Abstract
Objective:
Previous research has not investigated the role of prescription medication in sleep-obesity associations despite the fact that 56% of U.S. adults take at least one prescription medication.
Methods:
Data from n = 16,622 adults in the National Health and Nutrition Examination Survey (2007–2012) were used to examine how the association between obesity and self-reported sleep duration varied by total number of prescription medications used in the past 30 days and by select classes of prescription medications including anxiolytics/sedatives/hypnotics, antidepressants, sleep aids, anticonvulsants, thyroid agents, and metabolic agents.
Results:
Logistic regression analyses showed a significant inverse linear association of sleep duration and obesity, regardless of the total number of prescription medications individuals were taking. Each additional hour of sleep was associated with a 10% decrease in the odds of obesity. Results suggest that increased sleep duration is associated with lower odds of having obesity overall, even for long-duration sleepers (≥9 h), and this association does not differ for those taking antidepressants, thyroid agents, metabolic agents, and multiple prescription medications.
Conclusions:
The relationship between sleep duration and obesity was similar among all prescription medication users and nonusers. The potential for a nonlinear association between sleep duration and obesity may be important to examine in some specific prescription medication classes.
Introduction
Cross-sectional, longitudinal, and experimental research support a relation between short sleep duration and adult obesity (1,2). Experimental research restricting sleep duration has shown causal effects on weight gain, and improving sleep habits represents a potential intervention target for obesity treatment and prevention (2). Evidence supports potential mechanisms for a causal effect of short sleep on obesity including changes to hormones that control metabolism, hunger, and satiety (2). Although there is variation, short sleep duration is conservatively defined as sleeping less than 6 h per night (2). Less is known about long sleep duration (defined as sleeping 9 h or more per night). Some studies, however, suggest long sleep duration may also be associated with obesity (1) or that higher obesity prevalence in long-duration sleepers is secondary to other psychological and medical disorders that are associated with obesity (2). Contrary to short sleep duration and obesity, mechanisms to explain associations between long sleep duration and health status are lacking (3).
Data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2008 indicated that in the past month over 56% of U.S. adults used at least one prescription drug, and over 61% of adults with obesity used at least one prescription drug (4). Approximately 14% of all adults and 20% of adults with obesity (4) reported using five or more prescription medications. In addition, previous research has shown that prescription sleep aid use is higher among adults who report either a short or long sleep duration (5).
Few studies have investigated the role of prescription medication use in the context of sleep duration and obesity. While some studies of sleep duration and obesity have included covariates for prescription medication use (6), many have not included covariates or excluded participants taking certain medications, which limits research generalizability (7–9). Many classes of prescription medications, however, may be relevant to understanding the observed associations between sleep duration and obesity. For example, some commonly used prescription medications, including psychoactive and thyroid medications, may have side effects that include changes in sleep, may be used to treat conditions that include changes in sleep, or are associated with patients presenting with complaints of fatigue (10,11). Epilepsy and medications for epilepsy have known interactions with sleep (12). Metabolic agents (e.g., antidiabetes, antihyperlipidemic, antiobesity agents) may be important to examine to determine whether sleep-obesity associations differ among individuals who are likely to already have obesity and other comorbid chronic health conditions. Furthermore, drug interactions, off-label medication use, and individual differences in responses to medication may complicate research efforts to screen out individuals for whom medication use is likely to influence sleep-obesity associations (13,14).
Limitations of previous studies support the need for more research to clarify what role prescription medication use may play in the association between sleep duration and obesity, particularly with long-duration sleepers about whom less is known. The aims of the current study, therefore, were to: 1) examine whether the association between sleep duration and obesity is moderated by total number of prescription medications, and 2) examine whether the association between sleep duration and obesity is moderated by different classes of prescription medications.
