8 (6.9) | 50.4 (10.9) | 54.8 (7.4) | 48.9 (1.6)** | 53.9 (1.2) |
| Attention/Executive | 47.3 (8.2)* | 57.7 (8.9) | 51.8 (6.7) | 55.5 (7.8) | 49.8 (1.3)** | 54.2 (1.1) |
| Learning | 33.0 (12.5)* | 45.9 (4.9) | 39.5 (12.4) | 44.5 (12.4) | 36.6 (2.0)** | 42.4 (1.7) |
| Memory | 36.9 (10.8)* | 46.3 (8.7) | 39.5 (12.4) | 45.7 (11.7) | 37.2 (2.1)** | 44.1 (1.7) |
| Verbal | 54.7 (9.5) | 57.8 (7.8) | 54.0 (8.7) | 56.5 (8.7) | 55.1 (1.3) | 55.8 (1.2) |
| Motor | 49.5 (10.4) | 52.6 (2.6) | 48.4 (8.4) | 52.8 (10.6) | 50.4 (1.7) | 53.2 (1.6) |
Note: T-scores have a mean of 50 and a standard deviation of 10, such that higher scores indicate better performance. T-scores are for overall cognitive performance and by cognitive domain. Means (Standard Deviation). GCI = Global Cognitive Index (overall cognitive performance). EtOH-Heavy = Current heavy alcohol use according to the Timeline Follow Back; EtOH-Low = No current heavy alcohol use according to the Timeline Follow Back; AUD = Lifetime Alcohol Use Disorder according to KMSK alcohol sum score.
Significantly worse performance for the EtOH-Heavy + AUD group compared to EtOH-Low + No-AUD (p <.05).
significantly worse performance for age >50yrs compared to age <50yrs group (p<.05)
Planned univariate comparisons indicated that the AUD group had performance deficits in four cognitive domains compared to the no-AUD group (Processing Speed: p < .01; Attention-Executive: p < .001; Learning: p < .01; Memory: p < .01). The main effect of age was also significant for three domains (Processing Speed: p < .05; Attention-Executive: p < .05; Memory: p < .05). Older HIV+ adults had deficits compared to younger HIV+ adults in these domains. Tests of simple effects indicated that, older participants (regardless of AUD group) and young participants with AUD were weaker in these two domains compared to younger participants without AUD. In other words, AUD and older age were each associated with weaker performance, but their combined effect did not translate into further performance deficits. However, being younger and not having an AUD history was associated with stronger cognitive performance. Accordingly, results of these analyses indicate that AUD and age independently affect cognitive function in HIV+ adults, but that their combined effect may not be much greater than their individual effects.
Lifetime and Current Alcohol Use.
Chi-square analysis indicated an equal distribution of participants grouped on the basis of both current EtOH use and lifetime AUD (x2 > .05) and these variables had limited collinearity (r2 = 0.078). Comparisons of these groups performed via a 2 × 2 between-group MANOVA revealed a main effect for lifetime AUD and overall cognitive performance (F (6, 91) = 2.4, p < .05), and for the interaction of current EtOH use by lifetime AUD with overall performance (F (6, 91) = 2.0, p < .05). The main effect for lifetime AUD indicated weaker overall cognitive performance among heavy drinkers. Tests of simple effects of the interaction, indicated that individuals who were currently heavy drinkers and also had a lifetime history of AUD had weaker overall performance than the other group combinations (p < .05). Planned univariate comparisons for the main effect of lifetime alcohol use severity (EtOH-Low, EtOH-Moderate, EtOH-Heavy) indicated significant between-group differences in the cognitive domains of Processing Speed (p < .01), Attention-Executive functioning (p<.05), Learning (p <.05), and Memory (p<.05), but not for other domains.
The MANOVA conducted to examine the interaction of current heavy alcohol use and lifetime AUD indicated only a main effect for lifetime AUD (F (6, 89) = 2.9, p < .05). AUD was associated with weaker overall cognitive performance. Planned univariate analyses revealed results that were consistent with the results described above which used the KMSK lifetime heaviest alcohol use measure to group the participants. HIV+ adults with a history of AUD had weaker performance in the same four cognitive domains.
Hierarchical stepwise regression analyses conducted to examine the relationships of current alcohol consumption and lifetime AUD (quantity, frequency and duration) to neurocognitive performance indicated significant associations with overall cognitive function and also with performance in specific domains. In each case, factors associated with lifetime AUD were retained along with age as significantly associated with cognitive function. Lifetime AUD (β = −.23) and age (β = −.24) were both negatively associated with overall performance as measured by the Global Cognitive Composite Index (R = .32, p < .05). People who had previously consumed greater daily quantities of alcohol and who were older tended to have weaker performance. Notably, current heavy use was not retained as a significant factor in the regression analysis.
