Abstract
Germline heterozygous mutations in Pten (phosphatase and tensin homolog) are associated with macrocephaly and autism spectrum disorders (ASD). Pten germline heterozygous (Pten+/−) mice approximate these mutations, and both sexes show widespread brain overgrowth and impaired social behavior. Strikingly similar behavior phenotypes have been reported in oxytocin (Oxt) and/or oxytocin receptor (OxtR) knockout mice. Thus, we hypothesized that the behavioral phenotypes of germline Pten+/− mice may be caused by reduced Pten function in Oxt-expressing cells. To investigate this, we tested mice in which Pten was conditionally deleted using oxytocin-Cre (Oxt-Cre+; PtenloxP/+, Oxt-Cre+; PtenloxP/loxP) on a battery including assays of social, repetitive, depression-like, and anxiety-like behaviors. Minimal behavioral abnormalities were found; decreased anxiety-like behavior in the open field test in Oxt-Cre+; PtenloxP/loxP males was the only result that phenocopied germline Pten+/− mice. However, Oxt cell size was dramatically increased in Oxt-Cre+; PtenloxP/loxP mice in adulthood. Thus, conditional deletion of Pten using Oxt-Cre has a profound effect on Oxt cell structure, but not on ASD-relevant behavior. We interpret these results as inconsistent with our starting hypothesis that reduced Pten function in Oxt-expressing cells causes the behavioral deficits observed in germline Pten+/− mice.
Keywords: phosphatase and tensin homolog, autism spectrum disorder, oxytocin, social behavior, anxiety-like behavior, hypertrophy
Introduction
Deficits in social behavior and communication are one of the hallmarks of autism spectrum disorder (ASD), along with restricted, repetitive interests and behavior patterns [American Psychiatric Association, 2013; World Health Organization, 1992]. ASD is a neurodevelopmental disorder with high sexual dimorphism (~80% male) present in more than 1% of the population [Perou et al., 2013]. Up to 20% of the subset of individuals with ASD and macrocephaly (head circumference >2 standard deviations above the mean) have mutations in the gene Pten (phosphatase and tensin homolog) [Butler et al., 2005; Buxbaum et al., 2007; Klein, Sharifi-Hannauer, & Martinez-Agosto, 2013; McBride et al., 2010], which encodes a negative regulator of the PI3K–Akt–mTOR pathway [Sulis & Parsons, 2003]. Pten haploinsufficiency results in Pten hamartoma tumor syndromes, involving benign tumor-like malformations and brain overgrowth, which are often comorbid with ASD [Butler et al., 2005; Buxbaum et al., 2007; Matson & Shoemaker, 2009; Mester, Tilot, Rybicki, Frazier, & Eng, 2011]. The genomic lesions found in individuals with ASD and macrocephaly associated with germline heterozygous Pten mutations, which are generally missense mutations that reduce protein levels [Frazier et al., 2015], are approximated in Pten germline heterozygous (Pten+/−) mice. In addition to widespread brain overgrowth [Chen, Huang, Sejourne, Clipperton-Allen, & Page, 2015; Clipperton-Allen & Page, 2014; Page, Kuti, Prestia, & Sur, 2009a], these mice show social behavioral deficits and several sex-specific behavioral phenotypes related to ASD and comorbid disorders, including repetitive behavior, aggression, mood and anxiety phenotypes in males [Clipperton-Allen & Page, 2014, 2015; Page et al., 2009a; Sejourne, Llaneza, Kuti, & Page, 2015].
There are some striking similarities between the observed behavioral phenotypes of germline Pten+/− mice and those of oxytocin (Oxt) and/or oxytocin receptor (OxtR) knockout (KO) mice. Specifically, germline Pten+/−, OxtKO, and OxtRKO mice all show deficits in social recognition [Clipperton-Allen & Page, 2014; Ferguson et al., 2000; Ferguson, Aldag, Insel, & Young, 2001; Lee, Caldwell, Macbeth, Tolu, & Young, 2008; Macbeth, Lee, Edds, & Young, 2009; Page et al., 2009a; Takayanagi et al., 2005], and are less social than controls [Clipperton-Allen & Page, 2014, 2015; Lazzari et al., 2013; Pobbe et al., 2012a; Pobbe, Pearson, Blanchard, & Blanchard, 2012b]. Germline Pten+/− and OxtRKO mice fail to show a social preference in the three chamber social approach test [Amico, Mantella, Vollmer, & Li, 2004; Clipperton-Allen & Page, 2014; Page et al., 2009a; Pobbe et al., 2012a, 2012b; Sala et al., 2011, 2013; Sejourne et al., 2015], while OxtKO and OxtHT mice show normal social interest in this task [Crawley et al., 2007]. Germline Pten+/− and OxtKO mice both show increased repetitive behavior [Clipperton-Allen & Page, 2014, 2015; Lazzari et al., 2013], as well as decreased anxiety [Clipper-ton-Allen & Page, 2014; Mantella et al., 2003; Sala et al., 2011, 2013; Winslow et al., 2000]. Additionally, in a free social interaction or resident-intruder test, decreased aggression is observed in germline Pten+/− males [Clipper-ton-Allen & Page, 2015] and in OxtKO males born to Oxt heterozygous (OxtHT) females [Lazzari et al., 2013; Nishimori et al., 2008; Takayanagi et al., 2005], while OxtRKO males, and OxtKO males born to OxtKO females, show increased aggression [Dhakar, Rich, Reno, Lee, & Caldwell, 2012; Sala et al., 2011; Takayanagi et al., 2005; Winslow et al., 2000].
On the basis of these results, we hypothesized that the behavioral phenotypes of germline Pten+/− mice may reflect a role of Pten in the development or function of Oxt-expressing cells. Thus, we mated mice expressing Cre recombinase under control of the oxytocin promotor (Oxt-Cre) [Wu et al., 2012] with a floxed Pten line [Lesche et al., 2002], resulting in mice with conditional homozygous (Oxt-Cre+; PtenloxP/loxP) or heterozygous (Oxt-Cre+; PtenloxP/+) Pten mutations in Oxt cells. These mice, and littermate controls, were tested on a battery of behavioral assays, chosen based on observed phenotypes in germline Pten+/− mice and those of OxtKO and OxtRKO mice in the literature. These included social (three-chamber social approach and social novelty, social recognition, social aspects of resident-intruder), repetitive (marble burying, digging measure in resident-intruder), anxiety-like (dark-light emergence, open field), depression-like (tail suspension test) and motor learning (rotarod) tests. Additionally, as Pten mutations are known to alter cell number and size [Backman et al., 2001; Chen et al., 2015; Fraser et al., 2004; Goberdhan, Paricio, Goodman, Mlodzik, & Wilson, 1999; Groszer et al., 2001; Kazdoba et al., 2012; Kwon et al., 2001], we assessed the number and size of Oxt cells in the paraventricular nucleus of the hypothalamus (PVN) of mutant mice in adulthood, and developmentally at post-natal day 7 (P7) and P14.
Materials and Methods
Subjects
All mouse lines used have been described previously. B6;129S-Oxttm+.+(cre)Dolsn/J (Oxt-Cre+, https://www.jax.org/strain/024234) [Wu et al., 2012] was generously donated by Drs. Bradford B. Lowell and David P. Olson. This line arrived on a mixed C57BL/6 × 129S background and was backcrossed to C57BL/6J mice for at least five generations in our facility prior to use. The relevant geno-types used in this study were generated by crossing this line with mice carrying B6.129S4-Ptentm+Hwu/J (PtenloxP, https://www.jax.org/strain/006440) [Lesche et al., 2002] and/or B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Ai14+/+, https://www.jax.org/strain/007914) [Madisen et al., 2010], both of which were obtained from the Jackson Laboratory, where they were backcrossed and maintained on congenic C57BL/6J backgrounds. PCR using genomic DNA isolated from tail or ear samples was used to confirm mouse genotypes. In all cases, littermate controls (Oxt-Cre+; Pten+/+ or Oxt-Cre−) were used for experiments.