Methods
Data from the 2007 to 2012 cycles of NHANES were used in the current analysis. NHANES uses a complex sampling design to produce a nationally representative sample of the U.S. civilian noninstitutionalized population. Three 2-year data cycles were needed to obtain a large enough sample size of long-duration sleepers (9 or more hours). During 2007 to 2012, NHANES over-sampled some racial/ethnic minorities (Hispanic and non-Hispanic black), non- Hispanic white persons 80 years and older, and non-Hispanic white persons with low income. Beginning in 2011 to 2012, non-Hispanic Asian persons were also over-sampled. NHANES includes an inperson home interview during which demographic, socioeconomic, and health-related questions are assessed, including interviewer verified prescription medication data. Examination data, including medical, physiological, laboratory, and anthropometric measures, are collected during a separate visit to a Mobile Examination Center. The National Center for Health Statistics Research Ethics Review Board approved the protocol, and informed consent was obtained from all adult participants. The overall examination response rate for the 2007 to 2012 cycles ranged from 69.5% to 77.3%. Additional NHANES design and protocol details are available elsewhere (15).
Self-reported sleep duration
Sleep duration was assessed with a single question, “How much sleep do you usually get at night on weekdays or workdays?” Participants reported the number of hours in integers (i.e., 7 h or 8 h was allowed but nothing between 7 h and 8 h).
Medication use
Prescription medication use was collected in a personal interview in the home before physical examination. Participants were asked whether they have taken any medications in the past 30 days for which a prescription was needed. Those who answered “yes” were asked to provide the medication containers for all the products used. The product name was entered into the Computer-Assisted Personal Interviewing system. If no container was available, participants were asked to verbally report the name of the medication. Approximately 81% of all prescription medications reported in 2007 to 2012 were recorded based on interviewers viewing the container. In addition, approximately 92% of all prescription medications were automatically matched to the Lexicon Plus (Cerner Multum, Inc.) data collection drug database in Computer-Assisted Personal Interviewing, and the remaining medications were edited after data collection. The Lexicon Plus database is a comprehensive database of U.S. drug market prescription medications that is updated monthly (16). Nonmatch was most commonly due to incorrect spelling, insufficient detail, or reporting of a nonprescription product that was not in the database. Additional data processing and editing information can be found elsewhere (15). Total number of prescription medications reported was used categorically (0 medications, 1–2 medications, 3–4 medications, 5–6 medications, and ≥7 medications). Binary variables were created to indicate use of specific classes of drugs including: 1) antidepressants, 2) anxiolytics, sedatives, and hypnotics (ASH), 3) sleep aids (following previous use [5] of specific sleep aid medications), 4) anticonvulsants, 5) thyroid hormones, and 6) metabolic agents (including antihyperlipidemic agents, antidiabetic agents, and peripherally acting antiobesity agents). These drug classes were chosen for their associations with either sleep duration or obesity (e.g., side effects, presenting complaints, comorbidities). Sleep aids were a combination of some hypnotics and some antidepressants with a sedative function (5) and thus overlapped with the ASH category but may have a higher representation of individuals who present with sleep complaints rather than mood complaints. Antipsychotics, central nervous system stimulants, and natural/alternative sleep aids (e.g., melatonin and antihistamine from over-the-counter drug data from dietary supplement files) were considered but were not common enough to include.
Weight status
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared rounded to one decimal place using height and weight measures collected with standardized protocols in the Mobile Examination Center. Weight status was defined as normal weight/underweight = BMI <25 kg/m2, overweight = 25 kg/m ≤ BMI <30 kg/m2, and obesity = BMI ≥ 30 kg/m2.
Covariates
Covariates used in adjusted models included age (in years), race/ethnicity, and sex. Race/ethnicity was categorized as one of four categories: non-Hispanic white, non-Hispanic black, Hispanic (regardless of their race), and other (including non-Hispanic individuals reporting more than one race group and non-Hispanic individuals of other races). Those in the “other” group were included in calculations of the total population but were not reported separately.
Statistical analysis
All analyses were conducted in R v. 3.2.1 (17) using the Survey package (18) to account for the complex sampling design. Examination sample weights were used to account for over-sampling, nonresponse, and noncoverage. Standard errors were estimated using Taylor series linearization, and confidence intervals (CI) were calculated using the Korn and Graubard (19) method which may be more accurate for estimates near 0 and 1 compared with Wald-type methods.
