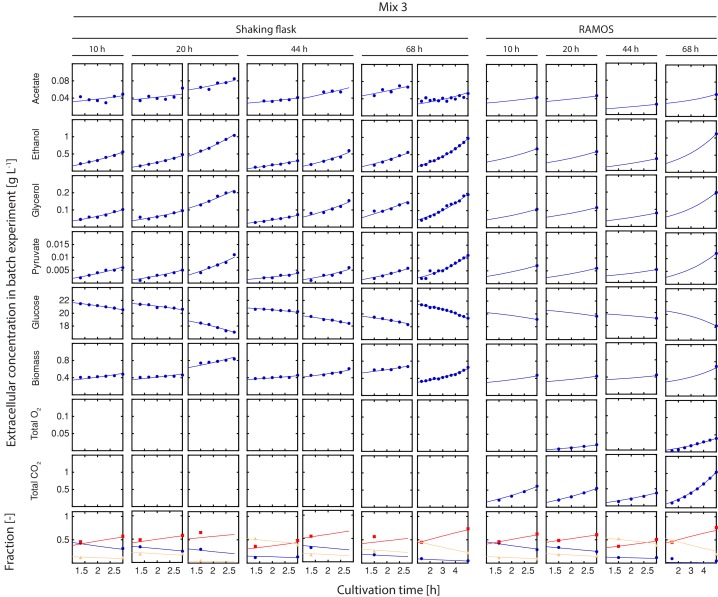
Figure 2. Changes in metabolite concentrations and physiological parameters during cellular aging.
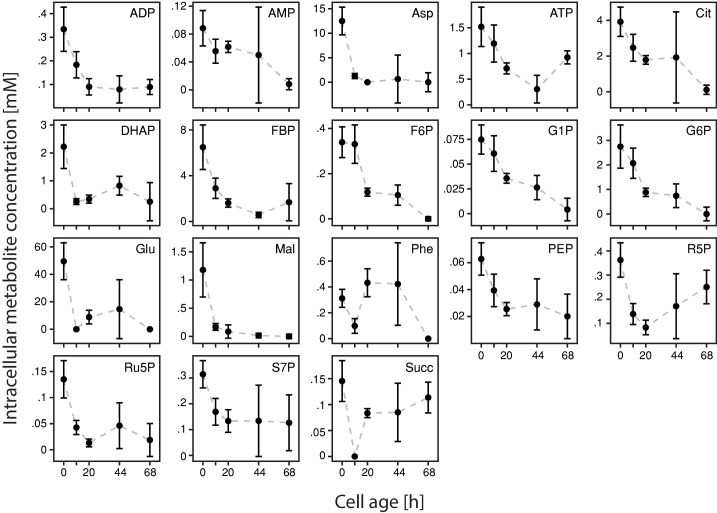
(a) The intracellular metabolite concentrations of 18 metabolites at various cell ages were inferred from LC-MS/MS measurements, cell volume measurements and the fractional abundances of each cell population using non-linear least square regression. Grey dashed lines depict the change of intracellular metabolite concentrations relative to concentrations determined from streptavidin-labeled cells (i.e. young cells at an age of 0 hr). The change in ATP concentration is highlighted in red, and FBP (fructose-1,6-bisphosphate) in blue. Figure 2—figure supplement 4 shows the data for each metabolite in absolute units. Figure 2—source data 1 contains the data. (b) The growth (µ), metabolite uptake and production rates at various cell ages were obtained by measuring the evolution of cell count and extracellular metabolites (including produced carbon dioxide and consumed oxygen) and fitting the acquired data to an ordinary differential equation model. A positive value indicates metabolite production and a negative uptake. To assess the validity of the inference approach physiological rates were independently determined from unlabeled and streptavidin-labeled cell cultures (time point 0 hr), consisting of predominantly young cells. The shading reflects the inverse of the relative uncertainty of the estimation (i.e. values which are depicted with a higher transparency are more uncertain). Figure 2—source data 2 contains the data.