Groups of three to five mice were housed on ventilated racks (Model No. MD75JU160MVPSHR, Allentown Inc., Allentown, NJ) in clear polyethylene cages (19.1 × 29.2 × 12.7 cm; Allentown Inc., Allentown, NJ) and provided with ¼″ corncob bedding, nestlets and food (Teklad Global 18% Protein Extruded Rodent Diet 2920X) and tap water ad libitum.
Behavioral Tests
At least 1 h prior to testing, mice were moved to a holding room in the behavior area. Automatic scoring of assays used the Ethovision XT video tracking system (Noldus, Leesburg, VA), and manual scoring was performed by a trained observer blind to sex and genotype. Unless specified, 70% ethanol (EtOH; Sigma–Aldrich, St. Louis, MO), 1% Micro-90 (International Products Corporation, Burlington, NJ) and/or quatricide (2oz/gallon; Pharmacal Research Laboratories, Inc., Waterbury, CT) were used to clean apparati between mice.
Experimental (Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP) and control (Oxt-Cre− and Oxt-Cre+; Pten+/+) mice of both sexes, unless specified, were tested in adulthood (P56–P148) during the dark (active) phase of a 12:12 h reversed light/dark cycle (lights on at 2100 h) under red light conditions, unless otherwise stated. Mice were run through a battery of behavioral tests in the following order: (1) dark-light emergence; (2) three-chamber social approach + social novelty; (3) marble burying; (4) social recognition; (5) tail suspension test; and (6) open field test. There were at least 3 days between tests. A subset of male mice also underwent the resident-intruder test 7 days after the open field test. Behavior assays are described below.
All research was conducted in accordance with National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care guidelines and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Three-chamber social approach 1 social novelty.
Mice were tested as previously described [Clipperton-Allen & Page, 2014; Page et al., 2009a, 2009b;] under white light conditions. Briefly, test mice were each given 5 min to acclimate to an acrylic arena with black walls and a white floor on each of two consecutive days. On the third day, mice were given 5 min acclimation, followed by 10 min social approach [choice between two clear acrylic tubes (20.25 cm tall, with twelve 14 mm diameter holes drilled in the bottom half of the tube), one containing a same-sex, novel conspecific (location counterbalanced across mice)] and 10 min social novelty (novel, same-sex conspecific placed in the previously empty tube) testing. Time spent in each chamber was automatically scored.
Social recognition.
The social recognition test was performed as described previously [Clipperton-Allen & Page, 2014]. Briefly, test mice were housed alone for 2 h in a home cage-like environment [Takayanagi et al., 2010], then presented with an acrylic tube with 12 holes near the bottom containing a same-sex, juvenile (P21–28) control mouse for 5 min. The first four exposures (habituation trials 1–4) were to the same mouse, whereas the fifth presentation (dishabituation trial) used a novel juvenile stimulus. The duration of investigation was scored manually, and mice spending less than 10 sec investigating the stimulus during the first exposure were removed from the analysis.
Resident-intruder test.
A subset of male mice (P70–P135) underwent this test following 7 days of isolation housing, as previously described [Clipperton-Allen & Page, 2015]. These males had not been housed with females since weaning, and had no sexual experience. Briefly, each resident had a group-housed WT male intruder placed into his home cage. Free social interactions were videotaped from above through clear acrylic lids for 15 min. Using The Observer XT 10 Video Analysis software (Noldus Information Technology, Leesburg, VA), an expert observer, blind to genotype, scored the videotaped interactions for 23 individual behaviors based on Grant and Mackintosh’s [1963] ethogram (see Table S1 in Supporting Information) [Clipperton-Allen & Page, 2015]. These behaviors, which focused on the resident (test) mouse, were also grouped into 10 categories to gain overall impressions of the duration, frequency, and latency of the mice’s behavior (see Table S2 in Supporting Information) [Clipperton-Allen & Page, 2015]. Because behavior can vary across the 15-min test, the social interaction was divided into three 5-min intervals, and the duration, frequency, and latency were analyzed both by 5 min bins and across the 15 min interaction.
Marble burying.
Mice were placed individually in a home-cage-like environment with 5 cm of ¼″ corncob bedding and 20 black marbles (14.3 mm in diameter) arranged in a 4 × 5 matrix, and left undisturbed for 30 min under white light conditions, as previously described [Clipperton-Allen & Page, 2014]. The number of marbles that were at least ⅔ buried at the end of the trial was counted [Thomas et al., 2009].
Dark-light emergence.
Mice were tested as previously described [Clipperton-Allen & Page, 2014]. Briefly, each mouse was placed into the dark chamber and allowed to explore for 5 min. Time spent in each compartment, number of crossings between chambers, and latency to enter the light compartment were manually scored from video.
Open field test.
Each mouse was placed in the center of the open field arena (43.8 × 43.8 × 32.8 cm) under approximately 240 lux for 5 min. Ethovision automatically recorded measures (time and number of entries) of center and thigmotaxis (occupying the corners and sides of the open field), velocity, and total distance moved.
Tail suspension test.
Mice spent 6 min suspended from a hook by medical tape attached approximately 2 cm from the tail tip, and immobility was recorded with Ethovision using a 7% immobility threshold.
Rotarod test.
Mice were placed on a 10.5 cm circumference rotating rod (ENV-577M, MedAssociates Inc., St. Albans, VT), and the rotation speed increased gradually from 4 to 40 rpm over 5 min. Mice received three trials, spaced at least 1 h apart, and their latency to fall, both over time and averaged across tests, was measured.
Neuroanatomical Assays
Immunohistochemistry.
Following transcardial per-fusion, mouse brains were fixed in 4% PFA overnight, incubated in 20% sucrose/PBS solution at 4°C for at least 24 h, then embedded in Tissue-Tek OCT compound (Sakura, Torrance, CA) and frozen at −80°C until sectioned. Twenty-five micrometer thick coronal sections were collected on Superfrost/Plus slides. Female adult (control, Oxt-Cre+; PtenloxP/+, and Oxt-Cre+; PtenloxP/loxP), P7 and P14 (control, Oxt-Cre+; PtenloxP/loxP) brain sections were immunostained with anti-Oxt (1:4000, Immunostar, Hudson, WI, catalog #20068), anti-phospho-S6 (1:2000, Cell Signaling Technology, Danvers, MA, catalog #4858), and/or anti-Pten (1:5000, Cell Signaling Technology, catalog #9556S), and AlexaFluor-488 or 2594 conjugated secondary antibodies (1:2000, Invitrogen, Carlsbad, CA), and then mounted with VectaShield Hard Set mounting media (Vector Laboratories, Burlingame, CA). Nuclear labeling used DAPI (Invitrogen, catalog #D3571) or DAPI-containing mounting media. Coronal sections from Oxt-Cre+; Pten+/+; Ai14+ (control) and Oxt-Cre+; PtenloxP/loxP; Ai14+ mice were mounted with VectaShield with DAPI. Images were obtained with an Olympus VS120 microscope and processed using the VSDESKTOP (Olympus) and ImageJ (http://imagej.nih.gov/ij/) software.
Oxytocin cell size and number analyses.
Using ImageJ, the medial PVN was delineated in plane-matched sections (Bregma −0.75 mm to −0.95 mm), and the size and mean grey value of Oxt signal in the PVN was measured. Within the region of interest of the PVN, Oxt+ cells were outlined, counted, and the size and mean grey value of Oxt signal for each cell were recorded. Cell density was calculated by dividing the number of Oxt+ cells by the PVN area.