Lifetime AUD was associated with performance across most individual cognitive domains as well. Quantity of alcohol consumed and age were negatively associated with performance in the Speed of Processing (R = .32, p < .05) and the Attention/Executive (R = .35, p < .05) domains. With respect to learning and memory, quantity of alcohol consumed (β = −.23) and age (β = −.24) were negatively associated with Learning (R = .32, p < .05). However, performance in the Memory domain was only associated with age (R = .26, p < .05). For these four cognitive domains, current heavy alcohol use was not retained as a significant factor in the regression analysis. Neither age nor current or lifetime AUD indices were significantly associated with performance in the Verbal or Motor domains (p > .05).
HIV status and Alcohol Use.
Overall cognitive functioning on the Global Cognitive Composite Index was significantly associated (R = .34, p < .05) with age (β = −.25) and duration of heaviest past alcohol use (β = −.26), but not quantity of alcohol consumed during this period or other clinical factors related to HIV. People with a longer duration of lifetime heavy alcohol use and older age had weaker overall cognitive performance.
Quantity of alcohol consumed and age were negatively associated with Speed of Processing (R = .34, p < .05) and performance in the Attention/Executive domain (R = .31, p < .05). Duration of lifetime heavy alcohol use (β = −.25), along with HCV status (β = −.29) and current CD4 level (β = −.21) were retained as factors significantly associated with Learning (R = .38, p < .05). Duration of lifetime heavy alcohol use (β = −.26) and age (β = −.30) were negatively associated with Memory performance (R = .37, p < .05). For both Learning and Memory, a longer duration of alcohol use corresponded with weaker performance. Lower current CD4 and a history of HCV co-infection were also associated with weaker learning performance, while older age was also associated with weaker memory performance. Again current heavy alcohol use was not retained as a significant factor in the regression analysis.
Notably, the inclusion of the HIV clinical variables in the analysis resulted in a significant association of these clinical variables with performance in the Verbal Domain (R = .45, p < .05). Specifically, current CD4 was the factor most strongly associated with verbal performance (β = −.47), though detectable HIV RNA viral load (β = −.25), and the lifetime quantity of alcohol consumed (β = −.22) were also retained in the model as factors associated with weaker verbal performance. None of the HIV or alcohol use factors were significantly associated with Motor performance, nor was current heavy alcohol use retained as a significant factor in any of these regression models.
Restricted VACS Index, Alcohol Use, and Cognitive Performance.
There was no significant bivariate relationship between the restricted VACS index (calculated with Age, CD4, HIV-RNA)(Tate et al., 2013) and any of the substance use variables or performance variables (p > .05). Backward linear regressions performed with each cognitive performance domain as the dependent variable were repeated with the restricted VACS index as an independent factor in place of its consistuent parts, as performed in other analyses herein. Results indicate that independent variables associated with the dependent cognitive performance were identical to that of the analyses described in Table 4, suggesting the restricted VACS index did not provide additional utility over the use of its individual components.
Table 4.
Regression Results for Lifetime AUD and Cognitive Performance (N=104)
| R | R2 | β | β standardized | 95% CI (β) | 95% CI (β) | p-val | |
|---|---|---|---|---|---|---|---|
| Global Cognition Index | 0.34 | 0.11 | .02 | ||||
| Age | −0.25 | −0.25 | −0.49 | −0.01 | .04 | ||
| Duration of Drinking | −3.07 | −0.26 | −5.95 | −0.19 | .03 | ||
| Speed of Processing | 0.34 | 0.11 | .02 | ||||
| Age | −0.26 | −0.25 | −0.52 | −0.01 | .04 | ||
| Quantity of Drinks | −2.05 | −0.25 | −4.04 | −0.06 | .04 | ||
| Attention/Exec. Function | 0.31 | 0.10 | .04 | ||||
| Age | −0.24 | −0.25 | −0.48 | −0.01 | .04 | ||
| Quantity of Drinks | −1.60 | −0.21 | −3.44 | 0.25 | .08 | ||
| Learning | 0.38 | 0.15 | .02 | ||||
| Duration of Drinking | −4.14 | −0.25 | −8.05 | −0.23 | .04 | ||
| HCV Status | −7.96 | −0.29 | −14.74 | −1.18 | .02 | ||
| CD4 Level | −0.01 | −0.21 | −0.02 | 0.01 | .09 | ||
| Memory | 0.37 | 0.14 | .01 | ||||
| Age | −0.42 | −0.30 | −0.76 | −0.08 | .01 | ||
| Duration of Drinking | −4.35 | −0.26 | −8.40 | −0.30 | .04 | ||
| Verbal | 0.45 | 0.21 | .004 | ||||
| CD4 Level | −0.01 | −0.47 | −0.02 | 0.00 | .001 | ||
| HIV RNA Viral Load | −4.32 | −0.25 | −8.83 | 0.19 | .06 | ||
| Quantity of Drinks | −1.46 | −0.22 | −2.96 | 0.04 | .05 |
Note: Only variables retained in the backward regression models are shown; Quantity of Drinks = number of drinks in one sitting during period of heaviest drinking; Duration of Drinking = Duration of heaviest drinking pattern (days); HCV = Hepatitis-C virus, lifetime; CD4 = current level (mg/L)
Binge Drinking.