Statistical significance was set at P < 0.05 using a two-tailed t statistic. For these analyses, participants 20 years and older were considered eligible (n = 17,713). Successively, participants who were pregnant (n = 182) or missing sleep duration data (n = 33), anthropometric data (n = 867), or prescription data (n = 9) were excluded from all analyses. Final unweighted sample sizes by weight status are shown in Table 1 (final n = 16,622). The unadjusted prevalence of obesity by self-reported sleep duration (grouped into five categories: ≤5 h, 6 h, 7 h, 8 h, and ≤9 h) was examined across categories of total and specific classes of prescription medications. For each medication class category, three mutually exclusive groups of medication users were identified: (1) those taking zero prescription medications, (2) those taking at least one medication in the specific drug class of interest, and (3) those taking other medications outside the specific drug class of interest. Contrast codes were used for each medication class variable to test for significant differences in those taking zero medications compared with those taking the specific drug of interest. Separate logistic regressions (for each drug class and one for total prescription medications) were used to test the differences in the odds of obesity in those not taking any prescription medications compared with those taking the drug classes of interest controlling for age, sex, and race/ethnicity. Interaction terms created with sleep duration (measured continuously) and the contrast coded medication use variables were used to test whether medication use moderated the sleep duration-obesity association including all lower order terms. Quadratic terms for sleep duration were considered and were not significant in any models. However, consistent with patterns seen in observed data, quadratic terms for sleep duration were included in models for anxiolytics, ASH, and anticonvulsants only as this improved the fit of the models to the observed data. It should be noted that odds ratios (OR) for obesity may overestimate the relative risk because the prevalence of obesity is relatively high (20). Predicted probabilities of obesity were calculated from the adjusted models using marginalized standardization. Sensitivity analyses were run that compared two categories of prescription medication class users: 1) those taking the specific medication of interest and 2) everyone else (i.e., those taking zero medications or taking medications outside the class of interest were combined). Results were similar and are therefore not presented. Stratified analyses including only those taking the specific medication of interest were considered, but some smaller sample sizes limited power (n ranged from 653 to 3,977 across medication categories).
TABLE 1.
Unweighted sample sizes by weight status, age, race/Hispanic origin, and sleep duration: U.S. adults aged 20 years and over, 2007–2012
| Weight status |
||||
|---|---|---|---|---|
| Underweight/normal weight | Overweight | Obesity | Total | |
| Whole sample | 4,898 | 5,577 | 6,147 | 16,622 |
| Age (yr) | ||||
| 20–39 | 2,067 | 1,635 | 1,825 | 2,695 |
| 40–59 | 1,370 | 1,930 | 2,211 | 12,720 |
| ≥60 | 1,461 | 2,012 | 2,111 | 1,207 |
| Race/Hispanic origin | ||||
| Non-Hispanic white | 2,317 | 2,493 | 2,535 | 5,527 |
| Non-Hispanic black | 888 | 1,030 | 1,652 | 5,511 |
| Hispanic | 920 | 1,640 | 1,714 | 5,584 |
| Other | 773 | 414 | 246 | 7,345 |
| Self-reported sleep duration | ||||
| Short sleepers (≤5 h) | 694 | 821 | 1,180 | 3,570 |
| Average sleepers (6–8 h) | 3,810 | 4,386 | 4,524 | 4,274 |
| Long sleepers (≥9 h) | 394 | 370 | 443 | 1,433 |
| Prescription medicationsa | ||||
| None | 2,432 | 2,446 | 2,123 | 7,001 |
| 1–2 Medications | 1,350 | 1,498 | 1,490 | 4,338 |
| 3–4 Medications | 605 | 777 | 1,036 | 2,418 |
| 5–6 Medications | 261 | 466 | 665 | 1,392 |
| ≥7 Medications | 250 | 390 | 833 | 1,473 |
Analytic sample Includes nonpregnant Individuals 20 years or older. Individuals missing data on sleep duration, anthropometries, or medication use were excluded. Underweight/normal weight = BMI <25 kg/m2; overweight = 25 kg/m2 ≤ BMI < 30 kg/m2; obesity = BMI ≥ 30 kg/m2.