Statistical Analysis
Three-chamber social approach and social novelty chamber preference, resident-intruder, marble burying, dark-light emergence, open field, tail suspension, and all neuroanatomical data were analyzed using oneway between-subjects analyses of variance (ANOVAs) for genotype. Social recognition [3 (genotype) × 5 (trial)] and rotarod [3 (genotype) × 3 (trial)] data were analyzed with mixed model ANOVAs. If trial × genotype interactions were found, within-subject ANOVAs (effect of trial on each genotype) and/or t-tests (effect of genotype on each trial) were used for post hoc comparisons. One-sample t-tests were used to determine if habituation, dishabituation, or dominance scores were significantly different from zero. Paired-sample t-tests were used for within-group comparisons of chamber time in the three-chamber social approach + social novelty (time in mouse chamber vs. time in empty chamber; time in novel mouse chamber vs. time in familiar mouse chamber) and dark-light emergence (time in light chamber vs. time in dark chamber) tests, as well as for center time in the open field test (time in center vs. time in thigmotaxis). Tukey’s corrected t-tests were used for post hoc analysis as appropriate.
Additionally, planned comparisons of mutants to control mice (control vs. Oxt-Cre+; PtenloxP/+, control vs. Oxt-Cre+; PtenloxP/loxP) were performed using independent-sample t-tests, as previously described [Clipperton-Allen & Page, 2014, 2015]. In the resident-intruder test, planned comparisons were only performed on behaviors across the whole 15 min trial unless one-way ANOVAs indicated a time bin-specific genotype effect.
For all statistical analyses, which were performed with PASW 18 (IBM Corporation, Armonk, NY), significance was set at P < 0.05, and statistics for nonsignificant results are not shown.
Results
Behavior Results: Tests Related to ASD Core Symptoms
Three-chamber social approach 1 social novelty.
Mice were tested on this assay because germline Pten+/−, OxtRKO, and OxtRHT mice all failed to show a signifi-cant social preference in this task [Amico et al., 2004; Clipperton-Allen & Page, 2014; Crawley et al., 2007; Page et al., 2009a; Pobbe et al., 2012b; Sala et al., 2011; Sala et al., 2013; Sejourne et al., 2015].
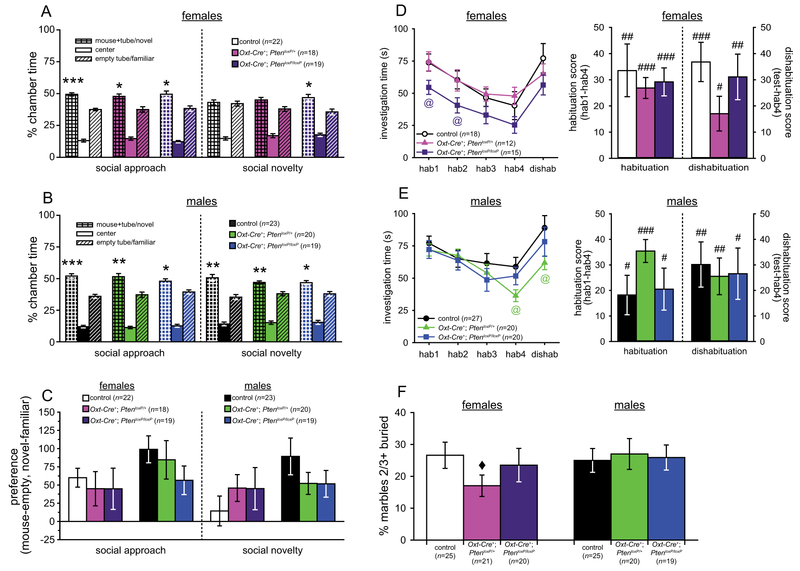
All genotypes in both sexes (females: control, n = 22; Oxt-Cre+; PtenloxP/+, n = 18; Oxt-Cre+; PtenloxP/loxP, n = 19; males: control, n = 23; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 19) spent significantly more time in the chamber containing the social stimulus during social approach (paired t-tests, all t > 2.46, all P < 0.025; Fig. 1a,b), and all groups except the control and Oxt-Cre+; PtenloxP/+ females spent significantly more time in the novel stimulus chamber during social novelty (paired t-tests, all t > 2.43, all P < 0.025; Fig. 1a,b). No significant genotype differences were found for chamber preference (mouse – object, or novel – familiar; Fig. 1c). Thus, unlike in germline Pten+/− mice [Clipperton-Allen & Page, 2014; Page et al. 2009a; Sejourne et al., 2015], no social approach deficits were found in any of the experimental mice.
Figure 1.
Results of social approach, social recognition, and repetitive behavior tests in Oxt-Cre+; PtenloxP mice. (A–C) Time spent in each chamber (A,B) and chamber preference (C) during the three-chamber social approach and social novelty in control (n = 22), Oxt-Cre+; PtenloxP/+ (n = 18), and Oxt-Cre+; PtenloxP/loxP (n = 19) female mice (A,C) and in control (n = 23), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 19) male mice (B,C). (D,E) Investigation of the stimulus mouse in the social recognition assay across trials, and habituation and dishabituation, in control (n = 18), Oxt-Cre+; PtenloxP/+ (n = 12), and Oxt-Cre+; PtenloxP/loxP (n = 15) female mice (D) and in control (n = 27), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 20) male mice (E). (F) Percentage of marbles at least ⅔ buried after 30 min by control (n = 25), Oxt-Cre+; PtenloxP/+ (n = 21), and Oxt-Cre+; PtenloxP/loxP (n = 20) females and control (n = 25), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 19) males. Significant difference between the mouse chamber and the object chamber or between the novel and familiar mouse chambers: *P < 0.05, **P < 0.01, ***P < 0.001. Significant difference between controls and Oxt-Cre+; PtenloxP/loxP females (D) or between controls and Oxt-Cre+; PtenloxP/+ males (E): @ P < 0.05. Significant habituation or dishabituation: #P < 0.05, ##P < 0.01, ###P < 0.001. Significant difference between controls and Oxt-Cre+; PtenloxP/+ females: ◆ P < 0.05.
Social recognition.
One of the best replicated phenotypes in OxtKO and OxtRKO mice is a social recognition deficit, which we also observed in germline Pten+/− male mice. Thus, we examined juvenile conspecific recognition in control, Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP mice.
One-sample t-tests showed that all groups (females: control, n = 18; Oxt-Cre+; PtenloxP/+, n = 12; Oxt-Cre+; PtenloxP/loxP, n = 15; males: control, n = 27; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 20) significantly habituated (all t > 2.35, all P < 0.027) and dishabituated (all t > 2.57, all P < 0.027) to the stimulus (see Fig. 1d,e). One-way ANOVAs and planned comparisons also found no effects of genotype on habituation or dishabituation. Significant group differences were found for specific trials with planned comparisons: Oxt-Cre+; PtenloxP/loxP females investigated the stimulus significantly less than controls in habituations 1 and 2 (all t > 2.10, all P < 0.045; see Fig. 1d), and Oxt-Cre+; PtenloxP/+ males spent less time than controls investigating the stimulus in habituation 4 (t(45) = 2.40, P = 0.021) and dishabituation (t(45) = 2.26, P = 0.028; see Fig. 1e). Mixed-model ANOVAs found significant effects of trial in both sexes (females: F(4,168) = 17.43, P < 0.001; males: F(4,256) = 13.31, P < 0.001), but no genotype effects or trial × genotype interactions.
Female Oxt-Cre+; PtenloxP/loxP mice therefore showed less initial investigation of the stimulus, unlike germ-line Pten+/− females, who showed no phenotype in this assay [Clipperton-Allen & Page, 2014]. Males, on the other hand, showed largely normal social recognition and equivalent initial social interest, unlike germline Pten+/− males [Clipperton-Allen & Page, 2014].
Resident-intruder test.
In the free social interaction of the resident-intruder test, decreased aggression is observed in germline Pten+/− males [Clipperton-Allen & Page, 2015], while OxtRKO, but not OxtRHT, males show increased aggression [Dhakar et al., 2012]. Aggression in OxtKO males differs depending on the genotype of their mother, likely due to the presence or absence of Oxt in utero; when born to an OxtHT female, OxtKO males show normal or decreased aggression, while OxtKO males born to OxtKO females show increased aggression [Dhakar et al., 2012; Sala et al., 2011, 2013; Takayanagi et al., 2005; Winslow et al., 2000]. We therefore tested males of all three genotypes on the resident-intruder test following 7 days of single housing.
