In vitro and animal studies revealed a potential protective role of CCR5 antagonists on reducing liver fibrosis progression and protecting from developing hepatocellular carcinoma.1 Hepatocytes bear CXCR4 and CCR5, the 2 main coreceptors for HIV entry into cells and the blockade of coreceptors on hepatic stellate cells, the major producers of extracellular matrix in the liver, will slow progression of liver fibrosis, especially due to HIV-envelope gp120–mediated fibrogenesis modulation.2–5
The aim of present analysis was to compare the evolution of liver fibrosis over time evaluated by surrogated biomarker assays in HIV-1–infected patients on a virologically successful antiretroviral therapy (stable HIV-1 RNA <50 copies/mL), randomized to switch to maraviroc + darunavir/r (MVC + DRV/r arm) qd or to continue the current MVC-free 3-drug antiretroviral therapy (ART) (3-drug ART arm).
Patients included in the study were enrolled in the GUided Simplification with Tropism Assay (GUSTA) trial, a multicenter, open-label, randomized study (www.clinicaltrials.gov, number NCT01367210), whose main results have been published.6
Briefly, GUSTA included patients with HIV-1 RNA <50 copies/mL for at least 6 months, R5 tropism and CD4 counts >200 cells/μL for at least 3 months before enrollment; hepatitis B virus–coinfected patients and those with Child-Pugh B/C cirrhosis were excluded.
We retrospectively evaluated Fibrosis-4 (FIB-4) Index and aspartate aminotransferase to Platelet Ratio Index (APRI) scores, at baseline and after 12, 24, 48, and 96 weeks. The cutoff points of serum marker tests of hepatic fibrosis were as follows: FIB-4 <1.45 (F0-F1), 1.45–3.25 (indeterminate), and >3.25 (F3-F4); APRI <0.5 (F0-F1), >1.5 (F2) and >2 (cirrhosis).
Differences between arms were assessed by χ2 and Student t test, longitudinal within-group differences by McNemar test. The FIB-4 Index and APRI scores were used as continuous variables; their predictors at baseline and their change over time were investigated by linear regression.
We included 150 patients, 76 randomized to MVC + DRV/r arm and 74 to 3-drug ART arm. Baseline characteristics were homogeneous between arms except for relative younger age in the MVC + DRV/r arm (median 47 yrs; interquartile range [IQR] 40–52) than in the 3-drug ART arm (50 yrs; IQR 44–57) (P = 0.08), more frequent African ethnicity in the 3-drug ART arm than in the MVC + DRV/r arm (8% vs. 1%) (P = 0.05), and FIB-4 median value higher in the MVC + DRV/r arm (1.15; IQR 0.82–1.32) than in the 3-drug ART arm (0.91; IQR 0.68–1.20) (P = 0.01). APRI score was similar between arms: 0.23 (IQR 0.18–0.29) in the MVC + DRV/r arm and 0.25 (IQR 0.20–0.33) in the 3-drug ART arm (P = 0.12).
Overall, 89% (134/150) were menmales and Caucasians; 41% (61/150) were heterosexuals; 38% (57/150) homosexuals/bisexuals; 7% (10/150) reported history of injected drug use, 11 years of HIV (IQR 7–18), 10 years of ART (IQR 6–15), CD4 at nadir 222 cells/mmc (IQR 132–319) and at baseline 654 cells/mmc (IQR 506–905). Eighteen patients presented positive serology for hepatitis C virus (HCV) and 8 had a detectable HCV RNA, 4 in each arms.
