Abstract
Recent improvements in risk-directed treatment and supportive care, together with greater reliance on both national and international collaborative studies, have made childhood acute lymphoblastic leukemia (ALL) one of the most curable human cancers. Next-generation sequencing studies of leukemia cells and the normal host genome provides exciting opportunities for precision medicine and thus the cure rate and quality of life of the patients. Efforts are under way to assess the global impact of childhood ALL and to develop initiatives that can meet the long-term challenge of providing quality care to the world’s children with this disease and improving cure rates globally. This ambitious task will rely greatly on increased collaborative research and international networking, so that the therapeutic gains in high-income countries can be translated to patients residing in low- and middle-income countries. Ultimately, the greatest obstacle to overcome will be to fully understand leukemogenesis, enabling measures to decrease the risk of leukemia development and thus close the last major gap in our ability to offer a cure to any child who may succumb to this disease.
Introduction
Advances in the biologic study and treatment of childhood acute lymphoblastic leukemia (ALL) are one of the most successful stories of modern medicine1. Over the past five decades, this once uniformly fatal disease has been transformed to one with a 5-year survival rate exceeding 90% among children receiving protocol-directed treatment in most developed countries (Table 1)2–14. This improved outcome can be attributed to advances in supportive care, more accurate diagnosis, and optimal risk-directed therapy incorporating consolidation treatment with high-dose or escalating-dose methotrexate, delayed intensification with vincristine, asparaginase and dexamethasone, and early use of intrathecal therapy -- treatment components that were largely established by the randomized clinical trials of major cooperative study groups15. As survival rates for ALL approach 100%, current research efforts have focused on improving the quality of life of patients by decreasing both acute and late morbidities, and on the development of curative treatment for the small subsets of patients who continue to develop drug-resistant leukemia, a major challenge that requires the concerted efforts of multiple pediatric oncology study groups15.
Table 1.
Patient characteristics and treatment results from selected clinical trials
| Study group | Years of study | No. of patients | Age range (years) | T-cell ALL (%) | 5-year cumulative rate of any relapse (%) | 5-year EFS (%) | 5-year survival (%) | Data source |
|---|---|---|---|---|---|---|---|---|
| AIEOP-BFM 2000 | 2000–2006 | 4,839 | 1–17 | 13.2 | 13.2 | 81.4±0.6 | 91.9±0.4 | Moricke et al.2 |
| CoALL-07–03 | 2003–2010 | 743 | 1–18 | 12.9 | NA | 83±0.3 | NA | Escherich et al.3 |
| COG | 2000–2005 | 7,153 | 0–21 | 7 | 7.22 | NA | 90.4±0.5 | Hunger et al.4 |
| DCOG-10 | 2004–2012 | 778 | 1–18 | 14.2 | 8.3 | 87±1.2 | 91.9±1.0 | Pieters et al.5 |
| DFCI 05–001 | 2005–2010 | 551 | 1–18 | 13 | 8.96 | 85 | 91 | Place et al.6 |
| EORTC 58951 | 1998–2008 | 1947 | 1–18 | 15.2 | 14.7 | 82.6±0.9 | 89.7±0.7 | Domenech et al.7 |
| IC-BFM 2002 | 2002–2007 | 5,060 | 1–18 | 13.3 | 19 | 74±1 | 82±1 | Stary et al.8 |
| JCCLSG ALL 2000 | 2000–2004 | 305 | 1–15 | 9.8 | 17 | 79.7±2.4 | 89.2±1.8 | Yamaji et al.9 |
| Ma-Spore ALL 2003 | 2002–2011 | 556 | 0–18 | 8.8 | NA | 80.6±3.5 | 89.2±2.7 | Yeoh et al.10 |
| MRC UKALL 2003 | 2003–2011 | 3,126 | 1–25 | 12 | 8.8 | 87.3±1.4 | 91.6±1.2 | Vora et al.11 |
| NOPHO-2008 | 2008–2014 | 1,022 266 |
1–9 10–17 |
9.1 25.2 |
10 3±1 |
89±1 80±3 |
1 2 ±± 47 98 | Toft et al.12 |
| SJCRH XV | 2000–2007 | 498 | 1–18 | 15 | 9.3 | 87.3±2.9 | 93.5±1.9 | Pui et al.13 |
| TPOG | 1999–2010 | 152 | 0–18 | 7.2 | NA | 84.2±3.0 | 90.2±2.4 | Liu et al.14 |
Abbreviations: ALL, acute lymphoblastic leukemia; AIEOP, Associazione Italiana di Ematologia Pediatrica Group; BFM, Berlin-Frankfurt-Münster; CNS, central nervous system; CoALL, Cooperative ALL Study Group; COG, Children’s Oncology Group; DCOG, Dutch Children’s Oncology Group; DFCI, Dana-Farber Cancer Institute consortium; EFS, event-free survival; EORTC – CLG, European Organisation for Research and Treatment of Cancer - Children Leukemia Group; IC-BFM, Intercontinental-BFM; JCCLSG, Japanese Children’s Cancer and Leukemia Study Group; Ma-Spore, Malaysia-Singapore; MRC UKALL, Medical Research Council United Kingdom acute lymphoblastic leukemia; NA, not available; NOPHO, Nordic Society of Pediatric Hematology and Oncology; SJCRH, St. Jude Children’s Research Hospital; TPOG, Taiwan Pediatric Oncology Group.
A sobering fact of childhood ALL treatment is that very few of the advances contributing to current high cure rates are available to children who live in low- and middle-income countries (LMIC) and who account for an unacceptable proportion of cancer-related deaths16,17. Indeed, data from CONCORD-2, a global comparison of population-based cancer survival, showed a very wide range in survival rates for childhood ALL patients diagnosed during 2005–09, from less than 50% in several countries in Africa, Asia and South America to approximately 90% in certain countries in Europe, North America, and Oceania (Table 2)18. Within Asia alone, the 5-year survival estimates ranged from 34.3% to 73.1% in LMIC compared to 77.1% to 85.0% in high-income countries (HIC). Thus, a major challenge in the coming decades will be to translate the therapeutic gains achieved in HIC to clinics in LMIC. In this review, we summarize advances in the treatment and biologic understanding of childhood ALL made by international collaborative groups, provide evidence and rationales to support the study of ethnic and racial influences on ALL subtypes and treatment responses, and suggest initiatives to address the global impact of this disease.
Table 2.
5-year survival for children (aged 0 to 14 years) diagnosed with acute lymphoblastic leukemia between 2005 and 2009 by continent and country.
| % Survival estimate (95% CI) | |
|---|---|
| Africa | |
| Algeria registries | 54.1 (31.3 – 76.8)* |
| Lesotho† | 39.5 (16.4 – 62.7) |
| Libya (Benghazi) | 70.1 (43.4 – 96.9) |
| Tunisia (Central) | 50.1 (26.0 – 74.2)* |
| America (Central and South) | |
| Argentina registries† | 66.9 (64.4 – 69.3) |
| Brazilian registries | 65.8 (57.7 – 74.0) |
| Chilean registries | 66.4 (51.3 – 81.5) |
| Colombian registries | 53.8 (43.9 – 63.6) |
| Ecuadorian registries | 62.6 (53.7 – 71.6) |
| Puerto Rico† | 80.1 (71.1 – 89.0) |
| America (North) | |
| Canada† | 90.6 (88.6 – 92.7) |
| US registries | 87.7 (86.9 – 88.4) |
| Asia | |
| Chinese registries | 61.1 (51.3 – 70.8) |
| Cyprus† | 83.2 (69.7 – 96.7) |
| Indian registries | 64.7 (50.1 – 79.2) |
| Indonesia (Jakarta) | 44.3 (13.4 – 75.3) |
| Israel† | 85.0 (80.5 – 89.4) |
| Japanese registries | 81.1 (76.8 – 85.4) |
| Jordan† | 16.4 (6.8 – 26.0)* |
| South Korea† | 77.1 (74.7 – 79.5) |
| Malaysia (Penang) | 69.4 (57.4 – 81.5) |
| Mongolia† | 34.3 (11.9 – 56.8) |
| Taiwan† | 77.9 (74.5 – 81.3) |
| Thai registries | 55.1 (45.5 – 64.6) |
| Turkey (Izmir) | 73.1 (66.1 – 80.2) |
| Europe | |
| Austria† | 91.1 (86.9 – 95.2) |
| Belarus† | 88.3 (83.6 – 93.0) |
| Belgium† | 89.7 (86.1 – 93.3) |
| Bulgaria† | 71.0 (64.2 – 77.7) |
| Croatia† | 85.9 (80.0 – 91.8) |
| Denmark† | 87.2 (81.5 – 92.9) |
| Estonia† | 62.6 (52.0 – 73.3) |
| Finland† | 81.9 (75.3 – 88.5) |
| French registries† | 89.2 (87.7 – 90.8) |
| German registries | 91.8 (89.8 – 93.7) |
| Iceland† | 84.1 (70.0 – 98.3) |
| Ireland† | 85.3 (79.1 – 91.5) |
| Italian | 87.7 (84.9 – 90.5) |
| Latvia† | 75.0 (64.3 – 85.8) |
| Lithuania† | 69.6 (59.1 – 80.1) |
| Malta† | 72.5 (59.5 – 85.4) |
| Netherland† | 85.9 (82.7 – 89.2) |
| Norway† | 89.7 (84.4 – 94.9) |
| Portugal† | 86.8 (80.7 – 92.9) |
| Slovakia† | 78.2 (69.5 – 87.0) |
| Slovenia† | 75.7 (63.8 – 87.6) |
| Spanish registries† | 83.3 (79.1 – 87.4) |
| Sweden† | 85.5 (80.9 – 90.1) |
| Swiss registries† | 88.4 (83.8 – 93.0) |
| UK† | 89.1 (87.6 – 90.7) |
| Oceania | |
| Australian registries | 88.6 (85.9 – 91.4) |
| New Zealand† | 89.3 (83.8 – 94.8) |
Survival estimate considered less reliable.