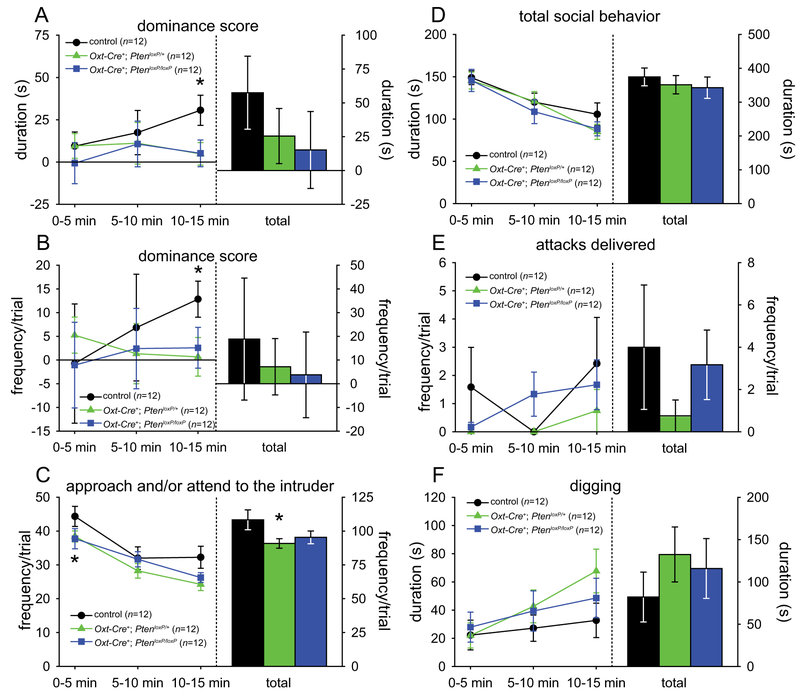
There were minimal genotype differences observed among males in the resident-intruder test (control males, n = 12; Oxt-Cre+; PtenloxP/+ males, n = 12; Oxt-Cre+; PtenloxP/loxP males, n = 12). One-way ANOVAs found significant main effects of genotype for both the duration and frequency (all F > 3.52, all P < 0.042) of dominance score in the last 5 min of the trial, which planned comparisons revealed to be due to significantly lower dominance scores in both Oxt-Cre+; PtenloxP/+ (all t > 2.31, all P < 0.031) and Oxt-Cre+; PtenloxP/loxP (all t > 2.13, all P < 0.045) mice than in controls (see Fig. 2a,b); in fact, only control mice showed significant dominance over the intruder for both frequency and duration in the last 5 min of the trial (all t > 3.43, all P < 0.007). No other significant differences were found on agonistic behaviors. However, planned comparisons indicated that Oxt-Cre+; PtenloxP/+ mice showed a decrease in the frequency of approaching and/or attending to the intruder (t(22) = 2.15, P = 0.043), particularly in the first 5 min (t(22) = 2.20, P = 0.039; see Fig. 2c). Unlike germ-line Pten+/− mice, no genotype differences were found in social behavior, attacks, or digging (see Fig. 2d–f).
Figure 2.
Resident-intruder test results of male Oxt-Cre+; PtenloxP mice. (A,B) Dominance score (agonistic behavior delivered minus agonistic behavior received) duration (A) and frequency (B). (C) Approaching and/or attending to the intruder. (D) Total social behavior. (E) Attacks delivered. (F) Digging. Significant difference from controls: *P < 0.05. Controls (n = 12), Oxt-Cre+; PtenloxP/+ (n = 12), and Oxt-Cre+; PtenloxP/loxP (n = 12).
Marble burying test.
Increased repetitive behavior was also observed in germline Pten+/− males, expressed as both increased digging in the resident-intruder test and more buried marbles in the marble burying test [Clipperton-Allen & Page, 2014, 2015], and OxtKO males showed a shorter latency to dig during a free social interaction [Lazzari et al., 2013]. To determine if our mutants also show increased repetitive behavior, we administered the marble burying test.
Planned comparison t-tests found that Oxt-Cre+; PtenloxP/+ females buried significantly fewer marbles than controls (t(44) = 2.09, P = 0.042; see Fig. 1f), but no other genotype differences were found with planned comparisons or one-way ANOVAs (females: control, n = 25; Oxt-Cre+; PtenloxP/+, n = 21; Oxt-Cre+; PtenloxP/loxP, n = 20; males: control, n = 25; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 19). This suggests that Oxt-Cre+; PtenloxP/+ females may show less repetitive, stereotyped behavior than control females, but unlike germline Pten+/− mice [Clipperton-Allen & Page, 2014], no differences in repetitive behavior were observed in males.
Anxiety-Like Behavior
In germline Pten+/− male mice, we observed decreased anxiety in the dark-light emergence and open field tests [Clipperton-Allen & Page, 2014]. Male OxtKO, but not OxtRKO or OxtRHT males, also showed decreased anxiety on the elevated plus maze [Mantella, Vollmer, Li, & Amico, 2003; Sala et al., 2011, 2013], and female OTKO mice performed more stretched approaches, an indicator of social anxiety [Choleris et al., 2003, 2006]. Thus, we assessed anxiety using the dark-light emergence and open field tests.
Dark-light emergence test.
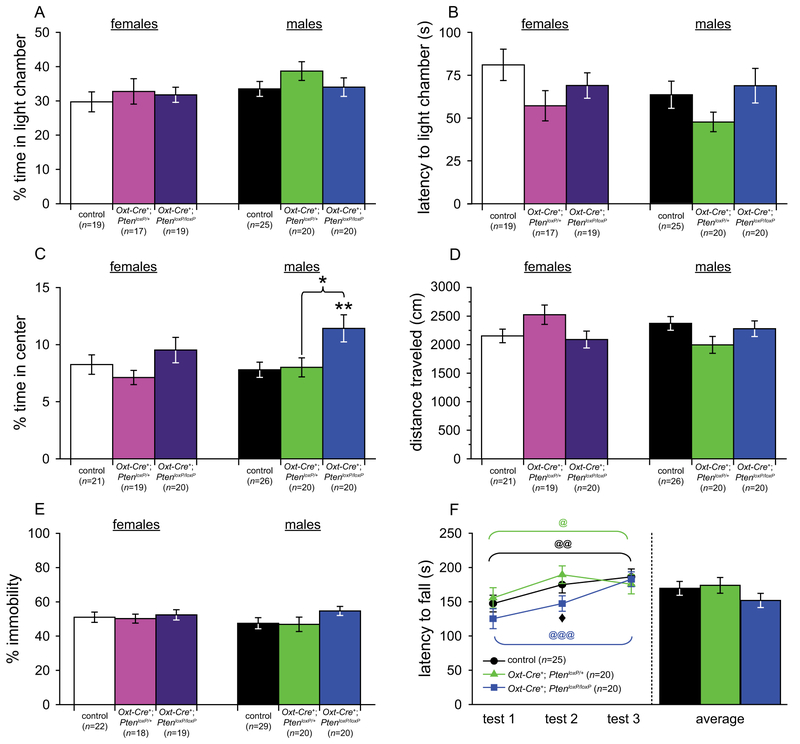
Both one-way ANOVAs and planned comparisons found no significant differences between genotypes for time in light chamber (see Fig. 3a), latency to enter the light chamber (see Fig. 3b), crossings between chambers, or any other measure (females: control, n = 19; Oxt-Cre+; PtenloxP/+, n = 17; Oxt-Cre+; PtenloxP/loxP, n = 19; males: control, n = 25; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 20). Additionally, all groups showed a significant preference for the dark chamber over the light chamber (all t > 4.10, all P < 0.002).
Figure 3.