To examine the effects of binge drinking, a MANOVA was conducted comparing participants who reported binge drinking greater than one time per week with those who did not. A significant between-group difference in overall cognitive performance was found (F (6, 74) = 2.8, p < .05). Univariate comparisons indicated between group differences (p < .05) in Attention-Executive (F (6, 74) = 3.1, p < .05) and Motor (F (6, 74) = 9.7, p < .05) performance as a function of drinking status. Binge drinking was associated with weaker performance for both domains.
Alcohol and other drug use.
In the final set of analyses, the KMSK indices obtained to assess lifetime cocaine and opiate use were examined relative to the alcohol and HIV measures. Heavy lifetime cocaine and/or cocaine use was significantly associated with performance in the Learning (R = .45, p < .05), Memory (R = .51, p < .05), and Verbal (R = .44, p < .05) domains. The regression model indicated that for Learning, opiate use severity (β = −.24) was retained as a significant factor, along with age (β = −.26) and duration of heaviest lifetime alcohol use (β = −.28). For Memory, opiate use severity (β = −.20) was retained as a significant factor, along with age (β = −.29), duration of heaviest lifetime alcohol use (β = −.29), and viral load (β = −.21). For Verbal , lifetime cocaine use severity (β = −.24) along with current CD4 and viral load (β = −.40) were retained as significant factors. Motor functioning was not significantly associated with lifetime alcohol, cocaine, or opiate use.
DISCUSSION.
Results detail the relationships between heavy alcohol consumption, age, and cognitive functioning in context of HIV infection. HIV+ adults who were currently heavy drinkers had poorer performance than HIV+ adults who were not or had never been heavy drinkers. Older HIV+ adults exhibited weaker cognitive performance than younger HIV+ adults, even after accounting for age in the standardized T-scores that were treated as dependent measures. Findings indicate that both heavy alcohol use and aging are risk factors associated with cognitive deficits in this cohort. Interactions between age and alcohol use groupings were not observed suggesting that these two factors may exert independent influences on cognitive function in the context of HIV.
Study findings indicate that cognitive deficits were evident as a function of both current heavy alcohol use and lifetime AUD, yetlifetime heavy AUD was consistently and more strongly associated with poorer cognitive performance. In fact, in most analyses lifetime heavy alcohol consumption was retained as a significant factor, whereas current heavy consumption was not. This suggests that while differences in cognitive performance occur based on current heavy alcohol use, history of heavy drinking cannot be ignored. This finding suggests that some adverse effects of past heavy consumption may persist and manifest as cognitive deficits in HIV+ adults who no longer consume large amounts of alcohol.
Our work and that of others’ has shown a number of cognitive domains to be affected by HIV and also in AUD (Patel et al., 2014, Cohen et al., 2001, Sacktor et al., 2002, Seider et al., 2014, Heaton et al., 2010), and we previously observed an interaction of age by current heavy alcohol use in a sample of adults who were not HIV-infected (Woods et al., 2016). The present study expands upon this work to indicate that current heavy alcohol use had an impact on attention/executive functions, processing speed, learning, and delayed recall (memory retrieval). Age and heavy alcohol use exerted independent influences on cognitive performance, although statistically significant interactions of alcohol use and age were not found. We found that HIV+ older adults who were currently heavy drinkers had overall cognitive functioning deficits compared to those who were not heavy drinkers. We have also demonstrated through our previous work that there exists an age-associated decline in verbal list-learning performance in HIV+ adults occurring in the fifth decade, with similar decline not evident in adults without HIV (Seider et al., 2016, Seider et al., 2014). The present study extends these findings in that not only was older age associated with weaker cognitive performance, but age effects were evident even with the use of age-adjusted test scores. This finding indicates that older HIV+ adults exhibit greater than expected age-associated reductions in cognitive function in a sample with a mean age of 47 years.
The fact that there was not a significant association between the restricted VACS index (Tate et al., 2013) and the cognitive measures was somewhat unexpected given previous findings using this index (Marquine et al., 2016, 2014). Althoughrestricted VACS has been validated and is highly correlated with the standard VACS index (c-statistic = .722), this study, several liver function (AST, ALT, FIB4), bone marrow function (hemoglobin), and renal function (eGFR) were not available for analysis. It is possible that these laboratory measures, , would have strengthened the relationship between the VACS index and the cognitive performance variables given that they have direct relevance to AUD and also HCV. Further, it is possible that heavy alcohol use, whether current or lifetime, causes other systemic abnormalities. Possible abnormalities include alterations in liver and gut function, such that the observed alcohol effects are meta-phenomena rather than a direct effect of alcohol. That we were unable to derive the full VACS index makes it difficult to know the contribution of liver and renal dysfunction severity to cognitive performance. Thus, the effects of AUD among those not currently drinking could porentially represent end-stage health complications (i.e. sick-quitters), and this possibility may have been resolved with consideration of the full VACS index.