Use in the past month.
Source: NHANES.
Results
Descriptive prevalence data across prescription medication classes are shown in Table 2. Approximately 49%, 43%, and 35% of individuals with underweight/normal weight, overweight, and obesity reported taking no prescription medication, respectively, and approximately 3%, 6%, and 12% took seven or more prescription medications, respectively (Table 2). The unadjusted prevalence of obesity across self-reported sleep duration by the number of total prescription medications and by specific prescription medication class is shown in Figures 1 and 2, respectively.
TABLE 2.
Past month prescription medication use prevalence by weight status according to drug class and total medication use in U.S. adults aged 20 years and over, 2007 to 2012
| Underweight/normal weight |
Overweight |
Obesity |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| % | Lower CI | Upper CI | % | Lower CI | Upper CI | % | Lower CI | Upper CI | |
| Total prescription medications | |||||||||
| None | 48.5 | 46.2 | 50.9 | 43.0 | 40.8 | 45.3 | 34.6 | 32.4 | 36.9 |
| 1–2 Medications | 32.0 | 30.3 | 33.8 | 30.5 | 28.8 | 32.2 | 27.0 | 25.7 | 28.4 |
| 3–4 Medications | 11.4 | 10.0 | 12.8 | 13.7 | 12.2 | 15.4 | 16.8 | 15.1 | 18.5 |
| 5–6 Medications | 4.7 | 3.8 | 5.8 | 7.2 | 6.2 | 8.4 | 10.0 | 9.0 | 11.0 |
| ≥7 Medications | 3.4 | 2.8 | 4.1 | 5.5 | 4.8 | 6.3 | 11.6 | 10.5 | 12.8 |
| Medication class | |||||||||
| Antidepressants | 5.9 | 5.1 | 6.9 | 5.9 | 4.9 | 7.0 | 6.5 | 5.6 | 7.5 |
| ASH | 9.4 | 8.2 | 10.6 | 10.8 | 9.3 | 12.5 | 15.1 | 13.5 | 16.8 |
| Sleep aids | 3.1 | 2.6 | 3.7 | 4.2 | 3.4 | 5.2 | 5.1 | 4.4 | 6.0 |
| Thyroid hormones | 5.9 | 5.1 | 6.8 | 6.0 | 5.1 | 7.1 | 8.6 | 7.5 | 9.8 |
| Anticonvulsants | 4.2 | 3.5 | 5.1 | 4.1 | 3.4 | 4.8 | 7.1 | 6.3 | 8.0 |
| Metabolic agents | 11.9 | 10.5 | 13.5 | 20.7 | 19.0 | 22.4 | 28.5 | 26.9 | 30.2 |
Total sample sizes for each medication class range from n = 653 to 3,977. Bold values do not denote significance.
ASH = anxiolytics, sedatives, and hypnotics. Metabolic agents include antihyperlipidemic agents, antidiabetic agents, and peripherally acting antiobesity agents.
Source: NHANES.
Figure 1.
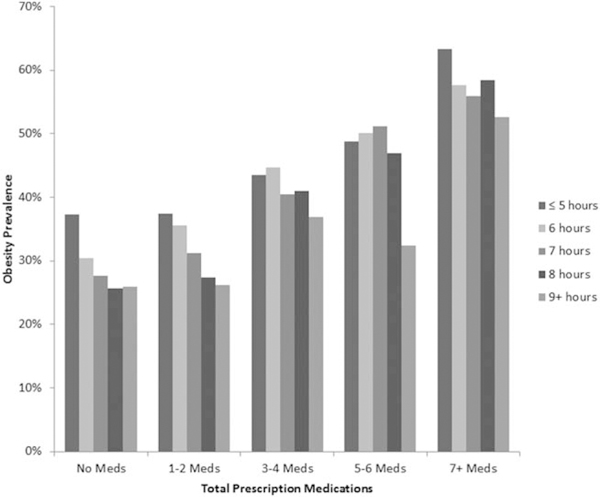
Unadjusted obesity prevalence by total number of prescription medications and hours of self-reported sleep duration in U.S. adults aged 20 years and over, 2007 to 2012. Source: NHANES.