Tests of anxiety-like, depression-like, and motor learning behavior in Oxt-Cre+; PtenloxP mice. (A,B) Dark-light emergence test for anxiety-like behavior in control (n = 19), Oxt-Cre+; PtenloxP/+ (n = 17), and Oxt-Cre+; PtenloxP/loxP (n = 19) females and control (n = 24), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 20) males. (C,D) Open field test for anxiety-like and locomotor behavior in control (n = 21), Oxt-Cre+; PtenloxP/+ (n = 19), and Oxt-Cre+; PtenloxP/loxP (n = 20) females and control (n = 26), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 20) males. (E) Tail suspension test of depression-like behavior in control (n = 22), Oxt-Cre+; PtenloxP/+ (n = 18), and Oxt-Cre+; PtenloxP/loxP (n = 19) females and control (n = 29), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 20) males. (F) Rotarod test of motor learning and behavior in control (n = 25), Oxt-Cre+; PtenloxP/+ (n = 20), and Oxt-Cre+; PtenloxP/loxP (n = 20) males. Significant differences between genotypes: *P < 0.05, **P < 0.01. Significant improvement over trials: @P < 0.05, @@P < 0.01, @@@P < 0.001. Significant difference between Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP males, ◆P < 0.05.
Open field test.
Planned comparisons revealed that Oxt-Cre+; PtenloxP/loxP males spent significantly more time in the center of the arena than control (t(44) = 2.81, P = 0.007) mice (see Fig. 3c), but no other genotype differences or differences in distance traveled or velocity (see Fig. 3d). Additionally, all groups (females: control, n = 21; Oxt-Cre+; PtenloxP/+, n = 19; Oxt-Cre+; PtenloxP/loxP, n = 20; males: control, n 5 26; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 20) spent significantly more time in thigmotaxis than in the center of the arena (all t > 32.50, all P < 0.001).
Thus, Oxt-Cre+; PtenloxP/loxP males did phenocopy germline Pten+/− mice, but only on one of the two tests on which germline Pten+/− males showed decreased anxiety-like behavior [Clipperton-Allen & Page, 2014].
Depression-Like Behavior
Tail suspension test.
We observed increased depression-like behavior in germline Pten+/− males; while depression-like behavior phenotypes have not been found in OxtKO or OxtRKO mice, Oxt has been shown to be related to depression, with decreased Oxt associated with higher depression scores [Scantamburlo et al., 2007], and Oxt administration having anti-depressant effects [Bakharev, Tikhomirov, & Lozhkina, 1986].
Both planned comparisons and one-way ANOVAs found no significant differences between genotypes (females: control, n = 22; Oxt-Cre+; PtenloxP/+, n = 18; Oxt-Cre+; PtenloxP/loxP, n = 19; males: control, n = 29; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 20) for immobility (see Fig. 3e).
Motor Coordination
Rotarod test.
To confirm that Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP mice had no motor deficits, and to ensure differences in motor ability did not lead to the genotype difference in open field center time, we tested motor learning on the rotarod test.
Mixed-model ANOVAs showed a significant main effect of test (F(2,122) = 17.66, P < 0.001), with all genotypes (males: control, n = 25; Oxt-Cre+; PtenloxP/+, n = 20; Oxt-Cre+; PtenloxP/loxP, n = 20) showing significant reductions in latency to fall across the three tests (all F > 3.74, all P < 0.034; see Fig. 3f). Planned comparisons revealed that Oxt-Cre+; PtenloxP/+ had a significantly longer latency to fall than Oxt-Cre+; PtenloxP/loxP males on test 2 (t(38) = 2.46, P = 0.019), but no other significant genotype effects on any, or across all, tests (see Fig. 3f).
Neuroanatomical results.
As Pten mutations are known to lead to altered cell number and size [Back-man et al., 2001; Chen et al., 2015; Fraser et al., 2004; Goberdhan et al., 1999; Groszer et al., 2001; Kazdoba et al., 2012; Kwon et al., 2001], we examined Oxt cell number and size, as well as PVN size, in conditional Pten mutants and controls following behavioral testing.
Oxytocin Cell Size and Number in Adults
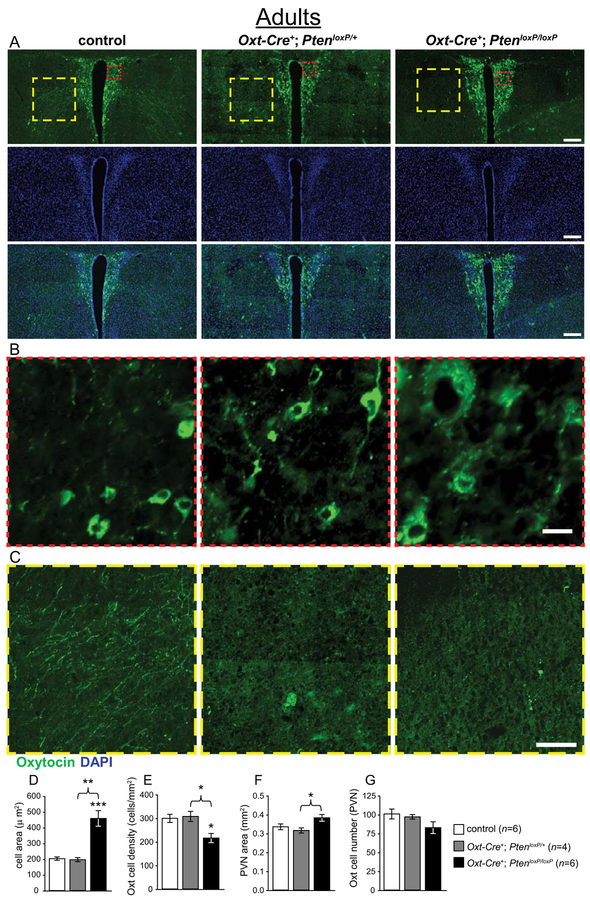
Planned comparisons indicated that Oxt-Cre+; PtenloxP/loxP mice (n = 6) had significantly larger soma in Oxt immunoreactive cells than controls (n = 6; t(5.5) = 5.37, P = 0.003; see Fig. 4a,b,d), and a significantly lower density of these cells in the PVN (t(10) = 3.20, P = 0.010; see Fig. 4a,b,e). One-way ANOVAs also found main effects of genotype for these measures (all F > 7.09, all P < 0.009), with post hoc tests revealing that Oxt-Cre+; PtenloxP/loxP also had larger cells and lower density than Oxt-Cre+; PtenloxP/+ mice (n = 4; all P < 0.020; see Fig. 4a,b,d,e). A main effect of genotype on PVN area was also found by one-way ANOVA (F(2,13) = 4.23, P = 0.038; see Fig. 4a,f), with post hoc tests indicating that the PVN of Oxt-Cre+; PtenloxP/loxP mice was significantly larger than that of Oxt-Cre+; PtenloxP/+ mice (P = 0.044; see Fig. 4a–f).
Figure 4.
Neuroanatomical analysis of Oxt immunoreactive cells in the paraventricular nucleus of the hypothalamus (PVN) of adult female Oxt-Cre+; PtenloxP mice. (A–C) Representative images of control, Oxt-Cre+; PtenloxP/+, and Oxt-Cre+; PtenloxP/loxP PVN immunostained with anti-Oxt (green) and DAPI (blue), with enlargements showing increased cell size (red square, B) and decreased immunoreactivity in the region lateral to the PVN (yellow square, C). (D) Increased Oxt cell soma area in Oxt-Cre+; PtenloxP/loxP mice. (E) Decreased Oxt cell density in the PVN of Oxt-Cre+; PtenloxP/loxP mice. (F) Oxt-Cre+; PtenloxP/loxP mice have a larger PVN than Oxt-Cre+; PtenloxP/+ mice, with a trend to a larger PVN than controls. (G) Non-significant trend to a decrease in Oxt cell number in Oxt-Cre+; PtenloxP/loxP mice. Significant differences between genotypes: *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 200 μm (A), 20 μm (B), 100 μm (C).