100% coverage of the national population.
Data extracted from Allemani et al.18
Recent Advances from International Collaborative Studies
Differences in risk classification, age eligibility, ethnic and racial distributions, and data reporting have made it difficult to compare results among study groups to identify effective treatment components or regimens that are particularly effective for certain groups of patients. Thus, two workshops were organized to address this issue. The first, held in Memphis in 1997, provided updated results from most of the major cooperative groups, while the second, held in Ponte di Legno in 1999, produced 12 reports on the long-term outcomes of clinical trials conducted in the 1980s and 1990s, using a standard format that enabled comparison of treatment results both overall and for well-defined subgroups of patients19. Continued participation in these workshops by 15 major study groups has led to a second series of 15 articles summarizing the results of clinical trials conducted between 1985 and 200020. The key findings of these meetings are described below and summarized in Table 3.
Table 3.
Clinical Research Findings from Selected Collaborative Studies (I will revise this table)
| Subgroup of ALL | Years of Study | No. of Study groups | No. of Patients | Major Findings | References |
|---|---|---|---|---|---|
| Infant ALL | 1995–2005 | 17 | 482 | Hybrid treatment regimen with drugs against both ALL and acute myeloid leukemia improved outcome. Transplantation benefited high-risk subgroup with MLL rearrangement, age < 6 months, and poor early steroid response or hyperleukocytosis. | Pieters et al21 Mann et al.22 |
| Down syndrome | 1995–2004 | 16 | 653 | These patients have increased risk of relapse and treatment-related mortality. More than half of the cases are characterized by aberrant expression of CRLF2, often associated with JAK-STAT activation. | Buitenkamp et al.23,25 Mullighan et al.24 |
| Induction failure | 1985–2000 | 14 | 1041 | Treatment outcome for patients with induction failure was highly heterogeneous. Only patients >6 years with B-ALL and those with T-cell ALL appeared to benefit from allogeneic transplantation. | Schrappe et al.26 |
| Early T-cell precursor | 1992–2006 | 2 | 30 | These patients have distinctive immunophenotype (CD1a−, CD8−, CD5weak with stem-cell or myeloid markers) and high levels of minimal residual disease after remission induction. | Coustan-Smith et al.28 |
| Philadelphia chromosome-positive | |||||
| 2004–2009 | 10 | 178 | Imatinib combined with intensive chemotherapy was well tolerated and might improve outcome. | Biondi et al.33 | |
| Philadelphia chromosome-like | |||||
| 2014 | 5 | 264 | Over 90% of the cases have kinase activating alterations, some amenable to inhibition with tyrosine kinase inhibitors. | Roberts et al.48,49 | |
| MLL-rearranged | |||||
| 1983–1995 | 12 | 450 | Secondary chromosomal abnormalities have no prognostic significance in patients with 11q23 rearrangements. | Moorman et al.40 | |
| Hypodiploid<44 chromosomes | |||||
| 2008–2013 | 2 | 126 | Near-haploid cases have genetic alterations targeting receptor tyrosine kinase and Ras pathway and IKZF3 mutations, and low-hypodiploid cases are characterized by IKZF2 alterations and TP53 mutations, half of which are inherited. | Holmfeldt et al.42 |
Clinical advances in specific subtypes of ALL
Infant ALL
Because of the rarity and poor prognosis of infant ALL, as well as its frequent co-expression of lymphoid and myeloid markers, 17 study groups collaborated to test a treatment regimen incorporating drugs that are effective against both ALL and acute myeloid leukemia. This study yielded a 4-year event-free survival (EFS) rate of 47.0%, which was superior to any previously reported result21. While delayed intensification failed to improve outcome21, allogeneic transplantation appeared to benefit the high-risk subgroup with MLL (KMT2A)-rearrangement, age less than 6 months, and either a poor response to glucocorticoids after 8 days of remission induction or a presenting leukocyte count ≥300 × 109/L22.
Down syndrome ALL
Children with Down syndrome have an estimated 20-fold increased risk of developing ALL that is associated with an older age (median 5.0 vs. 4.7 years); paucity of T-cell ALL (<1% vs. 12%); a low frequency of the Philadelphia chromosome (BCR-ABL1) (0.7% vs. 2.4%), the t(12;21)/ETV6-RUNX1 (8.3% vs. 25.8%), or hyperdiploidy >50 chromosomes (9% vs. 33%); and an increased cumulative risk of relapse (26% vs. 15%) and treatment-related mortality (7% vs. 2%), resulting in an inferior overall survival (74% vs. 89%) in one large collaborative study23. Favorable prognostic factors in that trial included younger age (<6 years), leukocyte count <10 × 109/L at diagnosis, and the presence of high hyperdiploidy or t(12;21)/ETV6-RUNX123. As many as 60% of ALL patients with Down syndrome are characterized by aberrant expression of CRLF2 (coding for cytokine receptor like factor 2), often associated with somatic activating mutations in the receptors or the downstream components of the JAK-STAT pathway24,25, suggesting that these patients may benefit from therapy targeting JAK or one or more of its downstream pathway elements. IKZF1 deletions occurred in approximately a third of these patients, and was associated with a particularly poor outcome25.
ALL with induction failure
A recent collaborative study showed that 2.4% of patients treated between 1985 and 2000 had induction failure defined by morphologic evidence of ≥ 5% blasts after 4 to 6 weeks of remission-induction treatment26. These patients often presented with unfavorable features, including older age (median, 8.1 years), high leukocyte count (median, 42 × 109/L), T-cell phenotype (38%), the Philadelphia chromosome (13%), and the 11q23/MLL rearrangement (10%)26. Treatment outcome was highly variable, with hyperdiploidy >50 chromosomes and age < 6 years (without an MLL rearrangement) associated with a favorable prognosis in patients with B-ALL. Only patients ≥ 6 years of age with B-ALL and those with T-cell ALL appeared to benefit from allogeneic transplantation. Another recent study indicated that minimal residual disease (MRD) measurement should be used to define induction failure because some patients with an M2 marrow (5% to 25% blasts) by morphologic examination actually had MRD <0.01% and a 5-year event-free survival of 100%, whereas some patients with an M1 marrow (<5% blasts) had high MRD levels (≥5%) and a 5-year event-free survival of only 38.1%27. Interestingly, approximately a third of B-ALL patients with induction failure had EBF1-PDGFRB fusion, which responds to ABL-class tyrosine kinase inhibitors27.
Early T-cell precursor (ETP) ALL
Based on immunophenotyping and gene expression profiling, a study identified a distinct T-cell ALL subtype (designated ETP-ALL) that was characterized by stem cell-like features, a poor early treatment response, and a high rate of induction failure and relapse28. Two recent studies showed that consolidation therapy with two cycles of cyclophosphamide, mercaptopurine, and cytarabine effectively reduced MRD levels in patients with ETP-ALL, resulting in an intermediate outcome (5-year EFS 86.2% and 76.7% at 3 and 5 years, respectively)29,30.
Philadelphia chromosome-positive ALL
Philadelphia chromosome-positive ALL with BCR-ABL1 fusion, typically found in 2% to 3% of children with ALL, was associated with a dismal outcome before the development of ABL tyrosine kinase inhibitors. In a collaborative study of 326 children and young adults treated between 1985 and 1996, the 5-year EFS rate was only 28%, and matched-related transplantation improved outcome31. In a second collaborative study of 610 patients treated between 1995 and 2005 without a tyrosine kinase inhibitor, the EFS had improved to 32%, and both matched-related and matched-unrelated transplantation improved outcome32. Subsequent studies showed that the addition of imatinib mesylate plus intensive chemotherapy improved the 5-year disease-free survival rate to 70% and could spare patients with a good initial response to remission induction from subsequent transplantation33–35. In a multi-center study, early achievement of a negative MRD status was associated with a superior outcome36. Whether second-generation ABL tyrosine kinase inhibitors, such as dasatinib37, given in conjunction with an intensive chemotherapy backbone, can further improve outcome is being investigated.