Sixteen (11%) presented diabetes mellitus: 12% (9/76) in the MVC + DRV/r arm and 9% (7/74) in the 3-drug ART arm (P = 0.04). At screening, nucleoside reverse transcriptase inhibitors (NRTIs) were used in 95% (143/150), nonnucleoside reverse transcriptase inhibitors (NNRTIs) in 12% (18/150), integrase strand transfer inhibitors (INSTIs) in 18% (17/150), and protease inhibitors (PIs) in 69% (103/150) of which boosted PI in 63% (94/150) and DRV/r in 31% (47/150). No differences between arms were observed in terms of dislypidemia (in 100/150, 66%), with total cholesterol 203 mg/dL (IQR 173–230), body mass index (23 kg/m2, IQR 22–26) and glucose 89 mg/dL (IQR 82–100). Median value of false positive rate at geno2pheno was 43 (IQR 24–69), with no differences between groups.
During observation in the 3-drug ART arm (n = 74), NRTIs were used in 92%, NNRTIs in 16%, INSTIs in 15%, PIs in 69%, boosted PI in 51%, and DRV/r in 43%.
According to the cutoff points of hepatic fibrosis, FIB-4 in the MVC + DRV/r arm was <1.45 in 83% (63/76), between 1.45 and 3.25 in 16% (12/76), and >3.25 in 1% (1/76); in the 3-drug ART arm, it was <1.45 in 88% (65/74) and between 1.45 and 3.25 in 12% (9/74) (no one had FIB-4 >3.25).
Overall, APRI was <0.5 in 91% (137/150), and no one had >1.5 at baseline.
Based on the FIB-4 score, at 48 weeks progression to a higher level was observed in 6% (4/63) in the MVC + DRV/r arm and in 6% (4/65) in 3-drug ART arm; in 3% (4/12) among those in MVC + DRV/r arm and in 3% (3/9) in 3-drug ART arm, FIB-4 improved by at least 1 stage, whereas the other patients did not modify their FIB-4 stratum.
Based on the APRI score, at 48 weeks, significant modification of the stratum was no observed.
In addition, no significant differences between arms were observed in platelet counts and alanine transaminase changes at 48 weeks from baseline. We observed a more profound decrease of aspartate transaminase (AST) levels in the MVC + DRV/r arm (mean change −4.19 IU/L, SD 7.2) vs. 3-drug ART arm (mean change +0.58 IU/L, SD 9.9) (P = 0.007).
In a multivariable model adjusting for risk factor for HIV acquisition and duration of ART exposure, longer time from HIV diagnosis (per 1 year increase +0.031, 95% confidence interval [CI]: +0.007 to +0.055, P = 0.01), lower nadir CD4+ cells count (+100 cells increase, −0.060, 95% CI −0.107 to −0.014, P = 0.01), and HCV antibody positive status (+0.321, 95% CI +0.000 to +0.642, P = 0.05) were associated with higher baseline FIB-4 values. No factor independently associated with baseline APRI values was observed. During follow-up, the APRI score decreased more prominently in the MVC + DRV/r arm vs 3-drug ART arm at week 12 (median change −0.02; IQR −0.06 to +0.12 vs −0.006; IQR −0.05 to +0.02; P = 0.28), at week 48 (−0.04; IQR −0.09 to +0.02 vs +0.001; IQR −0.037 to +0.049; P = 0.01), and at week 96 (−0.03; IQR −0.06 to +0.01 vs +0.02; IQR −0.01 to +0.10; P = 0.053) (Fig. 1A).
FIGURE 1.