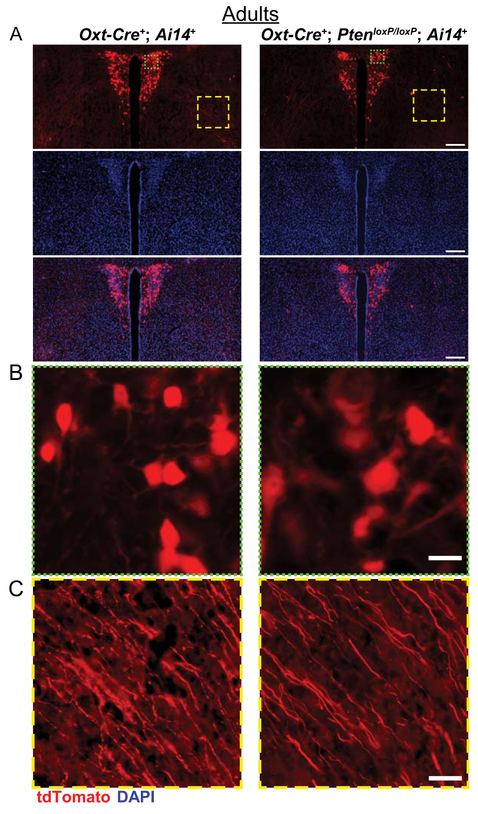
As a way to assess the characteristics of these cells without considering current Oxt levels, we examined the PVN of mice expressing the red fluorescent protein tdTomato in cells in which Oxt-Cre had been active (Oxt-Cre+; PtenloxP/loxP; Ai14+). Qualitatively, these mice also show increased soma size and lower density of tdTomato expressing cells (see Fig. 5a,b).
Figure 5.
Neuroanatomical assessment of Oxt cells in the PVN of adult female Oxt-Cre+; PtenloxP; Ai14+ mice. (A) Representative images of control (Oxt-Cre+; Pten+/+; Ai14+) and Oxt-Cre+; PtenloxP/loxP; Ai14+ PVN with tdTomato (red) expressed in cells in which Oxt-Cre has been active, stained with DAPI (blue). (B,C) Enlargements showing increased cell size (green square, B) and the presence of projections in the region lateral to the PVN (yellow square, C) in control and Oxt-Cre+; PtenloxP/loxP; Ai14+ mice. Scale bars: 200 μm (A), 20 μm (B), 50 μm (C).
Interestingly, we found that Oxt-Cre+; PtenloxP/loxP mice also appeared to lack Oxt immunoreactive projections in anterior hypothalamic area just lateral to the PVN (see Fig. 4c). However, tdTomato positive projections were present in this region (see Fig. 5c), suggesting that the lack of Oxt immunoreactivity in projections may be due to an Oxt trafficking deficit, not defects in Oxt neuronal projections per se.
Oxytocin Cell Size and Number in Juvenile Mice
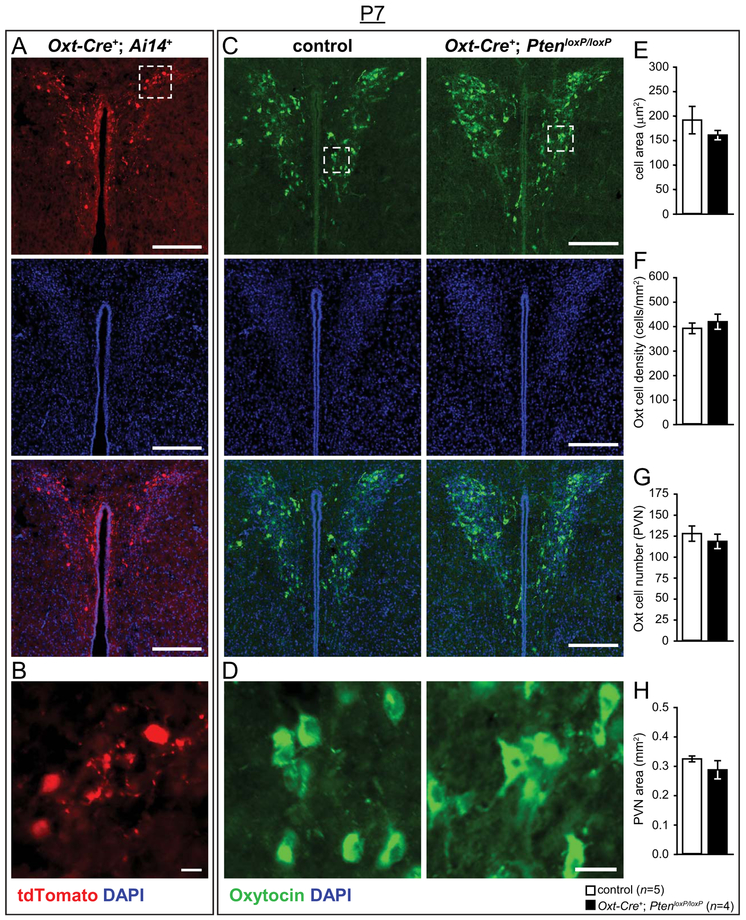
We first confirmed that Oxt-Cre had early developmental activity by examining the PVN in Oxt-Cre+; Ai14+ P7 and P0 brains, which displayed robust reporter expression in the PVN (see Fig. 6a,b and Supporting Information Fig. S1a). We next analyzed Oxt cellular characteristics in P7 mice to assess whether the neuroanatomical phenotype was present as the circuitry underlying social behavior is assembled. Unlike adult brains, no significant genotype differences were found for cell soma size, number, density, or grey value, or for PVN area or grey value (see Fig. 6c–h).
Figure 6.
Neuroanatomical analysis of Oxt immunoreactive cells at post-natal day 7 (P7) in the PVN of Oxt-Cre+; PtenloxP mice. (A,B) Representative images of control (Oxt-Cre+; Ai14+) and Oxt-Cre+; PtenloxP/loxP; Ai14+ PVN (A) with tdTomato (red) expressed in cells in which Oxt-Cre has been active, stained with DAPI (blue), and enlargements showing cellular resolution (white square, B). (C,D) Representative images of control and Oxt-Cre+; PtenloxP/loxP PVN (C) immunostained with anti-Oxt (green) and DAPI (blue), with enlargements showing cellular resolution (white square, D). (E–H) Normal Oxt cell soma area (E), Oxt cell density (F), Oxt cell number (G), and PVN size (H) in P7 Oxt-Cre+; PtenloxP/loxP mice. Scale bars: 200 μm (A,C), 20 μm (B,D).