A limitation of this study is that it was not possible to examine the influence of all possible co-morbidities on cognition given the statistical power that could be achieved with our sample. A common comorbidity in this population is other substance use, such as opioid and cocaine use. We excluded individuals with any other SUD , so as not to confound the effects, which may contribute to a selection bias among individuals chosen for the analyses. An early study suggested an interaction of marijuana use and HIV infection resulting in greater memory impairment (Cristiani et al., 2004). However, marijuana use was not examined in this study given that more recent epidemiological analysis of the MACS cohort (N=5,914) demonstrated no significant association between cumulative exposure to marijuana and cognitive function (Okafor, 2016).
While the large majority of individuals reported having an undetectable viral load, it is possible that the level of CNS penetration of ARV medication influenced cognitive outcome. Along these lines, analysis indicated there was not a sufficient frequency of participants taking other prescription medications to include this variable as a covariate in planned analyses. Bivariate correlation indicated the use of additional prescription medications was not a significant predictor of cognitive performance. Another factor that may have influenced the results is the possibility that individuals with the greatest disease burden may have a tendency to reduce their drinking in response to medication and disease effect (the so-called, sick-quitter phenomenon). Those who were heavier alcohol users may have been more likely to have poor adherence to cART. Yet, cART was not among the factors associated with cognitive performance, and the vast majority of participants (97%) were being actively treated with cART. Nonetheless, it is possible that reduced adherence to cART due to heavy drinking occurred in the earlier stages of disease which may have an influence on cognitive performance later in life. The influence of sick-quitters, treatment adherence early on in the disease, and prescription medications should be considered in future studies with larger samples.
While the results provide evidence of acquired cognitive deficits, determining how rates of decline change in the context of AUD and HIV requires research utilizing longitudinal assessment and inclusion of a control population of similar demographic and experiential background
Conclusions.
Current heavy alcohol use and a history of heavy drinking are associated with cognitive deficits in the context of HIV, and individuals exhibiting both a history of and current heavy alcohol use were most affected. Effects were evident even after accounting for various HIV-related clinical factors and past opiate and cocaine use. The fact that lifetime heavy alcohol use was a stronger correlate of cognitive function than was current heavy alcohol use, unfortunately suggests some detrimental impact of heavy drinking earlier in life may persist. Yet, the results also suggest that individuals with both current and lifetime heavy drinking had the poorest cognitive performance. Therefore, reducing ongoing alcohol consumption is likely to be beneficial. Our findings also provide evidence that HIV+ adults exhibit cognitive deficits in excess of what is expected relative to age-appropriate normative comparisons, emphazing the need for future studies to further delineate the effects of HIV and alcohol in the aging brain.
Figure 1.
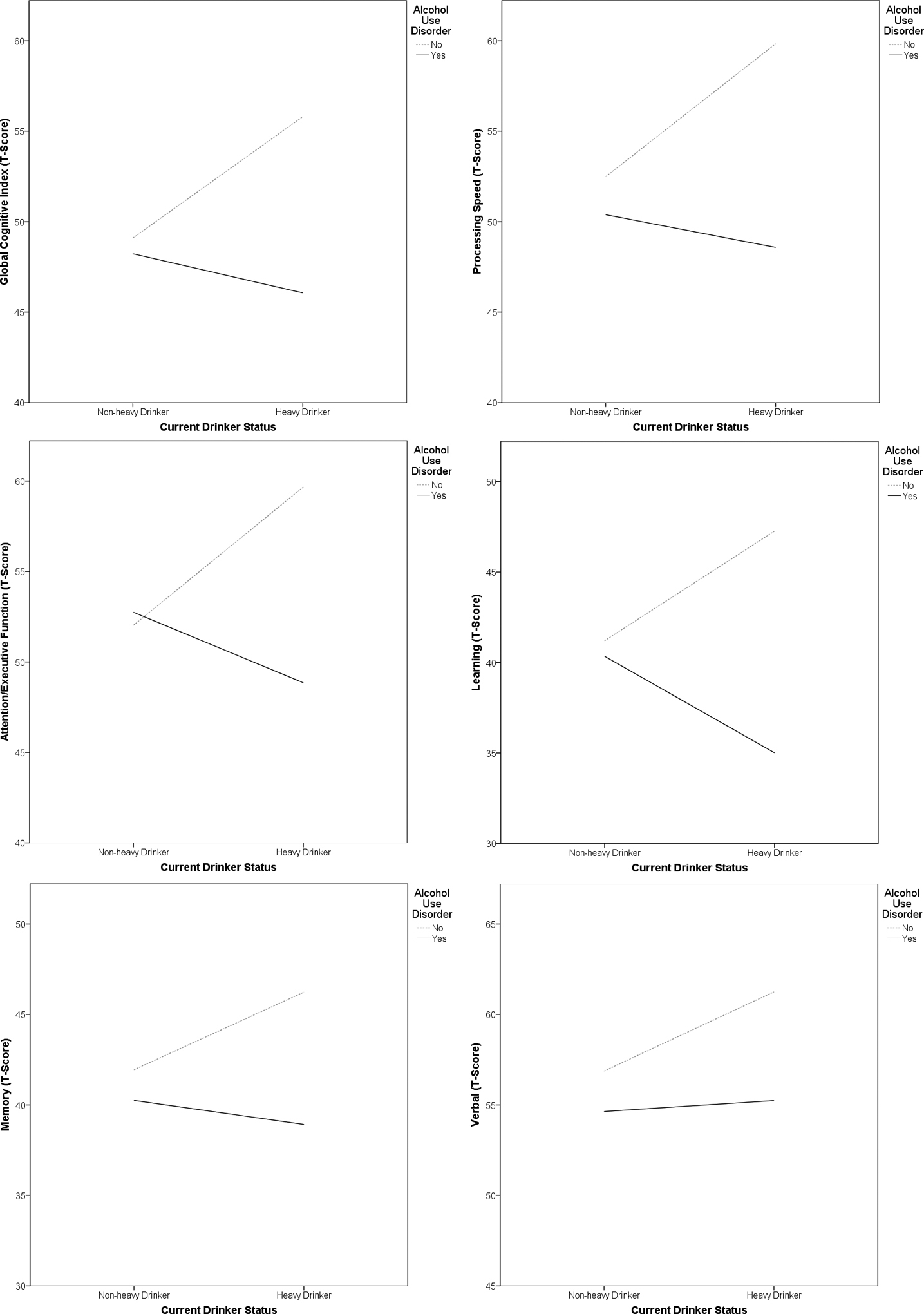