ALL with MLL (KMT2A) rearrangement
Patients with ALL and 11q23/MLL (KMT2A) rearrangements generally have a poor prognosis38. However, collaborative studies have revealed considerable heterogeneity among these patients in age at presentation, type of MLL rearrangement, and early response to glucocorticoids38,39. For example, infants with t(4;11) and MLL-AFF1 fusion had an especially dismal prognosis when they had poor early response to steroid treatment, and among patients with t(11;19) and MLL-MLLT1 fusion, those with T-lineage ALL and age over 1 year had an excellent outcome39. Secondary chromosomal changes lacked prognostic impact in this subgroup40. With the possible exception of a small subset of infant ALL cases22, allogeneic hematopoietic stem cell transplantation failed to improve outcome compared to chemotherapy alone38,39. Current clinical trials are testing molecularly targeted therapies for infant ALL with MLL rearrangement.
Hypodiploid ALL
Hypodiploidy <44 chromosomes has been associated with a poor prognosis; near-haploidy (24 to 31 chromosomes) and low-hypodiploidy (32 to 39 chromosomes) carry a particularly dismal outcome41. Near-haploid ALL is characterized by genetic alterations targeting a receptor tyrosine kinase, Ras signaling, and the lymphoid transcription factor IKZF3, while cases of low-hypodiploid ALL have genetic alterations in RB1, IKZF2, and TP53, some of which are often germline in nature42. In a recent single-institution study, the outcome of hypodiploid ALL was substantially improved by MRD-directed treatment, and patients with a negative MRD status at the end of remission induction were highly curable with intensive chemotherapy alone43. Although allogeneic transplantation is often recommended for patients with hypodiploid ALL, the efficacy of this treatment for patients with detectable MRD, especially those with a germline TP53 mutation, remains to be evaluated.
Biologic advances: emerging subtypes of ALL
Genomic profiling studies, based on microarray-based DNA copy number and gene expression analyses and, more recently, second-generation sequencing, have advanced our understanding of the genetic basis of leukemogenesis; identified new leukemia subtypes; and revealed genomic aberrations with diagnostic, prognostic or therapeutic implications15,44.
Among newly identified subtypes of ALL, Philadelphia chromosome-like ALL, with a gene expression profile and a high frequency of alterations of the IKZF1 (70% to 80%) similar to that of Philadelphia chromosome-positive ALL but lacking the BCR-ABL1 fusion protein, was characterized by resistance to daunorubicin and asparaginase, a high MRD level during remission induction, and a poor outcome45,46,47. A large collaborative study showed that this genotype increased in frequency from 10% among children with standard-risk ALL to 27% among adolescents and young adults with B-ALL48. This subtype has a complex genomic landscape, including over 30 rearrangements involving at least 13 kinase, cytokine, and cytokine-receptor genes49. Importantly, there are several key subgroups with therapeutic implications: (1) “ABL-class” rearrangements including ABL1, ABL2, CSF1R, PDGFRA and PDGFRB that are targetable by inhibitors of ABL1 (e.g., imatinib and dasatinib); (2) alterations activating JAK-STAT signaling including EPOR and JAK2 rearrangements that are sensitive to JAK inhibitors; (3) CRLF2 rearrangements, frequently with concomitant activating JAK point mutations that may also be sensitive to JAK inhibition; (4) Ras pathway mutations (KRAS, NRAS, NF1, PTPN11); and (5) infrequent targets of rearrangement including NTRK3, PTK2B, BLNK, and others49–51. Cases with a negative MRD after remission induction can be cured with low-intensity chemotherapy, those with low levels of MRD should be treated with intensive chemotherapy, while those with high levels of MRD require transplantation if they lack genetic lesions sensitive to molecularly targeted therapy49,51.
Once considered to be a unique ALL subtype with intragenic deletions of the ERG gene and favorable outcome, a recent study showed that this variant occurs in up to 7% of B-ALL cases; has DUX-rearrangement, an early initial event in leukemogenesis; and requires gene expression or sequencing approaches for accurate diagnosis and risk assignment50. Rearrangements of monocyte enhancer factor 2D (MEF2D) and zinc finger 384 (ZNF384) characterize two new subtypes of B-ALL, accounting for 3.5% and 4% of B-ALL cases, respectively44,52,53. MEF2D ALL is associated with an older age at presentation (12 years), pre-B immunophenotype, poor outcome, deregulation of histone deacetylase 9 gene (HDAC9), and exquisite sensitivity to histone deacetylase inhibitors in xenografts, such as panobinostat52,54. ZNF384 ALL is characterized by a high frequency of expression of myeloid-associated antigens (CD13, CD33) and CD10-negative phenotype, intermediate prognosis, and upregulation of JAK-STAT pathway, suggesting a potential target for therapy with a JAK inhibitor52,53.
Recent molecular profiling studies have also shed important light on the evolutionary process of ALL during the natural history of this disease and also during ALL therapy. Backtracking studies identified sentinel genomic abnormalities (ETV6-RUNX155, hyperdiploid56, BCR-ABL157) in pre-leukemia blood samples at birth, strongly arguing for an in-utero origin of these initiating genomic event. This was particularly evident in studies of identical twins who share a clonotypic founding genomic aberrations early in life but one can acquire secondary somatic changes related to overt leukemia while the other twin remains healthy in the absence of additional genomic aberrations57. Comprehensive profiling of these secondary events reveals ancestral relationships of ALL subclones and the evolutionary history of ALL in individual patient58. In ETV6-RUNX1 ALL, there is a striking over-representation of RAG-mediated genomic mutational process59, plausibly linked to inflammation/infection-related activation of these recombination events60. In contrast, leukemia evolution plays a completely different role during ALL therapy, often times as a primary means of evasion of cytotoxic effects of chemotherapy61. Somatic mutations in key drug targets of metabolizing genes (e.g., NT5C2 and PRPS1) have been identified specifically at the time of ALL relapse and are shown to confer dramatic resistance to anti-leukemic agents62,63,64.
Germline studies of inherited predisposition to ALL and ethnic and racial differences in disease subtypes and treatment responses
In addition to somatic genomic abnormalities in the leukemia cells of ALL patients, there is substantial genetic variation in the normal genome of the patients, mostly as inherited polymorphisms. Genome-wide association studies have shown that these germline genetic variants can influence inter-patient variability in a wide range of phenotypes65, including susceptibility to leukemia, treatment outcome, and side effects of ALL therapy. These studies require large numbers of patients for discovery and validation, and to adjust for the ancestral composition of study cohorts; indeed, many recent advances in this field have been the result of national and, in many instances, international collaborations, especially in studies of rare ALL subtypes.
Inherited genetic determinants of leukemogenesis and treatment response
Some of the early evidence for a genetic basis of ALL susceptibility came from global comparison of the prevalence of this cancer. Epidemiologic studies had long suggested that Africans possessed a significantly lower risk of ALL development compared to Asian/Pacific Islanders and individuals of European descent, who in turn had a lower risk than individuals with Hispanic ethnicity66–68. These racial and ethnic groups are characterized by germline genetic variants unique to their ancestral origin69–71; hence, it was hypothesized that ancestry-related genetic variants might be one of the causes of racial difference in ALL risk.
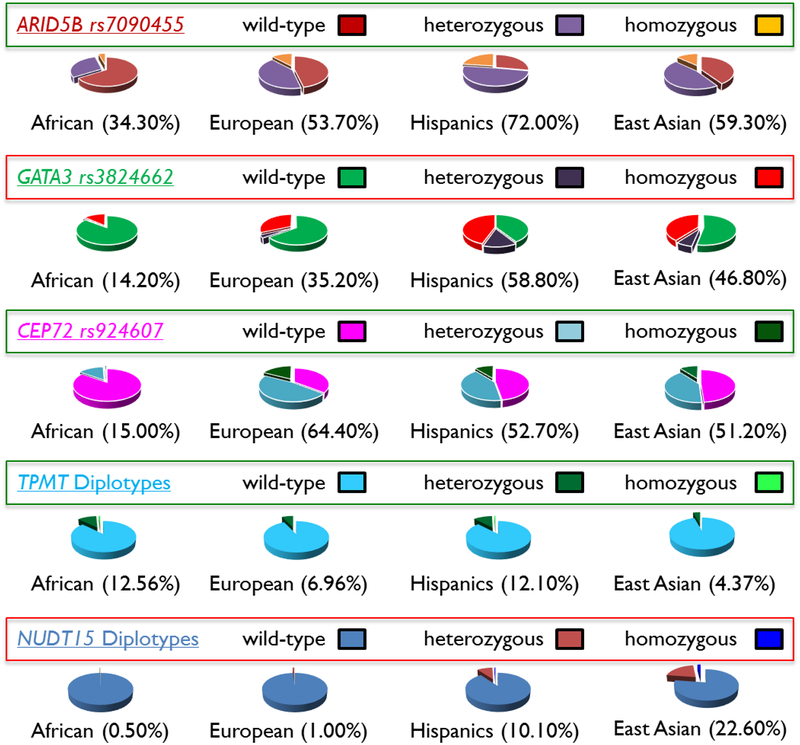
In 2009, we and others took a genome-wide approach to comprehensively search for germline variants associated with susceptibility to ALL, and identified intronic single nucleotide polymorphisms within the ARID5B, IKZF1, and CEBPE loci as having the most robust effects on ALL risk72. Subsequent larger genome-wide association studies identified additional susceptibility variants in CDKN2A73, BMII-PIP4K2A74, and GATA375. Interestingly, the ALL-related risk allele in ARID5B is unevenly distributed across populations: it is more frequent in Hispanics, followed by Europeans, and least frequent in Africans, paralleling the observed racial differences in ALL incidence (Figure 1)76. In fact, it was estimated that the ARID5B variant alone explains 30% of racial differences in ALL risk77.