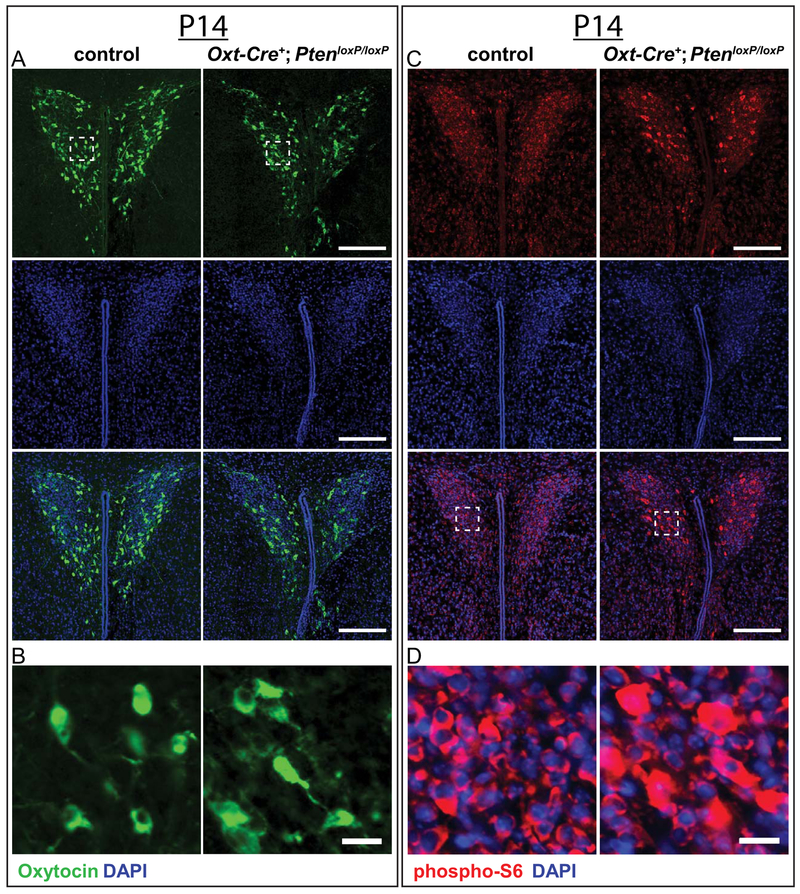
We then examined Oxt cell and PVN structure in control and Oxt-Cre+; PtenloxP/loxP brains at P14 (see Fig. 7a,b) and found no gross differences, indicating that conditional deletion of Pten induces hypertrophy in Oxt cells predominantly after the circuitry underlying social behavior is largely assembled. Because we did not detect significant phenotypic differences between controls and mutants at P14, we confirmed that Pten deletion resulted in elevated mTORC1 activity by immunostaining for phospho-S6, a readout of mTOR Complex 1 (mTORC1) activity [Chen et al., 2015; Zhou & Parada 2012], and found increased phospho-S6 levels in the PVN of Oxt-Cre+; PtenloxP/loxP mice (see Fig. 7c,d). To confirm that Pten was deleted in Oxt cells, we immunostained for Pten and Oxt and found that, while Oxt-immunoreactive cells were also immunoreactive for Pten in the PVN of control mice, Oxt-immunoreactive cells were not immunoreactive for Pten in the PVN of Oxt-Cre+; PtenloxP/loxP mice (see Supporting Information Fig. S1B). Taken together, these results are consistent with the progressive hypertrophy and elevated phospho-S6 that have been reported in numerous other Pten conditional knockout models [e.g., Backman et al., 2001; Fraser et al., 2004; Gregorian et al., 2009; Gregory et al., 2009; Kwon et al., 2001; Kwon, Zhu, Zhang, & Baker, 2003; Ljungberg, Sunnen, Lugo, Anderson, & D’Arcangelo, 2009; Pun et al., 2012; Takeuchi et al., 2013; Wen et al., 2013].
Figure 7.
Assessment of Oxt immunoreactive cells and phospho-S6 enrichment in the PVN at P14. (A,B) Representative images of P14 control and Oxt-Cre+; PtenloxP/loxP PVN (A) immunostained with anti-Oxt (green) and DAPI (blue), with enlargements showing cellular resolution (white square, B). (C,D) Representative images of P14 control and Oxt-Cre+; PtenloxP/loxP PVN (C) immunostained with anti-phospho-S6 (red) and DAPI (blue), with enlargements showing cellular resolution (white square, D). Scale bars: 200 μm (A,C), 20μm (B,D).
Discussion
Deletion of Pten in Oxt neurons had minimal effects on ASD-relevant behavior. No genotype effects were found in either sex for the three-chamber social approach or social novelty assay, in habituation or dishabituation in the social recognition assay, or for overall social behavior, social investigation, or related individual behaviors in males in the resident-intruder test. However, both Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP males did show a decreased dominance score in the latter part of the resident-intruder test, but no differences in attacking or individual aggressive behaviors, and Oxt-Cre+; PtenloxP/+ males also spent less time approaching and/or attending to the intruder. Male mutants did not differ from controls in repetitive behavior (marble burying or digging in the resident-intruder test), but Oxt-Cre+; PtenloxP/+ females did bury fewer marbles than controls. No genotype results were found for depression-like or motor behavior, and while Oxt-Cre+; PtenloxP/loxP males did show less anxiety-like behavior in the open field test, these differences were not found in a second anxiety test (dark-light emergence).
As many of the behavioral phenotypes observed in germline Pten+/− mice [Clipperton-Allen & Page 2014, 2015; Page et al., 2009a; Sejourne et al., 2015] are similar to those found in OxtKO and/or OxtRKO mice [Amico et al., 2004; Clipperton-Allen & Page, 2014, 2015; Ferguson et al., 2000, 2001; Lazzari et al., 2013; Lee et al., 2008; Macbeth et al., 2009; Mantella et al., 2003; Nishimori et al., 2008; Page et al., 2009a; Pobbe et al., 2012a, 2012b; Sala et al., 2011, 2013; Takayanagi et al., 2005; Winslow et al., 2000], we investigated whether conditional deletion of Pten in Oxt cells recapitulated these behavioral phenotypes. With the exception of decreased anxiety-like behavior in the open field test in males, germline Pten+/− phenotypes were not phenocopied by Oxt-Cre+; PtenloxP/+ or Oxt-Cre+; PtenloxP/loxP mice.
Despite the overall minimal impact on ASD-relevant behaviors, we observed increased Oxt cell size in Oxt-Cre+; PtenloxP/loxP mice, which more than doubled that of control or Oxt-Cre+; PtenloxP/+ mice. This was accompanied by an increase in PVN area and a decrease in Oxt cell density. The number of Oxt immunoreactive cells also trended towards a decrease, although this did not reach statistical significance.
Interestingly, we also observed a reduction in Oxt immunoreactive fibres in the anterior hypothalamic area in both the Oxt-Cre+; PtenloxP/+ and Oxt-Cre+; PtenloxP/loxP mice. We did not detect a decrease in tdTomato positive projections in Oxt-Cre+; PtenloxP/loxP; Ai14+ brains. This suggests that there may be defects in the intracellular trafficking of Oxt in these mice. Examining this phenotype is beyond the scope of this study; however, this may provide insight into the mechanisms of Oxt trafficking in future studies.
Given the substantial hypertrophy in Oxt cells, it is somewhat surprising that minimal behavioral phenotypes were observed. However, this may be accounted for by the lack of a developmental neuroanatomical phenotype. Alternatively, Oxt, a long-range, diffuse, and slow-acting neuromodulator, may have been present in sufficient quantities in the PVN and other brain areas to compensate for any local alterations in Oxt signaling.
Despite the lack of behavioral phenotypes, these results do not rule out the possibility that the Oxt system may play a role in germline Pten+/− behavioral abnormalities through a non-cell autonomous mechanism in Oxt cells or through an effect in OxtR-expressing cells. Additionally, Pten mRNA is expressed throughout the developing hypothalamus as early as E11.5, prior to the onset of Oxt mRNA expression between E13.5 and E15.5 (Allen Brain Atlas, http://developingmouse.brain-map.org/, last accessed 29 January 2016) [Thompson et al., 2014]. Thus, we cannot exclude the possibility that Pten may function early in the Oxt cell lineage, prior to Oxt-Cre mediated recombination.
In summary, our results indicate that conditional deletion of Pten using Oxt-Cre has a profound effect on Oxt cell structure, but leaves ASD-relevant behaviors largely unaffected. We interpret these results as inconsistent with our starting hypothesis, that reduced Pten function in Oxt-expressing cells causes the behavioral deficits observed in germline Pten+/− mice.
Supplementary Material
Supplementary Figure 1. Confirmation of Cre activity and Pten deletion in Oxt-Cre mice. A) Representative image of P0 Oxt-Cre+; Ai14+ PVN with tdTomato (red) expressed in cells in which Oxt-Cre has been active, stained with DAPI (blue). B) Representative images of adult control and Oxt-Cre+; PtenloxP/loxP PVN immuno-stained with anti-Oxt (red), anti-Pten (green), and DAPI (blue), confirming Pten deletion in Oxt-immunoreactive neurons. Scale bars: 100μm (A), 20μm (B).
Supplementary Table 1. Descriptions of behaviors scored in the resident-intruder test, modified from Clip-perton-Allen & Page, 2015.
Supplementary Table 2. Descriptions of categories and composite behaviors calculated for the resident-intruder test, modified from Clipperton-Allen & Page, 2015.