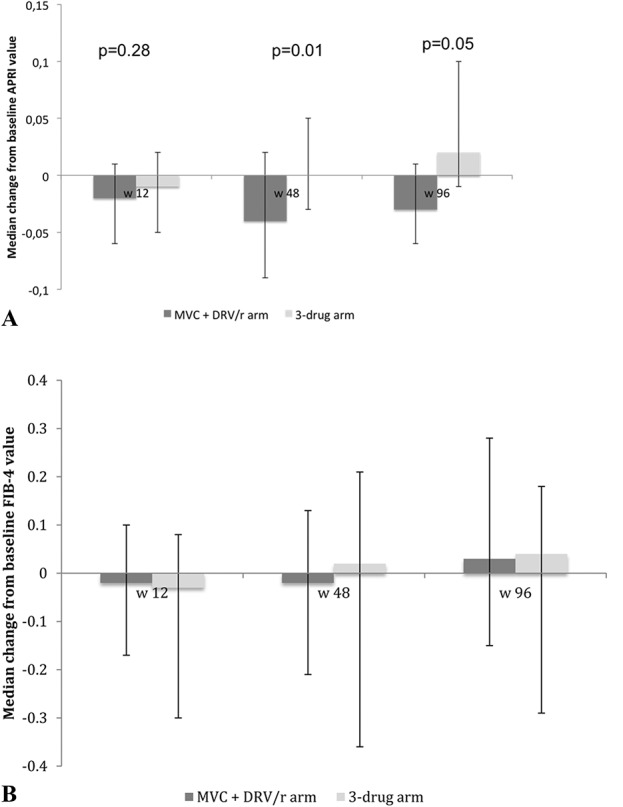
A, APRI score during follow-up. B, FIB-4 during follow-up. No significant difference between arms at each time-point.
In a multivariable model, predictors of APRI change at 48 weeks were baseline APRI (−0.391; 95% CI −0.515 to −0.266; P < 0.001) and MVC + DRV/r arm vs 3-drug ART arm (−0.040; 95% CI −0.006 to −0.074; P = 0.021).
FIB-4 also showed a trend toward a more prominent reduction in the MVC + DRV/r arm (−0.02; IQR −0.21 to +0.13) vs 3-drug ART arm (+0.02; IQR −0.23 to +0.20) (P = 0.35) at week 48 (Fig. 1B). Baseline FIB-4, but not study arm, predicted FIB-4 modifications during follow-up.
In conclusion, we observed that switch to MVC + DRV/r in HIV-1–infected, but virologically suppressed patients on 3-drug ART, was associated with a slight but significant improvement of the APRI score over time as compared with continuing 3-drug ART without MVC. This MVC-containing regimen did not significantly influence the longitudinal change of the FIB-4 score, possibly due to the presence of age as a component of the score, which was increasing over time in the study patients, although a trend toward an improvement was observed. Our observations are in agreement with experiments showing a reduction of hepatic stellate cells activation and fibrosis progression and an improved survival in a murine model of hepatocellular carcinoma1 and in vitro observations on the inhibitory effect of MVC on the accumulation of fibrillar collagens and extracellular matrix proteins by human hepatic stellate cells.7 Results from this study are also in line with a previous retrospective non-comparative analysis on 71 HIV/HCV-coinfected patients treated with MVC, showing a potential beneficial effect on liver fibrosis measured by the APRI score.8 In a previous prospective, non-controlled pilot study on 24 HIV/HCV-coinfected patients starting a MVC-based regimen, liver fibrosis was slightly but not significantly reduced, although observation was limited to 6 months.9 In addition, a recent study suggests that a validated marker of liver fibrosis was reduced in HIV-1–infected patients carrying the variant allele CCR5 delta-32, associated with reduced CCR5 expression, and in patients exposed to cenicriviroc, a CCR5/CCR2 blockade agent.10
Our study adds to previous evidence and has its strengths in the randomized comparison, the study arm treated with a homogeneous MVC-containing regimen and the prospective follow-up of the patients up to 96 weeks. Its main limitation is the lack of information on the liver histological pattern modification rather than indirect biomarkers, as it remains unclear whether their change truly reflects hepatic fibrosis change. The lack of information on patients' alcohol consumption and the absence of transient liver elastography measurements also represent limitations to this analysis.
Further studies are warranted to confirm an antifibrotic effect of CCR5 antagonist therapy.
ACKNOWLEDGMENTS
The authors thank the patients who shared their data, the GUSTA study group, ViiV Healthcare, Verona, that supported viral tropism determination, and TDM. Janssen who supported pharmacovigilance and gave darunavir.