Figure 1. Global variation in the frequency of key genetic variants associated with ALL susceptibility and treatment toxicities.
Numbers in parentheses denote the percentage of individuals with the risk allele.
These germline risk alleles can also exhibit their effects on ALL susceptibility in an age- and leukemia subtype-specific fashion78,79, and extend their influence to treatment outcome as well. For example, recent studies described variants in GATA3 that were significantly associated with the risk of developing Philadelphia chromosome-like ALL80. These non-coding variants in GATA3 predisposed children to the acquisition of somatic genomic aberrations specific to Philadelphia chromosome-like ALL (e.g., IKZF1 deletion, CRLF2 rearrangement, and JAK mutations)45,81,82. Importantly, GATA3 variants strongly correlated with early treatment response, as measured by MRD assay at the end of remission induction therapy, and the risk of ALL relapse across a number of different treatment regimens80,83. It should be noted that the GATA3 risk alleles were significantly more common in Hispanics (particularly those with strong Native American genetic ancestry) than in European Americans80 (Figure 1), which could contribute to the poor prognosis of ALL related to Native American genetic ancestry as we have described84.
A number of congenital abnormalities have been linked to ALL risk85–87, but the extent to which their associated genetic variants (e.g., chromosome 21 trisomy, variants in ATM and NBN genes associated with ataxia telangiectasia and Nijmegen breakage syndrome, respectively) influence the development and clinical behavior of ALL remains unclear. In addition to common variants with modest effects on ALL risk, there is growing evidence for rare germline genetic variants that are associated with highly penetrant familial malignancies. For example, germline loss-of-function variants in TP53, PAX5, and ETV6 were recently discovered in multiple independent pedigrees related to recurrent ALL; further studies identified damaging variants in these key risk genes even in children without a known family history of ALL, suggesting that the genetic predisposition to ALL is more common than originally thought. In fact, comprehensive whole-genome sequencing studies have shown that up to 5% children with ALL have potential pathogenic variants in cancer-susceptibility genes88. This information not only will influence ALL treatment, but will also impact families through genetic counseling and testing, and will facilitate measures for cancer prevention, surveillance, and early diagnosis and treatment89. For example, likely-pathogenic variants in TP53 were identified in 0.7% of children with ALL and were associated with 3.9-fold lower overall survival rate and a strikingly incidence of second cancers at 25.1%90.
Genomic determinants of drug toxicities
ALL therapy is associated with a spectrum of acute and long-term side effects91–93, that vary widely by treatment and genetic ancestry. For example, glucocorticoid-related osteonecrosis, which is significantly more common in patients of European descent than in those of African ancestry, was associated with inherited variations in glutamate receptor genes94. Asparaginase-related pancreatitis, by contrast, is associated with Native American genetic ancestry and is more frequent in Hispanics95; while vincristine-related peripheral neuropathy was less common in patients of African descent (possibly due to a lower frequency of the toxicity-related risk allele in the CEP72 gene)96 (Figure 1).
East Asians are particularly intolerant to mercaptopurine during maintenance therapy and have a high risk of hematopoietic toxicity97. This susceptibility is unrelated to inherited TPMT deficiency, a major genetic cause of mercaptopurine hematopoietic toxicity, as most individuals of East Asian descent have normal TPMT function (Figure 1). Thus, alternate hypotheses have been tested to identify the basis of mercaptopurine intolerance in this population, focusing largely on non-TPMT genetic polymorphisms97,98. A recent genome-wide association study found that germline variants in nucleoside diphosphate–linked moiety X-type motif 15 (NUDT15) also strongly predisposed patients to mercaptopurine-related toxicity, during ALL therapy, independent of TPMT99. Patients homozygous for the defective allele in NUDT15 tolerated only 6 mg/m2/day of mercaptopurine, compared to an average of 67.5 mg/m2/day for patients with wild-type NUDT15. Interestingly, the NUDT15 risk allele was markedly common in East Asians and thus responsible for their excessive mercaptopurine toxicity99 (Figure 1).
Global Impact of Childhood ALL
Childhood cancer and the global health agenda
Over the last two decades, the global health agenda led by the United Nations and the World Health Organization (WHO) (Table 4) has decreased the global child and adolescent mortality from 14.18 million deaths in 1990 to 7.26 million deaths in 2015,100 with a 52% decrease in the number of deaths among children younger than five.101 As competing causes of mortality decrease, the proportion of deaths from childhood cancer increases.17 By conservative estimates, at least 160,000 children from 0–14 years of age are diagnosed with cancer every year, and more than 90% live in LMICs.17 Asia accounts for half of the pediatric cancer burden, with another 25% of all expected cases arising in Africa. This geographic shift in the childhood cancer burden towards LMICs will likely continue due to the high birth rates in these countries. Due to these demographic trends, model forecasts suggest that pediatric cancer cases will increase by 30% by the end of this decade in LMICs, creating an imperative to prioritize initiatives for childhood cancer care and control.17,102–104
Table 4.
Timeline of global health agenda related to childhood health and childhood cancer
| September 2000 | United Nations Millennium Development Goals | |
| MDG4: To reduce under-five mortality rates by two thirds by 2015 | ||
| September 2011 | United Nations General Assembly | |
| Resolution 66/2: Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases | ||
| May 2013 | World Health Assembly | |
| Resolution 66/10: Follow-up to the Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases | ||
| WHO Global Action Plan for NCD - 25% reduction in NCD mortality by 2025 | ||
| January 2016 | United Nations Sustainable Development Goals | |
| SDG3: To ensure healthy lives and promote well-being for all at all ages | ||
| SDG3.2 | By 2030, end preventable deaths of newborns and children under 5 years of age, with all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births. | |
| SDG3.4 | By 2030, reduce by one-third premature mortality from NCD through prevention and treatment and promote mental health and well-being. | |
| SDG3.8 | Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all. | |
| May 2017 | World Health Assembly | |
| Resolution 70.12: Cancer Prevention and Control in the Context of an Integrated Approach | ||
In response to this global challenge, the member states of the WHO passed a resolution on May 31, 2017, to include pediatric cancer and cancer prevention and control to the 2013–2020 global action plan.105 As the most common and one of the most curable cancers, childhood ALL should provide an ideal model for guiding efforts to address the challenges and knowledge gaps in the WHO agenda.
Estimating the burden of childhood ALL worldwide
It is well recognized that the incidence of childhood ALL differs between high-income countries and LMICs, and also among the various ethnic and racial groups within a single country.67,103,106–109 Table 5 compares the four major pediatric leukemia burden studies that have systematically attempted to collect cancer registration data across all global regions. Two studies relied on different methods to generate global estimates of pediatric leukemia incidence and mortality: the GLOBOCAN study110, undertaken by the WHO International Agency for Research on Cancer (IARC), and the Global Burden of Disease (GBD) study111 from the Institute for Health Metrics and Evaluation. Two other published estimates from the International Incidence of Childhood Cancer 2001–2010 Study (IICC-3)109 and the CONCORD-2 study18 report registry-specific and regional variations for pediatric leukemias as a whole and do not provide estimates for all countries in the world. Thus, the best estimate available from these sources is that more than 50,000 children are diagnosed with ALL every year across the globe.17 In all likelihood, the number of individuals with this diagnosis is much higher in LMICs than commonly reported. These lack of core epidemiologic data stem from multiple factors. First, epidemiologic surveys historically grouping pediatric ALL with all leukemias. Additionally, there are very few population-based pediatric cancer registries in countries with lower incomes. In 2010, the WHO estimated that population-based cancer registration were active in just 17% of low-income countries, where the cancer burden is largest.104 Of the four major pediatric cancer disease burden estimates available, only between 3–12% of low and low-middle income country registries were included (Table 5). Finally, the accurate diagnosis of pediatric ALL remains a challenge in resource-limited settings.112 Many of the common signs and symptoms of ALL are similar to those of common communicable diseases such as malaria and dengue, resulting in substantial number of missed diagnoses. The lack of ready access to flow cytometry and cytogenetics, making it difficult to distinguish between ALL and acute myeloid leukemia, is another factor that could contribute to under-diagnosis of this lymphoid cancer.