Figure 2.
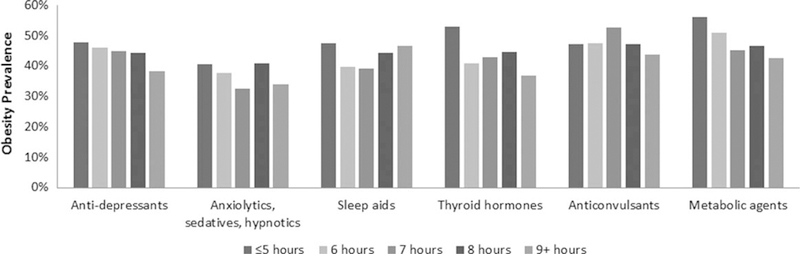
Unadjusted obesity prevalence by drug class and self-reported sleep duration in U.S. adults aged 20 years and over, 2007 to 2012. Source: NHANES.
When adjusting for covariates, logistic regression results with total prescription medication use showed there were significant main effects of sleep duration and medication use on odds of obesity such that sleep duration was inversely related to odds of obesity and medication use was positively related to odds of obesity (Table 3). There was no significant interaction between sleep duration and total medication use such that sleep duration and obesity were significantly inversely associated regardless of the total number of prescription medications used (Table 3). Predicted probabilities of obesity for individuals taking zero medications decreased from 35% to 22% as sleep duration increased from 4 h to 10 h (Supporting Information Table 1). Predicted probabilities of obesity for individuals taking ≥7 medications decreased from 68% to 53% with sleep duration increasing from 4 h to 10 h (Supporting Information Table 1). Analyses with total prescription medications stratified by sex or race/Hispanic origin were similar to the main analyses (see Supporting Information Table 2).
TABLE 3.
Logistic regression results of prescription medication use and selected prescription medication classes on obesity
| OR | Lower 95% CI |
Upper 95% CI |
|
|---|---|---|---|
| Total prescription medications | |||
| Intercept | 0.34 | 0.30 | 0.37 |
| Sleep duration | 0.91 | 0.89 | 0.94 |
| 1–2 Medications | 1.26 | 1.16 | 1.38 |
| 3–4 Medications | 2.07 | 1.78 | 2.41 |
| 5–6 Medications | 2.69 | 2.25 | 3.22 |
| ≥7 Medications | 4.14 | 3.43 | 5.00 |
| Antidepressants | |||
| Intercept | 0.54 | 0.49 | 0.59 |
| Sleep duration | 0.91 | 0.89 | 0.94 |
| Medication use | 2.19 | 1.89 | 2.53 |
| ASH | |||
| Intercept | 0.53 | 0.48 | 0.59 |
| Sleep duration | 0.93 | 0.90 | 0.95 |
| Medication use | 1.98 | 1.70 | 2.31 |
| Quadratic sleep | 1.00 | 0.99 | 1.02 |
| Medications × sleep | 1.08 | 1.00 | 1.17 |
| Sleep aids | |||
| Intercept | 0.55 | 0.50 | 0.61 |
| Sleep duration | 0.93 | 0.91 | 0.95 |
| Medication use | 2.30 | 1.95 | 2.72 |
| Quadratic sleep | 1.00 | 0.99 | 1.02 |
| Medications × sleep | 1.09 | 1.00 | 1.19 |
| Thyroid hormones | |||
| Intercept | 0.56 | 0.50 | 0.61 |
| Sleep duration | 0.92 | 0.89 | 0.94 |
| Medication use | 2.29 | 1.93 | 2.71 |
| Anticonvulsants | |||
| Intercept | 0.56 | 0.50 | 0.62 |
| Sleep duration | 0.93 | 0.90 | 0.95 |
| Medication use | 2.45 | 2.11 | 2.85 |
| Quadratic sleep | 1.00 | 0.99 | 1.02 |
| Medications × sleep | 1.08 | 1.00 | 1.18 |
| Metabolic agents | |||
| Intercept | 0.52 | 0.47 | 0.57 |
| Sleep duration | 0.91 | 0.89 | 0.94 |
| Medication use | 2.57 | 2.21 | 2.98 |
All models were adjusted for age, sex, and race/Hispanic origin. Prescription medication use was assessed as past month use.