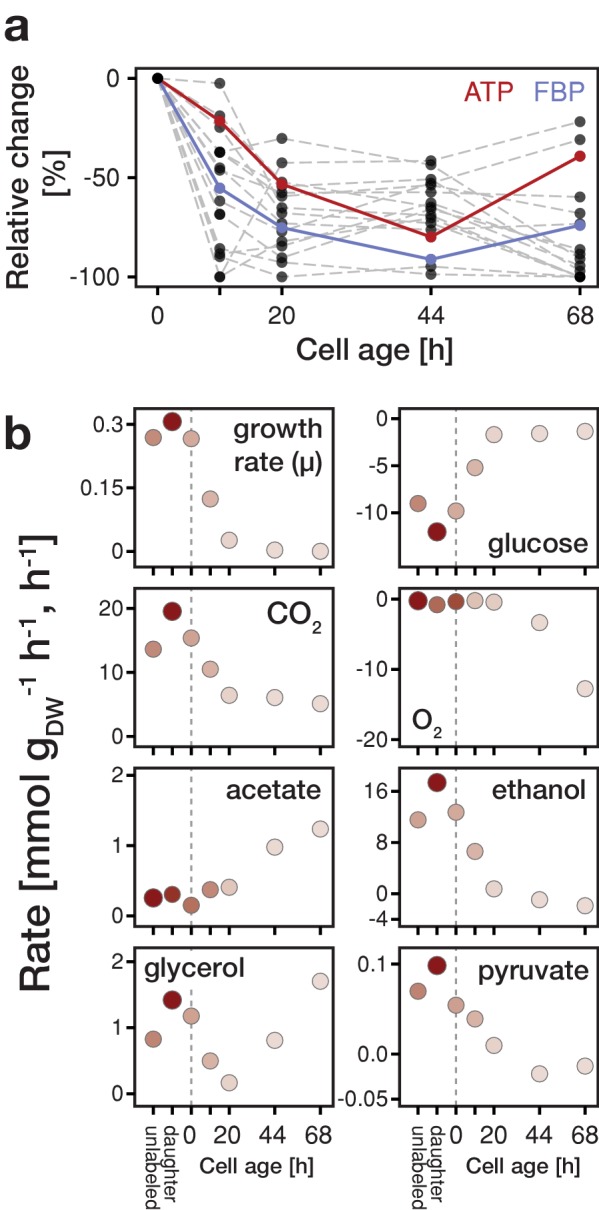
Figure 2—figure supplement 1. The cellular volume gradually increases with cellular age.
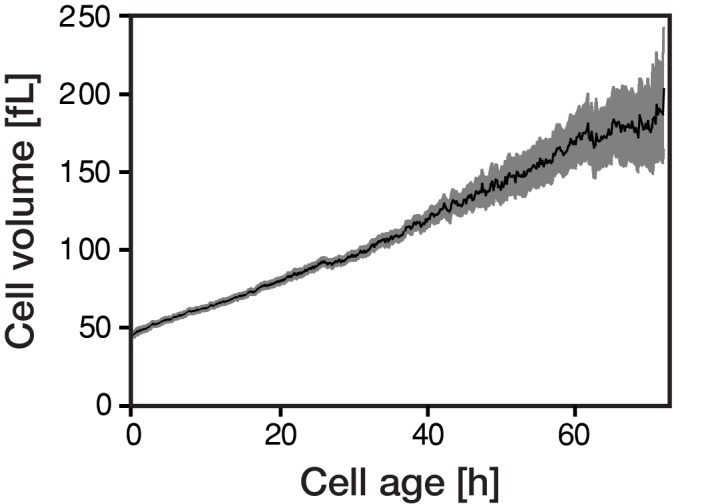
Figure 2—figure supplement 2. Inference of intracellular metabolite concentrations.
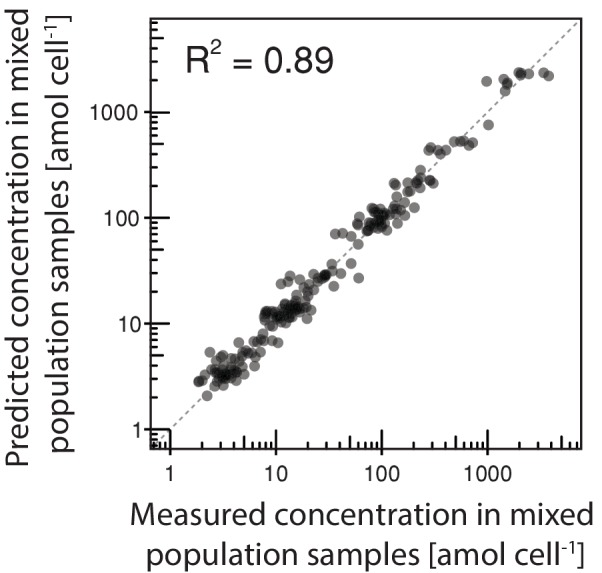
Figure 2—figure supplement 3. Comparison of inferred intracellular metabolite concentrations with independently determined concentrations of young cells.