Table 5:
Comparison of Current Global Pediatric Leukemia Burden Studies
| GBD 2015 | GLOBOCAN 2012 | IICC-3 | CONCORD-2 | |
|---|---|---|---|---|
| Global Estimates | Yes | Yes | No | No |
| Number of Registries Included | 562 | 290 | 153 | 215 |
| From Low and Low Middle Income Countries* | 12% | 7% | 11% | 3% |
| Outcomes Estimated | Incidence, Mortality, Disability Adjusted Life Years | Incidence, Mortality | Incidence | Survival |
| Global Annual Leukemia Incidence | 73,549 | 49,752 | Not Estimated | Not Estimated |
| ALL specific | 50,732 | Not Estimated | Not Estimated | Not Estimated |
| Global Annual Leukemia Mortality | 36,738 | 27,775 | Not Estimated | Not Estimated |
| ALL specific | 24,690 | Not Estimated | Not Estimated | Not Estimated |
| Disability Adjusted Life Years (ALL specific) | 1,997,861 | Not Estimated | Not Estimated | Not Estimated |
Based on 2017 World Bank LMIC Status
Treatment of Childhood ALL in Settings with Limited Resources
The ability to provide state-of-the-art curative treatments for children with cancer in LMICs is severely restricted by coexisting morbidities, as well as deficient supportive and palliative care, among other factors,17 as indicated by the lagging survival estimates (Table 2). The direct translation of effective protocols from developed countries to settings in LMICs is not a realistic option, so that steps must be taken to overcome inadequate health care capacities, socioeconomic limitations, and cultural barriers. Tiered system approaches have been proposed, with glucocorticoids plus two or three-drug induction regimens recommended according to local needs and the ability to deliver supportive care113,114. Early deaths due to infection, bleeding, and treatment abandonment often exceed the total cumulative frequency of relapse. Indeed, treatment refusal and abandonment may involve as many as 50–60% of children in some regions, thus often exceeding all other causes of treatment failure.115 Most of these patients withdraw from protocols early, usually soon after induction remission.116 An integrated multi-disciplinary approach that addresses abandonment comprehensively and that prioritizes family education and support is key to the success of any strategy. Hence, treatment strategies should be devised with clearly defined goals and trade-offs in mind. Modifications of the selected regimens should be driven by periodic review of local outcomes and should proceed as stepwise increments in diagnostic and stratification complexity, and in state-of-the-art treatment7,9,114,117–119. For example, while the Inter-Continental BFM-2002 study, conducted in 15 upper-middle and high-income countries on three continents produced 5-year event-free survival and overall survival rates of 74% and 82%, respectively,7 the adaptation of this regimen in lower-middle income countries in Central America resulted in 20% lower survival rates.118,119 The suboptimal results were not only due to delayed diagnosis, early death, and treatment abandonment, but also increased relapse rates reflecting in part a higher frequency of CNS leukemia and unfavorable genetic subtypes (e.g., BCR-ABL1 fusions) and lower frequency of favorable genetic subtypes (e.g., ETV6-RUNX1 fusions)119.
Thus, differences in outcome by ethnic group traditionally attributed to quality of care may need to be reassessed as better biologic disease characterization is performed and genome-wide studies are conducted. A recent U.S. study of more than 2,000 children with ALL identified 302 germline SNPs associated with relapse after adjusting for treatment and ancestry and 715 additional SNPs associated with relapse in an ancestry-specific manner.120 Therefore, it is critical to incorporate ancestry considerations into the development of global strategies and the implementation of adapted regimens. Finally, event-free survival should be analyzed in two ways, by treating abandonment as an adverse event and by censoring cases at the time of abandonment; these two estimates will reflect the upper and lower bounds of the true event-free survival estimate.121
The judicious adaptation of established diagnostic and therapeutic guidelines to local settings and the proper allocation of resources requires a well-trained and specialized healthcare workforce; the importance of investing in training and education while stepping up in diagnostic and treatment complexity cannot be underestimated. Models of successful implementation of training programs through partnerships with institutions in high-income countries have been well described.106 The application of cost-effective interventions and adjusting new technologies to local technical capacity should also be prioritized. Examples include the use of simplified and inexpensive assays for MRD that do not require extensive prior training in leukemia and allow for the use of risk-adapted treatment,122,123 or the implementation of early warning score systems to decrease hospital mortality in this vulnerable population.124
Delivering care: essential medicines for childhood ALL and cost of treatment
In 2004, the Ponte di Legno Working Group issued a statement on the right of children to have full access to essential treatment for ALL. This stance has prompted international agencies and regulatory authorities to provide the necessary antileukemic drugs at affordable costs worldwide, to support the development of centers of excellence, and to encourage authorities in LMICs to support measures that could increase the chances of a child to achieve cure of ALL125. Initiatives such as this will gain in importance if recent shortages in cancer drug availability persist.
Essential medicines
The WHO curates essential medicines lists to help countries prioritize and select the medicines to include in their national medication formularies and national reimbursable medicines lists -- a model that guides purchasing patterns for many governmental agencies, non-governmental organizations, and charities.126 In 2007, the WHO launched a parallel essential medicines list for children, including an expanded list of chemotherapeutic agents that has been updated every 2 years127. In 2015, the expert committee of the WHO undertook a comprehensive review of essential medicines for cancer, in both adults and children, as part of an international consultative process coordinated by the Union for International Cancer Control. This resulted in a comprehensive review of treatments for 29 cancer indications and a request to add 21 cytotoxic medicines and one supportive therapy128. Despite these efforts, there is considerable variation in the listing of anti-cancer medicines by geographical region, socioeconomic status, and burden of disease, with low-income and African countries showing more restrictions.126,128 The impact of these changes on the listing of ALL drugs cannot be ignored, as the lists often determine national access. In a global review of national essential medicines, the authors noted major deficiencies in ALL drugs128. While methotrexate and vincristine were included in 95% and 82% of the national lists, respectively, mercaptopurine, L-asparaginase, daunorubicin, and imatinib were included in only 64%, 50%, 41%, and 30%,128 further highlighting the need for coordinated efforts that include all stakeholders and prioritize the pediatric oncology needs. However, inclusion of childhood ALL drugs in national essential medicines lists is meaningless without a strong commitment by governments to guarantee access.
Access to medications for patients with cancer in LMIC is the subject of a recent review.129 The authors observed that “in low-resource settings, institutions might be weaker and problems with management and accountability might be prevalent, leading to corruption and perverse incentives that result in underfunding of and misallocations of expenditures”. These problems may be compounded by inappropriate utilization and poor inventory control.130 Solutions include the establishment of universal insurance coverage that empowers consumers,129 and the use of generic and bio-similar off-patent medications, especially if produced in LMIC and utilizing compulsory licensing as authorized by the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement negotiated by the World Trade Organization.131,132 Access may be enhanced also by participation in clinical trials, especially if funded by the pharmaceutical industry.129
Treatment costs
Costs of cancer treatment are rising so rapidly that even wealthy countries face economic difficulty in delivering high-quality cancer care equally to all of their citizens.104 The frailty of the healthcare systems in LMICs adds to their lack of essential resources; indeed, most have evolved into fragmented structures that provide only minimum urgent care. Further, the lack of health-care coverage for large segments of the population exposes them to a high risk of catastrophic and impoverishing health payments, which drives many families into poverty. In 2008, for example, one-third of the population in Latin America was at high risk for such impoverishment due to catastrophic health expenditures.133
Very few data exist regarding the cost of treating childhood cancer in LMIC. Two recent studies using a threshold analysis and global costing procedure suggest treating childhood cancers in LMICs is very cost-effective as per WHO criteria.134,135 Very few formal micro-costing studies of ALL specifically have been completed with published reports showing a wide variation in ALL treatment costs, ranging from $3,000 in Bangladesh136 to $10,000 to 20,000 in Brazil134, Mexico137, and China138,139 to $100,000 in North America and Europe140. A significant proportion of the costs (up to 50% in many cases) are associated with diagnostics, supportive care, and hospitalization. While access to chemotherapeutic agents is a limiting factor in many occasions, the costs related to drugs (including chemotherapy and supportive care drugs) accounts for less than one-third of the total costs135. In low-income countries, the monthly expenses related to the treatment of a child with ALL can be more than seven times the monthly per capita income, resulting in a dramatic loss of income and employment.141 The high price of some drugs for the treatment of cancer and supportive care are all-too-often a major obstacle to the provision of appropriate treatment for children with ALL in LMICs, higher than in HIC relative to family income, and yet treatment of childhood ALL is demonstrably very cost-effective134. Indeed the Institute of Medicine has identified the treatment of highly curable cancers in young people as one of the three main strategies to address the global cancer crisis142. The precedents offered by GAVI, the Global Fund and UNITAID suggest that an innovative financing approach, based on a value chain framework,143 is worthy of exploration.
An example of the coordinated efforts by multiple stakeholders to secure treatment for children with ALL is afforded by China, where 10,000 to 12,000 children are diagnosed with ALL every year. In the past few decades, China has moved from a system of universal insurance to a largely privatized health-care system with the majority of rural residents remaining uninsured144. In December 2004, a standardized, cost-efficient protocol was developed jointly by the Shanghai Children’s Medical Center, the Beijing Children’s Hospital, and St. Jude Children’s Research Hospital to treat underprivileged children with low- and intermediate-risk ALL with the support of a charitable foundation. In 2009, the effectiveness and the affordability (&14,000 USD) of the clinical trial were reported138, which prompted the Ministry of Health in 2010 to provide governmental funding toward the treatment of children with ALL145. With this drastic increase in the number of patients having access to treatment and the unprecedented opportunity of clinical and translational research, Shanghai Children’s Medical Center and St. Jude Children’s Research Hospital in October 2014 formed a national childhood ALL study group comprising 20 major hospitals and medical centers across China. A risk-adapted treatment protocol was initiated in early 2015 with a targeted enrollment of 1,200 patients a year.