ASH = anxiolytics, sedatives, and hypnotics. Metabolic agents include antihyperlipidemic agents, antidiabetic agents, and peripherally acting antiobesity agents.
Adjusted results with specific medication classes showed that there was no significant interaction between sleep duration and medication use for antidepressant, thyroid hormone, and metabolic agent medication use. Main effects, estimated with the interaction terms removed, showed that each additional hour of self-reported sleep was significantly associated with approximately 8% to 9% decrease in the odds of obesity for antidepressant, thyroid hormone, and metabolic agent medications (Table 3). For antidepressant, thyroid hormone, and metabolic agent medications the difference in predicted probability percentage points for those reporting 4 h of sleep compared with those reporting 10 h of sleep was 14% (42%−28%) for all three medication classes (Supporting Information Table 1). Interaction terms, indicating the slope of the relationship between sleep duration and odds of obesity differed depending on medication use, approached significance for ASH (P = 0.067), sleep aids (P = 0.061), and anticonvulsants (P = 0.071; Table 3). For ASH, sleep aids, and anticonvulsant medications the difference in predicted probability percentage points for those reporting 4 h of sleep compared with those reporting 10 h of sleep was 1% (39%−38%), 4% (42%−46%), and 1% (50%−49%), respectively (Supporting Information Table 1). Graphs of predicted probabilities for those taking ASH, sleep aids, and anticonvulsant medications versus zero medications by sleep duration are shown in Supporting Information Figure 1.
Discussion
The aims of this study were to examine the associations of sleep duration and obesity by prescription medication use, both in terms of number and different classes of prescription medications. There were three main findings.
First, results showed that longer sleep duration was associated with lower odds of obesity in U.S. adults, regardless of total prescription medication use. This is consistent with a previous study examining NHANES (2005–2010) sleep and BMI data that controlled for numerous self-reported chronic disease conditions and lifestyle behaviors (6). This study extends previous findings through the examination of detailed prescription medication data and includes the latest available sleep data. Our findings are in contrast to some studies that have shown an increased risk of obesity in self-reported long-duration sleepers (21). A recent review concluded that it is not yet known if long sleep duration is inherently unhealthy or could be indicative of other health problems, including medical or psychological issues (2). Some are beginning to speculate on potential mechanisms between long sleep duration and health status (22) though others have noted there is a paucity of research on long sleep duration mechanisms, which is in contrast to a large body of evidence on short sleep duration mechanisms (3). Long sleep duration may indicate other risk factors are present such as low socioeconomic status, depression (23,24), or low physical activity (25), and long sleep duration may be better conceptualized as a diagnostic marker rather than a cause of poor health (26). Results of this study are consistent with the interpretation that long sleep duration may be an indicator of select secondary health problems but not poorer health in general, as assessed by use of certain prescription medication classes but not total prescription medication use. In addition, results add to the extant and robust literature supporting an association between short sleep duration and obesity risk (2).