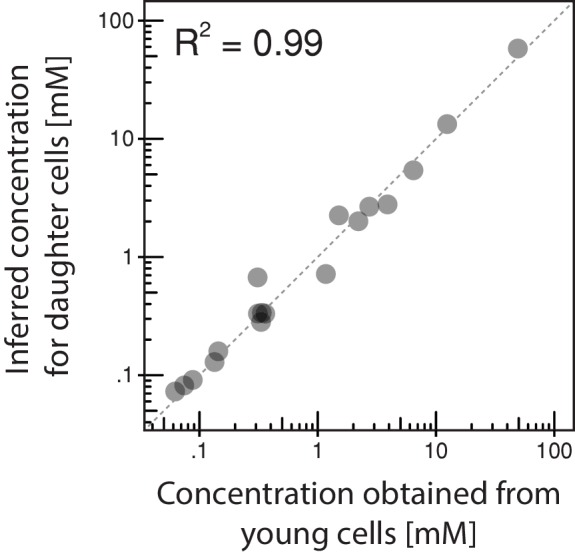
Figure 2—figure supplement 4. Inference of intracellular concentrations of 18 metabolites with cell age.
Figure 2—figure supplement 5. The energy charge remains constant with cell age.
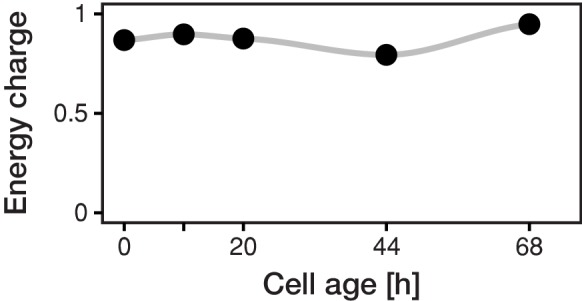
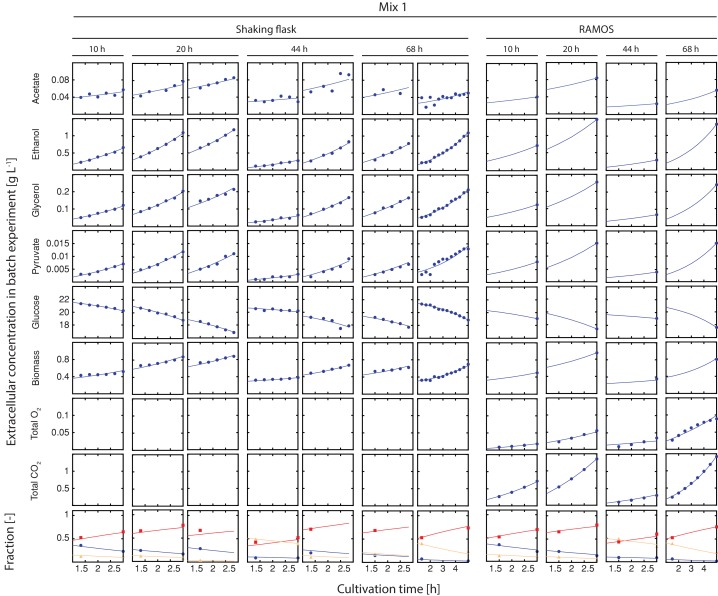
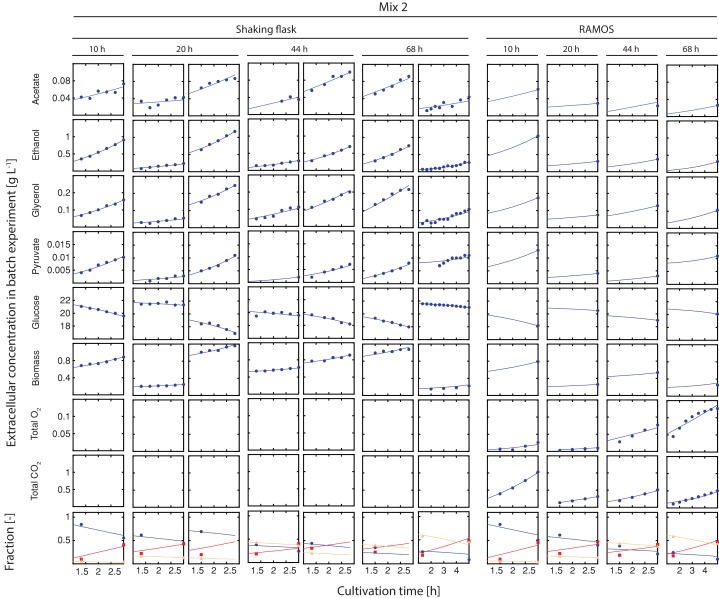
Figure 2—figure supplement 6. Inference of physiological parameters from dynamic changes in extracellular metabolites.
Figure 2—figure supplement 7. Inference of physiological parameters from dynamic changes in extracellular metabolites.
Figure 2—figure supplement 8. Inference of physiological parameters from dynamic changes in extracellular metabolites.