Collaborative Efforts and Network Development
International partnerships that integrate twinning between institutions in HIC and LMIC along with commitment from local governments and participation of global health agencies, advocacy groups, and local foundations, have provided successful models for building sustainable pediatric oncology programs in LMIC.106 The incorporation of the research methodology is a necessary step in their growth and in the regional initiatives that eventually may result; cooperative groups have been formed in different resource-limited regions of the Americas, Africa, East Mediterranean, Asia, and Oceania17. These partnerships can help design and conduct clinical research focused on the epidemiologic, biologic, clinical, and psychosocial questions relevant to the advancement of local care, the development of treatment guidelines and interventions, and to guiding public health priorities.17,146 The importance of incorporating a clinical research infrastructure early in the process of program building should be emphasized, and cost-effective data management tools should be developed. Ultimately, strengthening those networks and facilitating the development of new regional collaborations with support from larger consortia in high-income countries has the potential to create a new framework to advance care for children with ALL and other malignancies worldwide. Different levels of consortia with a step-wise increment in their level of complexity and range of initiatives developed have been proposed17.
Conclusions and Future Directions
Collaborative studies have refined the molecular diagnosis, improved risk assignment, validated new treatment strategies, and contributed to personalized therapy by identifying new targets for therapeutic intervention. In many instances, it has been possible to translate these advances to patients in LMIC. For example, the finding that systemic or intrathecal chemotherapy delivered effectively can safely replace prophylactic cranial irradiation147, and the development of simple and inexpensive assays to measure MRD levels during early remission induction to identify low-risk patients for reduced-intensity therapy, could be readily applied to the treatment of patients in limited-resource settings122. That a single year of chemotherapy cured two thirds of patients with newly diagnosed ALL, especially those with ETV6-RUNX1148, and that a single infusion of chimeric antigen receptor-engineered T-cells could be curative in patients with relapsed or refractory ALL149, are particularly encouraging. It is hope that such treatment strategies can eventually be applied to patients in LMIC to avoid the need of long-term continuation treatment.
Global comparison of ALL-related traits can often shed new light on the biology of leukemia as well as treatment toxicities. To this end, international efforts are under way to systematically map treatment-related toxicities across diverse regions to develop adapted guidelines and support related research initiatives150. Perhaps the greatest challenge for the future will stem from rapid demographic changes, which place even greater emphasis on devising ALL management strategies that can be rapidly employed in the face of large shifts in patient populations. Thus, there is a need for high-quality research that incorporates comprehensive epidemiologic studies and explores feasible, evidence-based, cost-effective, and resource-adapted therapies151. At the same time, efforts should be intensified to understand how inherited genetic polymorphisms underlie ethnic and racial differences in the incidence and outcome of ALL, and how the acquisitions of key somatic mutations could be blocked to lower the risk of ALL development15, the Holy Grail for pediatric oncologists. It will require a coordinated effort by academic institutions, global and public health governmental and non-governmental agencies, advocacy groups, and professional societies to address the global challenge of childhood ALL. Specific goals to guarantee access to care and to provide adequate diagnosis and treatment to all children with ALL within the next decade should be set, and the firm objective to cure all children, regardless of where they live, should concentrate all our efforts.
Search strategy and selection criteria
We searched PubMed for English-language articles published from Jan 1, 2000 to November 1, 2017, with the keywords “acute lymphoblastic leukemia”, “acute lymphocytic leukemia”, “acute lymphoid leukemia”, “pediatric”, and “childhood”. Further relevant articles were selected from the lists used in our previous publications. In some instances, review articles were selected over original articles because of space constraints.
Key messages.
More accurate diagnosis, improved risk-directed chemotherapy, and advances in supportive care have made childhood acute lymphoblastic leukemia (ALL) one of the most curable cancers. However, the 5-year survival rates of over 90% being achieved in most developed countries do not extend to countries with limited resources.
Factors contributing to the inferior outcomes in low-income countries include delayed diagnosis, treatment abandonment, increased numbers of infectious deaths and increased relapse rate due to a high proportion of patients with unfavorable genetic subtypes of ALL and a lack of access to effective treatment.
Inherited genetic variants influence the susceptibility to ALL, treatment response, and the side effects of therapy, and merit greater attention as means to reduce the global disparities in ALL.
Emerging ALL subtypes mandate research to devise effective targeted therapies for these disease variants and integrate them into frontline treatments.
Advancing the cure rates and quality of life of childhood cancer patients in low- and middle-income countries will require greater involvement by international organizations, both governmental and non-governmental.
Acknowledgments
This Review was supported in part by U.S. National Institutes of Health Grants Nos. CA21765, CA36401, CA176063, and GM115279, GM118578, and American Lebanese Syrian Associated Charities of St Jude Children’s Research Hospital. These funding sources had no role in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declared no conflicts of interest.
REFERENCES
- 1.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol 2013; 50(3): 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016; 127(17): 2101–12. [DOI] [PubMed] [Google Scholar]
- 3.Escherich G, Zimmermann M, Janka-Schaub G, Co ALLsg. Doxorubicin or daunorubicin given upfront in a therapeutic window are equally effective in children with newly diagnosed acute lymphoblastic leukemia. A randomized comparison in trial CoALL 07–03. Pediatr Blood Cancer 2013; 60(2): 254–7. [DOI] [PubMed] [Google Scholar]
- 4.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 2012; 30(14): 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pieters R, Groot-Kruseman Hd, Velden VVd, et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. Journal of Clinical Oncology 2016; 34(22): 2591–601. [DOI] [PubMed] [Google Scholar]
- 6.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol 2015; 16(16): 1677–90. [DOI] [PubMed] [Google Scholar]
- 7.Domenech C, Suciu S, De Moerloose B, et al. Dexamethasone (6 mg/m2/day) and prednisolone (60 mg/m2/day) were equally effective as induction therapy for childhood acute lymphoblastic leukemia in the EORTC CLG 58951 randomized trial. Haematologica 2014; 99(7): 1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stary J, Zimmermann M, Campbell M, et al. Intensive Chemotherapy for Childhood Acute Lymphoblastic Leukemia: Results of the Randomized Intercontinental Trial ALL IC-BFM 2002. Journal of Clinical Oncology 2014; 32(3): 174–84. [DOI] [PubMed] [Google Scholar]
- 9.Yamaji K, Okamoto T, Yokota S, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group. Pediatr Blood Cancer 2010; 55(7): 1287–95. [DOI] [PubMed] [Google Scholar]
- 10.Yeoh AE, Ariffin H, Chai EL, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol 2012; 30(19): 2384–92. [DOI] [PubMed] [Google Scholar]
- 11.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 2014; 15(8): 809–18. [DOI] [PubMed] [Google Scholar]
- 12.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2017. [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Pei D, Coustan-Smith E, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol 2015; 16(4): 465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HC, Yeh TC, Hou JY, et al. Triple intrathecal therapy alone with omission of cranial radiation in children with acute lymphoblastic leukemia. J Clin Oncol 2014; 32(17): 1825–9. [DOI] [PubMed] [Google Scholar]
- 15.Pui CH, Yang JJ, Hunger SP, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J Clin Oncol 2015; 33(27): 2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan R, Kowalczyk JR, Agarwal B, et al. New policies to address the global burden of childhood cancers. The Lancet Oncology 2013; 14(3): e125–e35. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. Journal of Clinical Oncology 2015; 33(27): 3065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). The Lancet 2015; 385(9972): 977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrappe M, Camitta B, Pui CH, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia 2000; 14(12): 2193–4. [DOI] [PubMed] [Google Scholar]
- 20.Schrappe M, Nachman J, Hunger S, et al. ‘Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000)’. Leukemia 2010; 24(2): 253–4. [DOI] [PubMed] [Google Scholar]
- 21.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet 2007; 370(9583): 240–50. [DOI] [PubMed] [Google Scholar]
- 22.Mann G, Attarbaschi A, Schrappe M, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood 2010; 116(15): 2644–50. [DOI] [PubMed] [Google Scholar]
- 23.Buitenkamp TD, Izraeli S, Zimmermann M, et al. Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood 2014; 123(1): 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullighan CG, Collins-Underwood JR, Phillips LA, et al. Rearrangement of CRLF2 in B-progenitor-and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 2009; 41(11): 1243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buitenkamp TD, Pieters R, Gallimore NE, et al. Outcome in children with Down’s syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia 2012; 26(10): 2204–11. [DOI] [PubMed] [Google Scholar]
- 26.Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 2012; 366(15): 1371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor D, Moorman AV, Wade R, et al. Use of Minimal Residual Disease Assessment to Redefine Induction Failure in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol 2017; 35(6): 660–7. [DOI] [PubMed] [Google Scholar]