Second, individuals taking antidepressants showed the same significant inverse sleep-obesity association compared with those taking no prescription medications. Some research has found long sleep duration to be associated with poor health outcomes only in individuals that had preexisting medical conditions but not in those who were healthy (27) and that self-reported psychiatric problems, but not other chronic health problems, were at play (21). This study complements past research by examining individuals who are receiving pharmacological treatment for different health conditions and showed that among individuals who were taking antidepressants sleep duration and obesity are significantly inversely associated. Studies of individuals who self-report emotional distress or depressive symptoms (8,28), of which only a fraction are receiving psychological or pharmacological treatment (29,30), have shown higher risk of poor health outcomes with long sleep duration. Some longitudinal research has suggested that self-reported sleep duration measures aspects of psychological distress and that associations of sleep duration with obesity may be secondary effects associated with psychological distress (8). This study’s results are partially consistent with this interpretation in that individuals being treated with antidepressants exhibit the same relationship between sleep duration and obesity as individuals taking no medications at all, who can be considered to be healthier than individuals taking prescription medications. However, there was no evidence of a general curvilinear association (i.e., only among users of ASH, sleep aids, and anticonvulsants), which indicates self-reported long sleep duration may only be an indicator of psychological distress or secondary health conditions in some subpopulations. Given that half of depressed individuals wait at least 6 to 8 years before seeking treatment (29), it is plausible that long sleep duration and obesity risk associations mean different things when considering self-reported depression symptoms compared with those engaged in treatment.
Lastly, results provided some support for ASH, sleep aids, and anticonvulsants modifying the relationship between sleep duration and obesity, though statistical significance was not achieved. Among those who use anxiolytic, sedative, and hypnotic and specific sleep aid medications, there is a U-shaped pattern in the relation between obesity and increased sleep duration. This is not surprising as anxiolytics, sedatives, and hypnotics directly target sleep and arousal, and the conditions these medications are used to treat are also commonly associated with sleep disturbance. The prevalence of using ASH, sleep aids, and anticonvulsants was relatively low (3.1%−4.1% of underweight/normal weight individuals and 4.1%−6.9% of individuals with obesity). However, the prevalence of some other types of medications that did not modify the sleep duration-obesity relationship (e.g., antidepressants, metabolic agents) was sometimes relatively high (8.6% of normal weight to 28.2% of individuals with obesity). Thus, practices such as routinely excluding individuals taking different classes of medications or estimating overall effects among different groups of medication users are likely to impact the generalizability of sleep-obesity research. Moderating versus main effects of certain classes of prescription medications and differing prevalence rates may also contribute to bias in studies that are not designed primarily to look at sleep duration-obesity associations and that may have had prescription medication exclusion criteria that targeted different health issues.
Strengths and limitations of this study should also be considered. Strengths included the nationally representative sample, the prescription medication data, and the examination of long-duration sleepers. The prescription medication data in NHANES are based on home interviews where medication containers physically located in the home are reviewed to obtain accurate information and reflect actual patient use more closely than data on what medications were prescribed, sold, or dispensed in the previous year. Previous research has relied on selfreports (7), prescription sales and doctor’s office data (31), or retrospectively linked drug dispensing records (32). Studies on long-duration sleepers are limited due to small sample sizes or combining long- duration and average-duration sleepers (2). Limitations include the use of a single self-reported sleep duration question, the use of past month prescription medication use, which may not reflect medications taken intermittently or for other acute conditions, and the cross-sectional design. This study was limited in the extent to which differences by sex and race/Hispanic origin could be investigated due to low sample sizes, which precluded examining sleep-medication interactions or specific medication classes in stratified analyses. While this study was focused on the effects of sleep on obesity, there may also be bidirectional effects whereby weight gain compounds sleep problems (33). In addition, the study cannot distinguish between associations with the prescription medications themselves versus the underlying medical conditions they were prescribed to treat.
This study sought to disentangle associations between sleep duration, obesity, and prescription medication use. Results showed a significant inverse association between sleep duration and risk of having obesity that was not modified by total prescription medication use, antidepressant medication use, thyroid hormones, or metabolic agents. This suggests that there is not an inherent increased risk of obesity associated with long sleep duration. The potential for a nonlinear association between sleep duration and obesity may be important to examine in individuals taking ASH, sleep aids, or anticonvulsants. Future research is needed to better elucidate the relations among sleep duration and obesity and the role of prescription medications.