Associations between drinker status and cognitive performance by history of Alcohol Use Disorder (N = 104)
Funding Support:
The research was supported in part from funding provided by the National Institutes of Alcohol Abuse and Alcoholism (NIAAA P01AA019072, T32AA025877), the McKnight Brain Research Foundation, and the UF Center for Cognitive Aging and Memory: Clinical Translational Research.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Reference List
- BECKER JT, LOPEZ OL, DEW MA & AIZENSTEIN HJ 2004. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS, 18 Suppl 1, S11–8. [PubMed] [Google Scholar]
- BLADOWSKA J, ZIMNY A, KNYSZ B, MALYSZCZAK K, KOLTOWSKA A, SZEWCZYK P, GASIOROWSKI J, FURDAL M & SASIADEK MJ 2013. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: a pilot study. J Hepatol, 59, 651–7. [DOI] [PubMed] [Google Scholar]
- CARDENAS VA, STUDHOLME C, MEYERHOFF DJ, SONG E & WEINER MW 2005. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res, 138, 115–30. [DOI] [PubMed] [Google Scholar]
- CHANG L, LEE PL, YIANNOUTSOS CT, ERNST T, MARRA CM, RICHARDS T, KOLSON D, SCHIFITTO G, JARVIK JG, MILLER EN, LENKINSKI R, GONZALEZ G, NAVIA BA & CONSORTIUM HM 2004. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage, 23, 1336–47. [DOI] [PubMed] [Google Scholar]
- COHEN RA, BOLAND R, PAUL R, TASHIMA KT, SCHOENBAUM EE, CELENTANO DD, SCHUMAN P, SMITH DK & CARPENTER CC 2001. Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS, 15, 341–5. [DOI] [PubMed] [Google Scholar]
- CONCHA M, GRAHAM NM, MUNOZ A, VLAHOV D, ROYAL W 3RD, UPDIKE M, NANCE-SPROSON T, SELNES OA & MCARTHUR JC 1992. Effect of chronic substance abuse on the neuropsychological performance of intravenous drug users with a high prevalence of HIV-1 seropositivity. Am J Epidemiol, 136, 1338–48. [DOI] [PubMed] [Google Scholar]
- CONIGLIARO J, MADENWALD T, BRYANT K, BRAITHWAITE S, GORDON A, FULTZ SL, MAISTO S, SAMET J, KRAEMER K, COOK R, DAY N, ROACH D, RICHEY S & JUSTICE A 2004. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res, 28, 313–21. [DOI] [PubMed] [Google Scholar]
- CRISTIANI SA, PUKAY-MARTIN ND, BORNSTEIN RA, 2004. Marijuana Use and Cognitive Function in HIV-Infected People. J. Neuropsychiatry Clin. Neurosci, 16(3), 330–335. [DOI] [PubMed] [Google Scholar]
- DOWNER B, JIANG Y, ZANJANI F & FARDO D 2015. Effects of alcohol consumption on cognition and regional brain volumes among older adults. Am J Alzheimers Dis Other Demen, 30, 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE AI, BUTTERS N, SHEAR PK, SMITH TL, BONDI M, IRWIN M & SCHUCKIT MA 1995. Cognitive recovery with abstinence and its relationship to family history for alcoholism. J Stud Alcohol, 56, 104–9. [DOI] [PubMed] [Google Scholar]
- DURAZZO TC, MEYERHOFF DJ & NIXON SJ 2013a. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depend, 133, 704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURAZZO TC, PENNINGTON DL, SCHMIDT TP, MON A, ABE C & MEYERHOFF DJ 2013b. Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcohol Clin Exp Res, 37, 1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURAZZO TC, ROTHLIND JC, CARDENAS VA, STUDHOLME C, WEINER MW & MEYERHOFF DJ 2007. Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology. Alcohol, 41, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALS-STEWART W, O’FARRELL TJ, FREITAS TT, MCFARLIN SK & RUTIGLIANO P 2000. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol, 68, 134–44. [DOI] [PubMed] [Google Scholar]