- 28.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 2009; 10(2): 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 2014; 166(3): 421–4. [DOI] [PubMed] [Google Scholar]
- 30.Conter V, Valsecchi MG, Buldini B, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: a retrospective analysis. Lancet Haematol 2016; 3(2): e80–6. [DOI] [PubMed] [Google Scholar]
- 31.Arico M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med 2000; 342(14): 998–1006. [DOI] [PubMed] [Google Scholar]
- 32.Arico M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol 2010; 28(31): 4755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 2012; 13(9): 936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia 2014; 28(7): 1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeha S, Coustan-Smith E, Pei D, et al. Impact of tyrosine kinase inhibitors on minimal residual disease and outcome in childhood Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2014; 120(10): 1514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazzaniga G, De Lorenzo P, Alten J, et al. Predictive value of MRD in Ph+ ALL treated with imatinib in the EsPhALL study, based on IG/TR and BCR/ABL1 methodologies. Haematologica 2017. [Google Scholar]
- 37.Zwaan CM, Rizzari C, Mechinaud F, et al. Dasatinib in children and adolescents with relapsed or refractory leukemia: results of the CA180–018 phase I dose-escalation study of the Innovative Therapies for Children with Cancer Consortium. J Clin Oncol 2013; 31(19): 2460–8. [DOI] [PubMed] [Google Scholar]
- 38.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 2002; 359(9321): 1909–15. [DOI] [PubMed] [Google Scholar]
- 39.Pui CH, Chessells JM, Camitta B, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 2003; 17(4): 700–6. [DOI] [PubMed] [Google Scholar]
- 40.Moorman AV, Raimondi SC, Pui CH, et al. No prognostic effect of additional chromosomal abnormalities in children with acute lymphoblastic leukemia and 11q23 abnormalities. Leukemia 2005; 19(4): 557–63. [DOI] [PubMed] [Google Scholar]
- 41.Nachman JB, Heerema NA, Sather H, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 2007; 110(4): 1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmfeldt L, Wei L, Diaz-Flores E, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 2013; 45(3): 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullighan CG, Jeha S, Pei D, et al. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood 2015; 126(26): 2896–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iacobucci I, Mullighan CG. Genetic Basis of Acute Lymphoblastic Leukemia. J Clin Oncol 2017; 35(9): 975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 2009; 360(5): 470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol 2009; 10(2): 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood 2013; 122(15): 2622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014; 371(11): 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts KG, Pei D, Campana D, et al. Outcomes of children with BCR-ABL1-like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol 2014; 32(27): 3012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, McCastlain K, Yoshihara H, et al. Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat Genet 2016; 48(12): 1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pui C-H, Roberts KG, Yang JJ, Mullighan CG. Philadelphia Chromosome–like Acute Lymphoblastic Leukemia. Clinical Lymphoma Myeloma and Leukemia 2017; 17(8): 464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu YF, Wang BY, Zhang WN, et al. Genomic Profiling of Adult and Pediatric B-cell Acute Lymphoblastic Leukemia. EBioMedicine 2016; 8: 173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian M, Zhang H, Kham SK-Y, et al. Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res 2017; 27(2): 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu Z, Churchman M, Roberts K, et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat Commun 2016; 7: 13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alpar D, Wren D, Ermini L, et al. Clonal origins of ETV6-RUNX1+ acute lymphoblastic leukemia: studies in monozygotic twins. Leukemia 2014; 29: 839. [DOI] [PubMed] [Google Scholar]
- 56.Bateman CM, Alpar D, Ford AM, et al. Evolutionary trajectories of hyperdiploid ALL in monozygotic twins. Leukemia 2014; 29: 58. [DOI] [PubMed] [Google Scholar]
- 57.Cazzaniga G, van Delft FW, Lo Nigro L, et al. Developmental origins and impact of BCR-ABL1 fusion and IKZF1 deletions in monozygotic twins with Ph+ acute lymphoblastic leukemia. Blood 2011; 118(20): 5559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 2010; 469: 356. [DOI] [PubMed] [Google Scholar]
- 59.Papaemmanuil E, Rapado I, Li Y, et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nature Genetics 2014; 46: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swaminathan S, Klemm L, Park E, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nature Immunology 2015; 16: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spinella J-F, Richer C, Cassart P, Ouimet M, Healy J, Sinnett D. Mutational dynamics of early and late relapsed childhood ALL: rapid clonal expansion and long-term dormancy. Blood Advances 2018; 2(3): 177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma X, Edmonson M, Yergeau D, et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun. England DOI - 10.1038/ncomms7604: Nature Pub. Group; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer JA, Wang J, Hogan LE, et al. Relapse specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nature genetics. United States DOI - 10.1038/ng.2558; 2013. p. 290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li B, Li H, Bai Y, et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med. United States DOI - 10.1038/nm.3840; 2015. p. 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moriyama T, Relling MV, Yang JJ. Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood 2015; 125(26): 3988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metayer C, Milne E, Clavel J, et al. The Childhood Leukemia International Consortium. Cancer Epidemiol 2013; 37(3): 336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chow EJ, Puumala SE, Mueller BA, et al. Childhood cancer in relation to parental race and ethnicity. Cancer 2010; 116(12): 3045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giddings BM, Whitehead TP, Metayer C, Miller MD. Childhood leukemia incidence in California: High and rising in the Hispanic population. Cancer 2016; 122(18): 2867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li JZ, Absher DM, Tang H, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science 2008; 319(5866): 1100–4. [DOI] [PubMed] [Google Scholar]
- 70.Pagani L, Lawson DJ, Jagoda E, et al. Genomic analyses inform on migration events during the peopling of Eurasia. Nature 2016; 538(7624): 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reich D, Patterson N, Campbell D, et al. Reconstructing Native American population history. Nature 2012; 488(7411): 370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trevino LR, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009; 27(35): 5972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu H, Zhang H, Yang W, et al. Inherited coding variants at the CDKN2A locus influence susceptibility to acute lymphoblastic leukaemia in children. 2015; 6: 7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu H, Yang W, Perez-Andreu V, et al. Novel Susceptibility Variants at 10p12.31–12.2 for Childhood Acute Lymphoblastic Leukemia in Ethnically Diverse Populations. JNCI Journal of the National Cancer Institute 2013; 105(10): 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet 2009; 41(9): 1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu H, Cheng C, Devidas M, et al. ARID5B Genetic Polymorphisms Contribute to Racial Disparities in the Incidence and Treatment Outcome of Childhood Acute Lymphoblastic Leukemia. Journal of Clinical Oncology 2012; 30(7): 751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang W, Trevino LR, Yang JJ, et al. ARID5B SNP rs10821936 is associated with risk of childhood acute lymphoblastic leukemia in blacks and contributes to racial differences in leukemia incidence. Leukemia 2010; 24(4): 894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trevino LR, Yang W, French D, et al. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet 2009; 41(9): 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Garcia A, Ambesi-Impiombato A, Hadler M, et al. Genetic loss of SH2B3 in acute lymphoblastic leukemia. Blood 2013; 122(14): 2425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez-Andreu V, Roberts KG, Harvey RC, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet 2013; 45(12): 1494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood 2010; 116(23): 4874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2009; 106(23): 9414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Migliorini G, Fiege B, Hosking FJ, et al. Variation at 10p12.2 and 10p14 influences risk of childhood B-cell acute lymphoblastic leukemia and phenotype. Blood 2013; 122(19): 3298–307. [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet 2011; 43(3): 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izraeli S The acute lymphoblastic leukemia of Down Syndrome - Genetics and pathogenesis. Eur J Med Genet 2016; 59(3): 158–61. [DOI] [PubMed] [Google Scholar]
- 86.Suarez F, Mahlaoui N, Canioni D, et al. Incidence, presentation, and prognosis of malignancies in ataxia-telangiectasia: a report from the French national registry of primary immune deficiencies. J Clin Oncol 2015; 33(2): 202–8. [DOI] [PubMed] [Google Scholar]
- 87.Pastorczak A, Szczepanski T, Mlynarski W. Clinical course and therapeutic implications for lymphoid malignancies in Nijmegen breakage syndrome. Eur J Med Genet 2016; 59(3): 126–32. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Walsh MF, Wu G, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med 2015; 373(24): 2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porter CC, Druley TE, Erez A, et al. Recommendations for Surveillance for Children with Leukemia-Predisposing Conditions. Clin Cancer Res 2017; 23(11): e14–e22. [DOI] [PubMed] [Google Scholar]
- 90.Qian M, Cao X, Devidas M, et al. TP53 Germline Variations Influence the Predisposition and Prognosis of B-Cell Acute Lymphoblastic Leukemia in Children. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. United States DOI - 10.1200/JCO.2017.75.5215; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011; 29(5): 551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Semin Hematol 2009; 46(1): 52–63. [DOI] [PubMed] [Google Scholar]