GUSTA study group: S Di Giambenedetto, N Ciccarelli, R Gagliardini, S Lamonica, I Fanti, F Lombardi, D'Avino Alessandro, Fabbiani Massimiliano (Clinic of Infectious Diseases, Catholic University of Sacred Heart, Rome); P Navarra, L Lisi, GMP Ciotti, (Pharmacology Department, Catholic University of Sacred Heart, Rome); A De Luca , B Rossetti, C Bianco, M Masini, (Infectious Diseases Unit, Azienda Ospedaliera Universitaria Senese, Siena), M Zazzi, G Meini (Department of Medical Biotechnology, University of Siena, Siena); D Francisci, A Tosti, B Belfiori, L Malincarne (Clinic of Infectious Diseases, University of Perugia, S. Andrea delle Fratte, Perugia); J Vecchiet, F Vignale, C Ucciferri, K Falasca (Clinic of Infectious Diseases, G. D'Annunzio University, Chieti,); A Di Biagio, S Grignolo, LA Nicolini, R Prinapori, P Tatarella, (Infectious Diseases Unit, IRCCS S. Martino-IST, Genova), B Bruzzone (Virology IRCCS S. Martino-IST, Genova); M Galli, S Rusconi, M Franzetti, V Di Cristo, (Infectious and Tropical Diseases Unit, DIBIC L. Sacco Hospital, University of Milano, Milano), V Micheli (Microbiology and Virology Laboratory, L. Sacco Hospital, Via G.B Grassi, Milano); A Latini, C Giuliani, M Colafigli, A Pacifici, A Cristaudo (Infectious Dermatology and Allergology IRCCS IFO, via Elio Chianesi, Roma); I Mezzaroma, A Fantauzzi, (Department of Clinical Medicine, Sapienza University of Rome, Rome); V Vullo, G D'Ettorre, EN Cavallari (Department of Public Health and Infectious Diseases, Sapienza University of Rome, Roma), G Antonelli, O Turriziani, (Virology, Sapienza University of Rome, Roma); P Grima, (Division of Infectious Diseases, S. Caterina Novella Hospital, Galatina, Lecce); P Viale, V Colangeli, L Calza, C Valeri, V Donati, N Girometti, G Vandi, E Magistrelli, (Clinic of Infectious Diseases, Azienda Ospedaliera Universitaria S.Orsola Malpighi, Bologna); MC Re, I Bon (Microbiology, Azienda Ospedaliera Universitaria S.Orsola Malpighi, Bologna); P Caramello, G Orofino, M Farenga, S Carosella (Infectious Diseases Unit A, Amedeo di Savoia Hospital, Torino), Valeria Ghisetti (Microbiology and Virology Laboratory, Amedeo di Savoia Hospital, Torino); E Petrelli, B Canovari (Infectious Diseases Unit, Pesaro Hospital, Pesaro); C Catalani, M Trezzi (Infectious Diseases Unit, Pistoia Hospital, Pistoia); C Mastroianni, M Lichtner, R Marocco (Infectious Disease Unit, SM Goretti Hospital, Department of Public Health and Infectious Diseases, Sapienza University, Latina); A Bartoloni, G Sterrantino, S Tekle Kiros, I Campolmi (Clinic of Infectious Diseases, Azienda Ospedaliera Universitaria Careggi, Firenze); A D'Arminio Monforte, T Bini, G Ancona, S Solaro (Infectious and Tropical Diseases Institute, Department of Health Sciences, University of Milan San Paolo Hospital, Milano); A Antinori, R Acinarupa, S Ottou, R Libertone, S Mosti, C Pinnetti, (Infectious Diseases Unit, IRCCS L. Spallanzani, Roma); CF Perno, Ada Bertoli (Department of Experimental Medicine and Surgery, University of Rome Tor Vergata, Roma). We are grateful to Alessandro Cozzi-Lepri, Annamaria Geretti and Jonathan Schapiro for their invaluable work in the Data Safety and Monitoring Board.