- FAMA R, ROSENBLOOM MJ, SASSOON SA, THOMPSON MA, PFEFFERBAUM A & SULLIVAN EV 2011. Remote semantic memory for public figures in HIV infection, alcoholism, and their comorbidity. Alcohol Clin Exp Res, 35, 265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONGVATANA A, MORGAN EE, IUDICELLO JE, LETENDRE SL, GRANT I, WOODS SP & GROUP, H. I. V. N. R. P. 2014b. A history of alcohol dependence augments HIV-associated neurocognitive deficits in persons aged 60 and older. J Neurovirol, 20, 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN JE, SAVEANU RV & BORNSTEIN RA 2004. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry, 161, 249–54. [DOI] [PubMed] [Google Scholar]
- GRIFFIN WC 3RD, MIDDAUGH LD, COOK JE & TYOR WR 2004. The severe combined immunodeficient (SCID) mouse model of human immunodeficiency virus encephalitis: deficits in cognitive function. J Neurovirol, 10, 109–15. [DOI] [PubMed] [Google Scholar]
- GRIFFIN WC 3RD, MIDDAUGH LD & TYOR WR 2007. Chronic cocaine exposure in the SCID mouse model of HIV encephalitis. Brain Res, 1134, 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLETT JM, LAMB DG, PORGES E, WOODS AJ, RIEKE J, THOMPSON P, JAHANSHAD N, NIR TM, TASHIMA K, COHEN RA 2018. The Impact of Alcohol Use on Frontal White Matter in HIV. Alcohol. Clin. Exp. Res 42(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNA DB, BUCHACZ K, GEBO KA, HESSOL NA, HORBERG MA, JACOBSON LP, KIRK GD, KITAHATA MM, KORTHUIS PT, MOORE RD, NAPRAVNIK S, PATEL P, SILVERBERG MJ, STERLING TR, WILLIG JH, LAU B, ALTHOFF KN, CRANE HM, COLLIER AC, SAMJI H, THORNE JE, GILL MJ, KLEIN MB, MARTIN JN, RODRIGUEZ B, ROURKE SB, GANGE SJ, NORTH AMERICAN, A. C. C. O. R. & DESIGN OF THE INTERNATIONAL EPIDEMIOLOGIC DATABASES TO EVALUATE, A. 2013. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis, 56, 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATON R, MILLER SW, TAYLOR MJ, GRANT I 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults, Lutz, FL, Psychological Assessment Resources. [Google Scholar]
- HEATON RK, CLIFFORD DB, FRANKLIN DR JR., WOODS SP, AKE C, VAIDA F, ELLIS RJ, LETENDRE SL, MARCOTTE TD, ATKINSON JH, RIVERA-MINDT M, VIGIL OR, TAYLOR MJ, COLLIER AC, MARRA CM, GELMAN BB, MCARTHUR JC, MORGELLO S, SIMPSON DM, MCCUTCHAN JA, ABRAMSON I, GAMST A, FENNEMA-NOTESTINE C, JERNIGAN TL, WONG J, GRANT I & GROUP, C. 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75, 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKIN CH, HARDY DJ, MASON KI, CASTELLON SA, DURVASULA RS, LAM MN & STEFANIAK M 2004. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS, 18 Suppl 1, S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSTON RJ, DERRICK JL, LEONARD KE, TESTA M, QUIGLEY BM & KUBIAK A 2014. Effects of heavy drinking on executive cognitive functioning in a community sample. Addict Behav, 39, 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB T, BLONIGEN DM, UPAH R & JUSTICE A 2013. Lifetime drinking trajectories among veterans in treatment for HIV. Alcohol Clin Exp Res, 37, 1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAEGER LB & NATH A 2012. Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech, 5, 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUSTICE AC, MCGINNIS KA, ATKINSON JH, HEATON RK, YOUNG C, SADEK J, MADENWALD T, BECKER JT, CONIGLIARO J, BROWN ST, RIMLAND D, CRYSTAL S, SIMBERKOFF M & VETERANS AGING COHORT 5-SITE STUDY PROJECT, T. 2004. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS, 18 Suppl 1, S49–59. [PubMed] [Google Scholar]