Acknowledgments
We thank Ms. Nancy Lurie Marks and the American Honda and Children’s Healthcare Charity Inc. for gift funds to support this work. This work was also funded by NIH grant number R01MH105610. Additionally, we appreciate the invaluable administrative assistance of Mrs. Trina L. Kemp. We are extremely grateful for the assistance of Julien Sejourne and Alan Sales Barbosa with immunohistochemistry.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- Amico JA, Mantella RC, Vollmer RR, & Li X (2004). Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology, 16, 319–324. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, … Mak TW (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nature Genetics, 29, 396–403. [DOI] [PubMed] [Google Scholar]
- Bakharev VD, Tikhomirov SM, & Lozhkina TK (1986). Psychotropic properties of oxytocin. Neuroscience and Behavioral Physiology, 16, 160–164. [DOI] [PubMed] [Google Scholar]
- Butler M, Dasouki M, Zhou X-P, Talebizadeh Z, Brown M, Takahashi T, … Eng C (2005). Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline Pten tumour suppressor gene mutations. Journal of Medical Genetics, 42, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, … Betancur C (2007). Mutation screening of the Pten gene in patients with autism spectrum disorders and macrocephaly. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 144B, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang WC, Sejourne J, Clipperton-Allen AE, & Page DT (2015). Pten mutations alter brain growth trajectory and allocation of cell types through elevated beta-catenin signaling. Journal of Neuroscience, 35, 10252–10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, & Ogawa S (2003). An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 100, 6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, & Pfaff DW (2006). Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes, Brain, and Behavior, 5, 528–539. [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE & Page DT (2014). Pten haploinsufficient mice show broad brain overgrowth but selective impairments in autism-relevant behavioral tests. Human Molecular Genetics, 23, 3490–3505. [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE & Page DT (2015). Decreased aggression and increased repetitive behavior in Pten haploinsufficient mice. Genes, Brain, and Behavior, 14, 145–157. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, … Young WS (2007). Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides, 41, 145–163. [DOI] [PubMed] [Google Scholar]
- Dhakar MB, Rich ME, Reno EL, Lee HJ, & Caldwell HK (2012). Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Hormones and Behavior, 62, 86–92. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience, 21, 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, & Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nature Genetics, 25, 284–288. [DOI] [PubMed] [Google Scholar]
- Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, & Baker SJ (2004). Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Research, 64, 7773–7779. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, & Eng C (2015). Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Molecular Psychiatry, 20, 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, & Wilson C (1999). Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes & Development, 13, 3244–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC & Mackintosh JH (1963). A comparison of the social postures of some common laboratory rodents. Behaviour, 21, 246–259. [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, … Wu H (2009). Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. Journal of Neuroscience, 29, 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, … Pericak-Vance MA (2009). Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Medicine, 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, … Wu H (2001). Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science, 294, 2186–2189. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Sunnen CN, Crowell B, Lee GH, Anderson AE, & D’Arcangelo G (2012). Development and characterization of NEX-Pten: A novel forebrain excitatory neuron-specific knockout mouse. Developmental Neuroscience, 34, 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Sharifi-Hannauer P, & Martinez-Agosto JA (2013). Macrocephaly as a clinical indicator of genetic subtypes in autism. Autism Research, 6, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, … Baker SJ (2001). Pten regulates neuronal soma size: A mouse model of Lhermitte-Duclos disease. Nature Genetics, 29, 404–411. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, & Baker SJ (2003). mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proceedings of the National Academy of Sciences of the United States of America, 100, 12923–12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari VM, Becker RO, de Azevedo MS, Morris M, Rigatto K, Almeida S, … Giovenardi M (2013). Oxytocin modulates social interaction but is not essential for sexual behavior in male mice. Behavioural Brain Research, 244, 130–136. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, & Young WS III (2008). A conditional knockout mouse line of the oxytocin receptor. Endocrinology, 149, 3256–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, & Wu H (2002). Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis, 32, 148–149. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, & D’Arcangelo G (2009). Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Disease Models & Mechanisms, 2, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, & Young WS III (2009). Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes, Brain, and Behavior, 8, 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, … Zeng H (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience, 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, & Amico JA (2003). Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology, 144, 2291–2296. [DOI] [PubMed] [Google Scholar]
- Matson JL & Shoemaker M (2009). Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities, 30, 1107–1114. [DOI] [PubMed] [Google Scholar]
- McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, & Herman GE (2010). Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Research, 3, 137–141. [DOI] [PubMed] [Google Scholar]
- Mester JL, Tilot AK, Rybicki LA, Frazier TWI, & Eng C (2011). Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. European Journal of Human Genetics, 19, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, & Kawamata M (2008). New aspects of oxytocin receptor function revealed by knockout mice: Sociosexual behaviour and control of energy balance. Progress in Brain Research, 170, 79–90. [DOI] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, & Sur M (2009a). Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proceedings of the National Academy of Sciences of the United States of America, 106, 1989–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, & Sur M (2009b). Computerized assessment of social approach behavior in mouse. Frontiers in Behavioral Neuroscience, 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, … Huang LN (2013). Mental health surveillance among children — United States, 2005–2011. MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report, 62, 1–35. [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS III, Lee HJ, … Blanchard RJ (2012a). Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Hormones and Behavior, 61, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RLH, Pearson BL, Blanchard DC, & Blanchard RJ (2012b). Oxytocin receptor and Mecp2308/Y knockout mice exhibit altered expression of autism-related social behaviors. Physiology & Behavior, 107, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, … Danzer SC (2012). Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron, 75, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Donzelli A, Martucci R, Busnelli M, Bulgheroni E, … Chini B (2013). Mice heterozygous for the oxytocin receptor gene (Oxtr(1/-)) show impaired social behaviour but not increased aggression or cognitive inflexibility: Evidence of a selective haploinsufficiency gene effect. Journal of Neuroendocrinology, 25, 107–118. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, … Chini B (2011). Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biological Psychiatry, 69, 875–882. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, … Legros JJ (2007). Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology, 32, 407–410. [DOI] [PubMed] [Google Scholar]
- Sejourne J, Llaneza D, Kuti OJ, & Page DT (2015). Social behavioral deficits coincide with the onset of seizure susceptibility in mice lacking serotonin receptor 2c. PLoS One, 10, e0136494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis ML & Parsons R (2003). PTEN: From pathology to biology. Trends in Cell Biology, 13, 478–483. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Fujita E, Yu Z, Yamagata T, Momoi MY, Momoi T, … Onaka T (2010). Impairment of social and emotional behaviors in Cadm1-knockout mice. Biochemical and Biophysical Research Communications, 396, 703–708. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, … Nishimori K (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 102, 16096–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Gertner MJ, Zhou J, Parada LF, Bennett MV, & Zukin RS (2013). Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proceedings of the National Academy of Sciences of the United States of America, 110, 4738–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, & Paylor R (2009). Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology, 204, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Ng L, Menon V, Martinez S, Lee CK, Glattfelder K, … Jones AR (2014). A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron, 83, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Li W, Choudhury GR, He R, Yang T, Liu R, … Yang SH (2013). Astroglial PTEN loss disrupts neuronal lamination by dysregulating radial glia-guided neuronal migration. Aging and Disease, 4, 113–126. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1992). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization. [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, & Insel TR (2000). Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones and Behavior, 37, 145–155. [DOI] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, … Tong Q (2012). An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One, 7, e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J & Parada LF (2012). PTEN signaling in autism spectrum disorders. Current Opinion in Neurobiology, 22, 873–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Confirmation of Cre activity and Pten deletion in Oxt-Cre mice. A) Representative image of P0 Oxt-Cre+; Ai14+ PVN with tdTomato (red) expressed in cells in which Oxt-Cre has been active, stained with DAPI (blue). B) Representative images of adult control and Oxt-Cre+; PtenloxP/loxP PVN immuno-stained with anti-Oxt (red), anti-Pten (green), and DAPI (blue), confirming Pten deletion in Oxt-immunoreactive neurons. Scale bars: 100μm (A), 20μm (B).
Supplementary Table 1. Descriptions of behaviors scored in the resident-intruder test, modified from Clip-perton-Allen & Page, 2015.
Supplementary Table 2. Descriptions of categories and composite behaviors calculated for the resident-intruder test, modified from Clipperton-Allen & Page, 2015.