Footnotes
Supported by grants from Ministero della Salute, ISS, for Programma Nazionale AIDS project number 40H94. Janssen Europe provided Darunavir (DRV) tablets for patients in the study arm and supported the pharmacovigilance of the study, and ViiV Healthcare Italy supported tropism testing for all patients for conducting the study. ViiV Healthcare Italy also supported plasma antiretroviral drug monitoring for patients in the study arm for conducting the study. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Presented as poster at the 9° Italian conference on AIDS and Antiviral Research; June 12–14, 2017, Siena, Italy (P67).
A.B. reports nonfinancial support from Bristol-Myers Squibb; personal fees from Gilead Sciences; and nonfinancial support from ViiV Healthcare. A.D.L. reports consulting fees from Gilead Sciences, Abbvie, Janssen, Bristol-Myers Squibb, ViiV Healthcare Italy, and Merck Sharp and Dohme, outside the submitted work. B.R. reports nonfinancial support from Janssen, ViiV Healthcare Italy, Abbvie, and Gilead and consulting fees from Merck Sharp and Dohme, outside the submitted work. A.D.M. reports grants and consulting fees from Bristol-Myers Squibb, Merck Sharp and Dohme, and Gilead and consulting fees from ViiV Healthcare Italy, outside the submitted work. C.M. reports consulting fees and nonfinancial support from ABBVIE; consulting fees from Merck Sharp and Dohme, Gilead Sciences, ViiV Healthcare Italy, and BMS; and nonfinancial support from ASTELLAS, outside the submitted work. F.V. reports nonfinancial support from Bristol-Myers Squibb, ViiV Healthcare Italy, and Gilead Sciences and consulting fees from Merck Sharp and Dohme and BMS, outside the submitted work. M.C. reports consulting fees from Gilead Sciences, Janssen-Cilag, Merck Sharp and Dohme, Bristol-Myers Squibb, and ViiV Healthcare Italy, outside the submitted work. I.M. reports grants and consulting fees from ViiV Healthcare Italy. S.R. reports grants and consulting fees from ViiV Healthcare Italy, Bristol-Myers Squibb, Merck Sharp and Dohme, Gilead Sciences, and Janssen, outside the submitted work. S.D.G. reports personal fees from Bristol-Myers Squibb, Janssen-Cilag, ViiV Healthcare Italy, Gilead, and Merck Sharp and Dohme, outside the submitted work. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ochoa-Callejero L, Pérez-Martínez L, Rubio-Mediavilla S, et al. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One. 2013;8:e53992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Preface. Clin Liver Dis. 2008;12:xiii–xiv. [DOI] [PubMed] [Google Scholar]
- 3.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berres ML, Koenen RR, Rueland A, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno R, Galastri S, Sacchi P, et al. Gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–520. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti B, Gagliardini R, Meini G, et al. Switch to maraviroc with darunavir/r, both QD, in patients with suppressed HIV-1 was well tolerated but virologically inferior to standard antiretroviral therapy: 48-week results of a randomized trial. PLoS One. 2017;12:e0187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppola N, Perna A, Lucariello A, et al. Effects of treatment with Maraviroc a CCR5 inhibitor on a human hepatic stellate cell line. J Cell Physiol. 2018;233:6224–6231. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales E, Boix V, Deltoro MG, et al. The effects of Maraviroc on liver fibrosis in HIV/HCV co-infected patients. J Int AIDS Soc. 2014;17(4 suppl 3):19643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macos J, Viloria MM, Rivero A, et al. Lack of short-term increase in serum mediators of fibrogenesis and in non-invasive markers of liver fibrosis in HIV/hepatitis C virus-coinfected patients starting maraviroc-based antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2012;31:2083–2088. [DOI] [PubMed] [Google Scholar]
- 10.Sherman KE, Abdel-Hameed E, Rouster SD, et al. Improvement in hepatic fibrosis biomarkers associated with chemokine receptor inactivation through mutation or therapeutic blockade. Clin Infect Dis. 2018. 10.1093/cid/ciy807. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