- JUSTICE AC, MCGINNIS KA, SKANDERSON M, CHANG CC, GIBERT CL, GOETZ MB, RIMLAND D, RODRIGUEZ-BARRADAS MC, OURSLER KK, BROWN ST, BRAITHWAITE RS, MAY M, COVINSKY KE, ROBERTS MS, FULTZ SL, BRYANT KJ & TEAM VP 2010. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med, 11, 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG SH, MCHUGH PF, BELL K, SCHLUGER JH, SCHLUGER RP, LAFORGE KS, HO A & KREEK MJ 2003. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend, 69, 137–50. [DOI] [PubMed] [Google Scholar]
- KIST N, SANDJOJO J, KOK RM & VAN DEN BERG JF 2014. Cognitive functioning in older adults with early, late, and very late onset alcohol dependence. Int Psychogeriatr, 26, 1863–9. [DOI] [PubMed] [Google Scholar]
- KLOVE H 1963. Clinical Neuropsychology. Med Clin North Am, 47, 1647–58. [PubMed] [Google Scholar]
- KORTHUIS PT, FIELLIN DA, MCGINNIS KA, SKANDERSON M, JUSTICE AC, GORDON AJ, DOEBLER DA, ASCH SM, FIELLIN LE, BRYANT K, GIBERT CL, CRYSTAL S, GOETZ MB, RIMLAND D, RODRIGUEZ-BARRADAS MC & KRAEMER KL 2012. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr, 61, 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR R, PEREZ-CASANOVA AE, TIRADO G, NOEL RJ, TORRES C, RODRIGUEZ I, MARTINEZ M, STAPRANS S, KRAISELBURD E, YAMAMURA Y, HIGLEY JD & KUMAR A 2005. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr, 39, 386–90. [DOI] [PubMed] [Google Scholar]
- LEWIS-DE LOS ANGELES CP, WILLIAMS PL, HUO Y, WANG SD, UBAN KA, HERTING MM, MALEE K, YOGEV R, CSERNANSKY JG, NICHOLS S, VAN DYKE RB, SOWELL ER, WANG L, PEDIATRIC, H. I. V. A. C. S., THE PEDIATRIC IMAGING, N. & GENETICS, S. 2017. Lower total and regional grey matter brain volumes in youth with perinatally-acquired HIV infection: Associations with HIV disease severity, substance use, and cognition. Brain Behav Immun, 62, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOFTIS JM, TAYLOR J, RAUE HP, SLIFKA MK & HUANG E 2016. Alcohol intake alters immune responses and promotes CNS viral persistence in mice. Behav Brain Res, 312, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCONDES MC, WATRY D, ZANDONATTI M, FLYNN C, TAFFE MA & FOX H 2008. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res, 32, 1583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARQUINE MJ, MONTOYA JL, UMLAUF A, FAZELI PL, GOUAUX B, HEATON RK, ELLIS RJ, LETENDRE SL, GRANT I, MOORE DJ & GROUP, H. I. V. N. R. P. 2016. The Veterans Aging Cohort Study (VACS) Index and Neurocognitive Change: A Longitudinal Study. Clin Infect Dis, 63, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYERHOFF DJ, MACKAY S, SAPPEY-MARINIER D, DEICKEN R, CALABRESE G, DILLON WP, WEINER MW & FEIN G 1995. Effects of chronic alcohol abuse and HIV infection on brain phosphorus metabolites. Alcohol Clin Exp Res, 19, 685–92. [DOI] [PubMed] [Google Scholar]
- MONNIG MA, KAHLER CW, LEE H, PANTALONE DW, MAYER KH, COHEN RA & MONTI PM 2016. Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men. AIDS Care, 28, 300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON JA, DOU H, ELLISON B, UBERTI M, XIONG H, ANDERSON E, MELLON M, GELBARD HA, BOSKA M & GENDELMAN HE 2005. Coregistration of quantitative proton magnetic resonance spectroscopic imaging with neuropathological and neurophysiological analyses defines the extent of neuronal impairments in murine human immunodeficiency virus type-1 encephalitis. J Neurosci Res, 80, 562–75. [DOI] [PubMed] [Google Scholar]
- OKAFOR CN, 2016. Marijuana Use among HIV-Positive and HIV-Negative Men Who Have Sex with Men : Long Term Trends, Predictors of Use and Impact on Cognitive Function. University of Florida. [Google Scholar]