Supplementary Material
Acknowledgments
We acknowledge Dr. Brian Kit for his assistance with the prescription medication data.
Footnotes
Disclosure: The authors declared no conflict of Interest.
References
- 1.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity 2008;16:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlin JW, Smith MT. Sleep, obesity, and weight loss in adults: is there a rationale for providing sleep interventions in the treatment of obesity? Int Rev Psychiatry 2014;26:177–188. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep 2006;29:878–879. [DOI] [PubMed] [Google Scholar]
- 4.Kit BK, Ogden CL, Flegal KM. Prescription medication use among normal weight, overweight, and obese adults, United States, 2005–2008. Ann Epidemiol 2012;22: 112–119. [DOI] [PubMed] [Google Scholar]
- 5.Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005–2010 NCHS Data Brief, no. 127 Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 6.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity 2014;22: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Selfreported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep 2008;31:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Fernandez-Mendoza J, Miksiewicz T, et al. Unveiling the longitudinal association between short sleep duration and the incidence of obesity: the Penn State Cohort. Int J Obes 2014;38:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput JP, Bouchard C, Tremblay A. Change in sleep duration and visceral fat accumulation over 6 years in adults. Obesity 2014;22:E9–E12. [DOI] [PubMed] [Google Scholar]
- 10.Bottini P, Tantucci C. Sleep apnea syndrome in endocrine diseases. Respiration Int Rev Thorac Dis 2002;70:320–327. [DOI] [PubMed] [Google Scholar]
- 11.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry 2005; 66(10):1254–1269. [DOI] [PubMed] [Google Scholar]
- 12.Placidi F, Scalise A, Marciani MG, Romigi A, Diomedi M, Gigli GL. Effect of antiepileptic drugs on sleep. Clin Neurophysiol 2000;111:S115. [DOI] [PubMed] [Google Scholar]
- 13.Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev 2004;8:7–17. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005;352:2211. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Health and Nutrition Examination Surveys: NHANES 2011–2012. US Department of Health and Human Services; 2012. http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx Accessed December 15, 2014. [Google Scholar]
- 16.Cerner Multum. Lexicon Plus. Cerner Multum, Inc.; 2012. http://www.multum.com/Lexicon.html. [Google Scholar]
- 17.R Core Development Team. A Language and Environment for Statistical Computing. 2015. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 18.Lumley T Analysis of complex survey samples. J Stat Softw 2004;9:1–19. [Google Scholar]
- 19.Korn EL, Graubard B. Analysis of Data from Health Surveys. New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 20.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998(19);280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 21.Léger D, Beck F, Richard J-B, Sauvet F, Faraut B. The risks of sleeping “too much”. Survey of a national representative sample of 24671 adults (INPES health barometer). Barometer 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev 2007;11:341–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosom Med 2002;64:337–344. [DOI] [PubMed] [Google Scholar]
- 24.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep 2006;29:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stranges S, Dorn JM, Shipley MJ, et al. Correlates of short and long sleep duration: a cross-cultural comparison between the United Kingdom and the United States the Whitehall II Study and the Western New York Health Study. Am J Epidemiol 2008; 168:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 27.Magee CA, Holliday EG, Attia J, Kritharides L, Banks E. Investigation of the relationship between sleep duration, all-cause mortality, and preexisting disease. Sleep Med 2013;14:591–596. [DOI] [PubMed] [Google Scholar]
- 28.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol 2009;169:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:603–613. [DOI] [PubMed] [Google Scholar]
- 30.Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005–2008. J Am Board Fam Med 2011;24:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf 2011;20:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milder I, Klungel O, Mantel-Teeuwisse A, Verschuren W, Bemelmans W. Relation between body mass index, physical inactivity and use of prescription drugs: the Doetinchem Cohort Study. Int J Obes 2010;34:1060–1069. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev 2011;1;12:78–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