Figure 2—figure supplement 9. FBP (fructose-1,6-bisphosphate) concentration as function of sugar uptake rate.
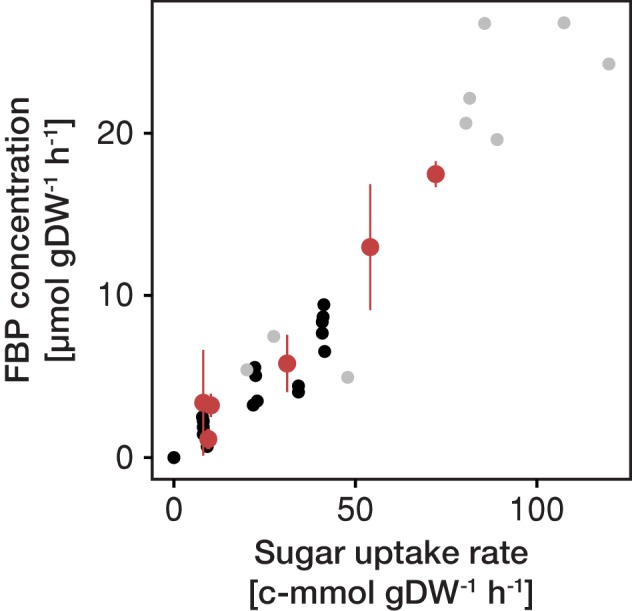
Figure 2—figure supplement 10. The decreasing growth rate was confirmed using single cell analysis.
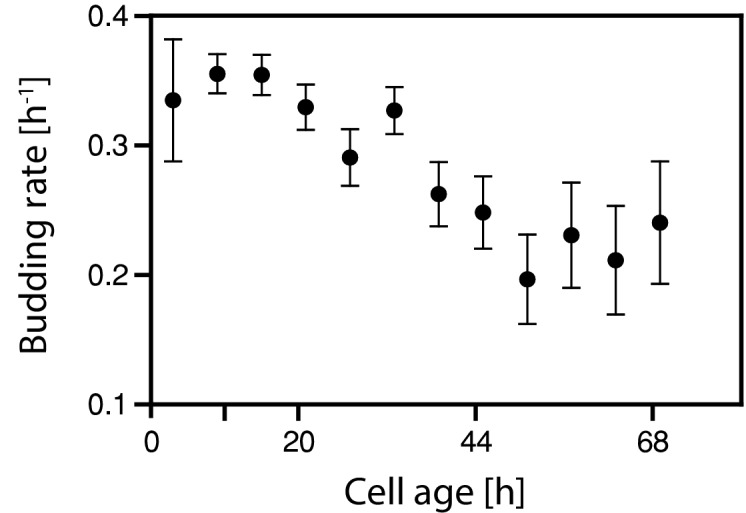
Figure 2—figure supplement 11. The yeast proteome progressively transforms form a fermentation- to a respiration-associated state during aging.
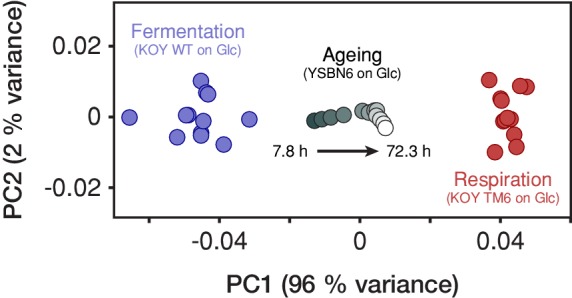
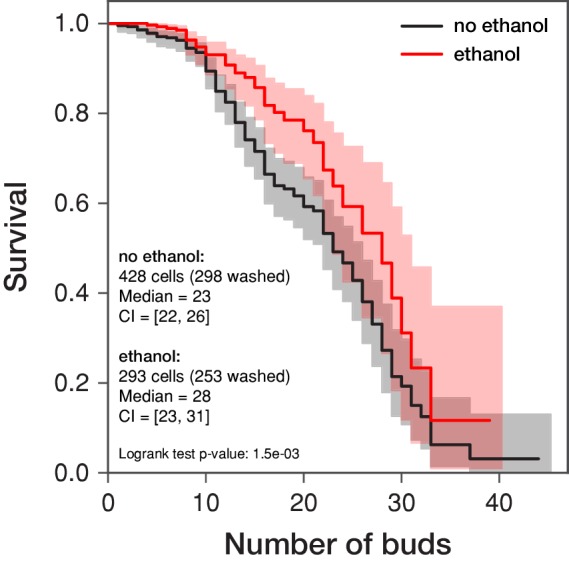
Figure 2—figure supplement 12. Replicative lifespan is increased in the presence of ethanol.