- PATEL K, WANG J, JACOBSON DL, LIPSHULTZ SE, LANDY DC, GEFFNER ME, DIMEGLIO LA, SEAGE GR 3RD, WILLIAMS PL, VAN DYKE RB, SIBERRY GK, SHEARER WT, YOUNG L, SCOTT GB, WILKINSON JD, FISHER SD, STARC TJ & MILLER TL 2014. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation, 129, 1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSIDSKY Y, LIMOGES J, RASMUSSEN J, ZHENG J, GEARING A & GENDELMAN HE 2001. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol, 114, 57–68. [DOI] [PubMed] [Google Scholar]
- PFEFFERBAUM A, ADALSTEINSSON E & SULLIVAN EV 2005. Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology, 30, 1392–9. [DOI] [PubMed] [Google Scholar]
- PFEFFERBAUM A, ROSENBLOOM M & SULLIVAN EV 2002. Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol Clin Exp Res, 26, 1031–46. [DOI] [PubMed] [Google Scholar]
- PFEFFERBAUM A, ROSENBLOOM MJ, ADALSTEINSSON E & SULLIVAN EV 2007. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain, 130, 48–64. [DOI] [PubMed] [Google Scholar]
- POTULA R, HAORAH J, KNIPE B, LEIBHART J, CHRASTIL J, HEILMAN D, DOU H, REDDY R, GHORPADE A & PERSIDSKY Y 2006. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. Am J Pathol, 168, 1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBLOOM MJ, SULLIVAN EV & PFEFFERBAUM A 2010. Focus on the brain: HIV infection and alcoholism: comorbidity effects on brain structure and function. Alcohol Res Health, 33, 247–57. [PMC free article] [PubMed] [Google Scholar]
- SACKTOR N, MCDERMOTT MP, MARDER K, SCHIFITTO G, SELNES OA, MCARTHUR JC, STERN Y, ALBERT S, PALUMBO D, KIEBURTZ K, DE MARCAIDA JA, COHEN B & EPSTEIN L 2002. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol, 8, 136–42. [DOI] [PubMed] [Google Scholar]
- SEIDER TR, GONGVATANA A, WOODS AJ, CHEN H, PORGES EC, CUMMINGS T, CORREIA S, TASHIMA K & COHEN RA 2016. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol, 22, 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDER TR, LUO X, GONGVATANA A, DEVLIN KN, DE LA MONTE SM, CHASMAN JD, YAN P, TASHIMA KT, NAVIA B & COHEN RA 2014. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol, 36, 356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN EV, ROSENBLOOM MJ, ROHLFING T, KEMPER CA, DERESINSKI S & PFEFFERBAUM A 2011. Pontocerebellar contribution to postural instability and psychomotor slowing in HIV infection without dementia. Brain Imaging Behav, 5, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATE JP, JUSTICE AC, HUGHES MD, BONNET F, REISS P, MOCROFT A, NATTERMANN J, LAMPE FC, BUCHER HC, STERLING TR, CRANE HM, KITAHATA MM, MAY M, STERNE JAC 2013. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS, 27(4), 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYBOH K, JENABIAN MA, MEHRAJ V & ROUTY JP 2015. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res, 2015, 614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER BM & KOOB GF 2008. Regarding “Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake”. FASEB J, 22, 2113; author reply 2113–4. [DOI] [PubMed] [Google Scholar]
- WEYERER S, SCHAUFELE M, WIESE B, MAIER W, TEBARTH F, VAN DEN BUSSCHE H, PENTZEK M, BICKEL H, LUPPA M, RIEDEL-HELLER SG & GERMAN AGECODE STUDY, G. 2011. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing, 40, 456–63. [DOI] [PubMed] [Google Scholar]
- WOODS AJ, PORGES EC, BRYANT VE, SEIDER T, GONGVATANA A, KAHLER CW, DE LA MONTE S, MONTI PM & COHEN RA 2016. Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcohol Clin Exp Res, 40, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKHARI S, HERALD DR, GENTRY T, RADAEVA S, WANG J, HUNG MK, GUO M, GAO P. 2009. Gut-Liver-Brain Interactions in Alcohol-Induced Pathogenesis: NIAAA Briefing Book. In: EFFECTS, D. O. M. A. H. (ed.). Bethesda, MD: National Institute of Alcohol Abuse and Alcoholism. [Google Scholar]