- 93.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007; 297(11): 1207–15. [DOI] [PubMed] [Google Scholar]
- 94.Karol SE, Yang W, Van Driest SL, et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood 2015; 126(15): 1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu C, Yang W, Devidas M, et al. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol 2016; 34(18): 2133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 2015; 313(8): 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. Journal of gastroenterology and hepatology 2009; 24(7): 1258–64. [DOI] [PubMed] [Google Scholar]
- 98.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood 2014; 124(15): 2345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 2015; 33(11): 1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.The Global Burden of Disease C, Adolescent Health C. Child and adolescent health from 1990 to 2015: Findings from the global burden of diseases, injuries, and risk factors 2015 study. JAMA Pediatrics 2017; 171(6): 573–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collaborators GBDCM. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 2016; 388(10053): 1725–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heck JE, Park AS, Contreras ZA, et al. Risk of Childhood Cancer by Maternal Birthplace: A Test of the Hispanic Paradox. JAMA pediatrics 2016; 170(6): 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Katz AJ, Chia VM, Schoonen WM, Kelsh MA. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes & Control 2015; 26(11): 1627–42. [DOI] [PubMed] [Google Scholar]
- 104.Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. The Lancet 2014; 383(9916): 564–73. [DOI] [PubMed] [Google Scholar]
- 105.Burki TK. WHA passes resolution on cancer prevention and control. The Lancet Oncology 2017; 18(7): 858. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez-Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Current Opinion in Pediatrics 2013; 25(1): 3–15 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 107.Valery PC, Moore SP, Meiklejohn J, Bray F. International variations in childhood cancer in indigenous populations: a systematic review. The Lancet Oncology 2014; 15(2): e90–e103. [DOI] [PubMed] [Google Scholar]
- 108.Bhatia S Disparities in cancer outcomes: Lessons learned from children with cancer. Pediatric Blood & Cancer 2011; 56(6): 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. The Lancet Oncology 2017; 18(6): 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferlay J, Soerjomataram II, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2014: n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 111.Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncology 2017; 3(4): 524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Access Healthcare and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. The Lancet 2017; 390(10091): 231–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hunger SP, Sung L, Howard SC. Treatment strategies and regimens of graduated intensity for childhood acute lymphoblastic leukemia in low-income countries: A proposal. Pediatric Blood & Cancer 2009; 52(5): 559–65. [DOI] [PubMed] [Google Scholar]
- 114.Yeoh AEJ, Tan D, Li C-K, Hori H, Tse E, Pui C-H. Management of adult and paediatric acute lymphoblastic leukaemia in Asia: resource-stratified guidelines from the Asian Oncology Summit 2013. The Lancet Oncology 2013; 14(12): e508–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Friedrich P, Lam CG, Itriago E, Perez R, Ribeiro RC, Arora RS. Magnitude of Treatment Abandonment in Childhood Cancer. PLoS ONE 2015; 10(9): e0135230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sitaresmi MN, Mostert S, Schook RM, Sutaryo, Veerman AJP. Treatment refusal and abandonment in childhood acute lymphoblastic leukemia in Indonesia: an analysis of causes and consequences. Psycho-Oncology 2010; 19(4): 361–7. [DOI] [PubMed] [Google Scholar]
- 117.Karachunskiy A, Herold R, von Stackelberg A, et al. Results of the first randomized multicentre trial on childhood acute lymphoblastic leukaemia in Russia. Leukemia 2008; 22: 1144–53. [DOI] [PubMed] [Google Scholar]
- 118.Navarrete M, Rossi E, Brivio E, et al. Treatment of childhood acute lymphoblastic leukemia in central America: A lower-middle income countries experience. Pediatric Blood & Cancer 2014; 61(5): 803–9. [DOI] [PubMed] [Google Scholar]
- 119.Antillón FG, Blanco JG, Valverde PD, et al. The treatment of childhood acute lymphoblastic leukemia in Guatemala: Biologic features, treatment hurdles, and results. Cancer 2017; 123(3): 436–48. [DOI] [PubMed] [Google Scholar]
- 120.Karol SE, Larsen E, Cheng C, et al. Genetics of ancestry-specific risk for relapse in acute lymphoblastic leukemia. Leukemia 2017; 31(6): 1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedrich P, Ortiz R, Strait K, et al. Pediatric sarcoma in Central America. Cancer 2012: n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coustan-Smith E, Ribeiro RC, Stow P, et al. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood 2006; 108(1): 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vinhas E, Lucena-Silva N, Pedrosa F. Implementation of a simplified flow cytometric assays for minimal residual disease monitoring in childhood acute lymphoblastic leukemia. Cytometry Part B: Clinical Cytometry 2016: n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 124.Agulnik A, Mora Robles LN, Forbes PW, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource-limited pediatric oncology hospital. Cancer 2017; 123(15): 2965–74. [DOI] [PubMed] [Google Scholar]
- 125.Pui CH, Schrappe M, Masera G, et al. Ponte di Legno Working Group: statement on the right of children with leukemia to have full access to essential treatment and report on the Sixth International Childhood Acute Lymphoblastic Leukemia Workshop. Leukemia 2004; 18(6): 1043–53. [DOI] [PubMed] [Google Scholar]
- 126.Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. The Lancet 2017; 389(10067): 403–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Organization WH. WHO Model List of Essential Medicines for Children (3rd List, March 2011). http://wwwwhoint/medicines/publications/essentialmedicines/en/indexhtml.
- 128.Robertson J, Barr R, Shulman LN, Forte GB, Magrini N. Essential medicines for cancer: WHO recommendations and national priorities. Bulletin of the World Health Organization 2016; 94(10): 735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lopes GdL, de Souza JA, Barrios C. Access to cancer medications in low- and middle-income countries. Nat Rev Clin Oncol 2013; 10(6): 314–22. [DOI] [PubMed] [Google Scholar]
- 130.Clements B, Coady D, Gupta S. The Economics of Public Health Care Reform in Advanced and Emerging Economies. International Monetary Fund 2012. [Google Scholar]
- 131.WHO. Trade-related aspects of intellectual property rights. http://www.wto.org/english/tratop_e/trips_e/trips_e.htm. 2013.
- 132.WTO. Compulsory licensing of pharmaceuticals and TRIPS. World Trade Organization. http://wwwwtoorg/english/tratop_e/trips_e/public_health_faq_ehtm 2006.
- 133.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. The Lancet Oncology 2013; 14(5): 391–436. [DOI] [PubMed] [Google Scholar]
- 134.Bhakta N, Martiniuk ALC, Gupta S, Howard SC. The cost effectiveness of treating paediatric cancer in low-income and middle-income countries: a case-study approach using acute lymphocytic leukaemia in Brazil and Burkitt lymphoma in Malawi. Archives of Disease in Childhood 2013; 98(2): 155–60. [DOI] [PubMed] [Google Scholar]
- 135.Fuentes-Alabi S, Bhakta N, Vasquez RF, Gupta S, Horton SE. The cost and cost-effectiveness of childhood cancer treatment in El Salvador, Central America: A report from the Childhood Cancer 2030 Network. Cancer 2018; 124: 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Islam A, Akhter A, Eden T. Cost of treatment for children with acute lymphoblastic leukemia in Bangladesh. Journal of Cancer Policy 2015; 6: 37–43. [Google Scholar]
- 137.Jaime-Pérez JC, Fernández LT, Jiménez-Castillo RA, et al. Hospitalization rate and costs in acute lymphoblastic leukemia of childhood in a low-income group: Financial impact in Northeast Mexico. Pediatric Blood & Cancer 2017; 64(12): e26673–n/a. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Chen J, Tang J, Ni S, Xue H, Pan C. Cost of childhood acute lymphoblastic leukemia care in Shanghai, China. Pediatric Blood & Cancer 2009; 53(4): 557–62. [DOI] [PubMed] [Google Scholar]
- 139.Luo X-Q, Ke Z-Y, Guan X-Q, Zhang Y-C, Huang L-B, Zhu J. The comparison of outcome and cost of three protocols for childhood non-high risk acute lymphoblastic leukemia in China. Pediatric Blood & Cancer 2008; 51(2): 204–9. [DOI] [PubMed] [Google Scholar]
- 140.Rae C, Furlong W, Jankovic M, et al. Economic evaluation of treatment for acute lymphoblastic leukaemia in childhood. European Journal of Cancer Care 2014; 23(6): 779–85. [DOI] [PubMed] [Google Scholar]
- 141.Ghatak N, Trehan A, Bansal D. Financial burden of therapy in families with a child with acute lymphoblastic leukemia: report from north India. Supportive Care in Cancer 2016; 24(1): 103–8. [DOI] [PubMed] [Google Scholar]
- 142.Sloan F, Gelband H, eds. Cancer control opportunities in low- and middle-income countries. Washington DC, Institute of Medicine of the National Academies, National Academies Press, 2007 2007. [PubMed] [Google Scholar]
- 143.Atun R, Knaul FM, Akachi Y, Frenk J. Innovative financing for health: what is truly innovative? The Lancet 2012; 380(9858): 2044–9. [DOI] [PubMed] [Google Scholar]
- 144.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. The Lancet Oncology 2014; 15(5): 489–538. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Z, Mi J, Gum L, et al. Using sound Clinical Paths and Diagnosis-related Groups (DRGs)-based payment reform to bring benefits to patient care: A case study of leukemia therapy. Front Med China 2010; 4: 8–15. [Google Scholar]
- 146.Denburg AE, Joffe S, Gupta S, et al. Pediatric oncology research in low income countries: Ethical concepts and challenges. Pediatric Blood & Cancer 2012; 58(4): 492–7. [DOI] [PubMed] [Google Scholar]
- 147.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360(26): 2730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kato M, Ishimaru S, Seki M, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia 2017; 31(3): 580–4. [DOI] [PubMed] [Google Scholar]
- 149.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol 2016; 17(6): e231–9. [DOI] [PubMed] [Google Scholar]
- 151.Joffe S, Miller FG. Ethics of Cancer Clinical Trials in Low-Resource Settings. Journal of Clinical Oncology 2014; 32(28): 3192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]