Abstract
All species, including horses, suffer from alterations that increase intestinal permeability. These alterations, also known as “leaky gut,” may lead to severe disease as the normal intestinal barrier becomes compromised and can no longer protect against harmful luminal contents including microbial toxins and pathogens. Leaky gut results from a variety of conditions including physical stressors, decreased blood flow to the intestine, inflammatory disease, and pathogenic infections, among others. Several testing methods exist to diagnose these alterations in both a clinical and research setting. To date, most research has focused on regulation of the host immune response due to the wide variety of factors that can potentially influence the intestinal barrier. This article serves to review the normal intestinal barrier, measurement of barrier permeability, pathogenesis and main causes of altered permeability, and highlight potential alternative therapies of leaky gut in horses while relating what has been studied in other species. Conditions resulting in barrier dysfunction and leaky gut can be a major cause of decreased performance and also death in horses. A better understanding of the intestinal barrier in disease and ways to optimize the function of this barrier is vital to the long-term health and maintenance of these animals.
Keywords: Horse, Intestinal permeability, Leaky gut, Barrier function, Decreased performance
1. Introduction
The intestinal tract serves many vital functions that include the selective absorption of essential nutrients, ions, and other compounds while serving as a barrier against harmful, noxious substances. Dysfunction of this barrier and alterations in intestinal permeability, also known as “leaky gut” is an important topic for clinicians and researchers in all species including horses and humans. In humans, changes in intestinal permeability have been linked in the pathogenesis of debilitating inflammatory bowel diseases (IBDs) such as Crohn’s disease (CD) [1] and in autoimmune diseases such as Celiac disease [2]. Currently conditions resulting in leaky gut are known to occur in equine species, yet few scientific studies have been conducted focusing on this condition. In horses, gastrointestinal issues are reported second to only old age as the leading cause of death [3]. Death or illness can result from the systemic effects of microbial toxins and pathogens that “leak” through the intestinal wall and the subsequent immune response that includes the production of inflammatory mediators. Leaky gut is therefore described in both severe, life-threatening intestinal obstructions as well as in long-term, insidious disorders that result in weight loss and decreased performance (Fig. 1). Thus, a better understanding of the intestinal barrier in disease and how to improve its function remains vital to the long-term health and the maintenance of high-level athletic performance of these animals. This paper serves to review the normal intestinal barrier, measurement of barrier permeability, pathogenesis and main causes of altered permeability, along with highlight potential alternative therapies of leaky gut in horses while relating what is known in other species including poultry, porcine, rodent, and human.
Fig. 1.
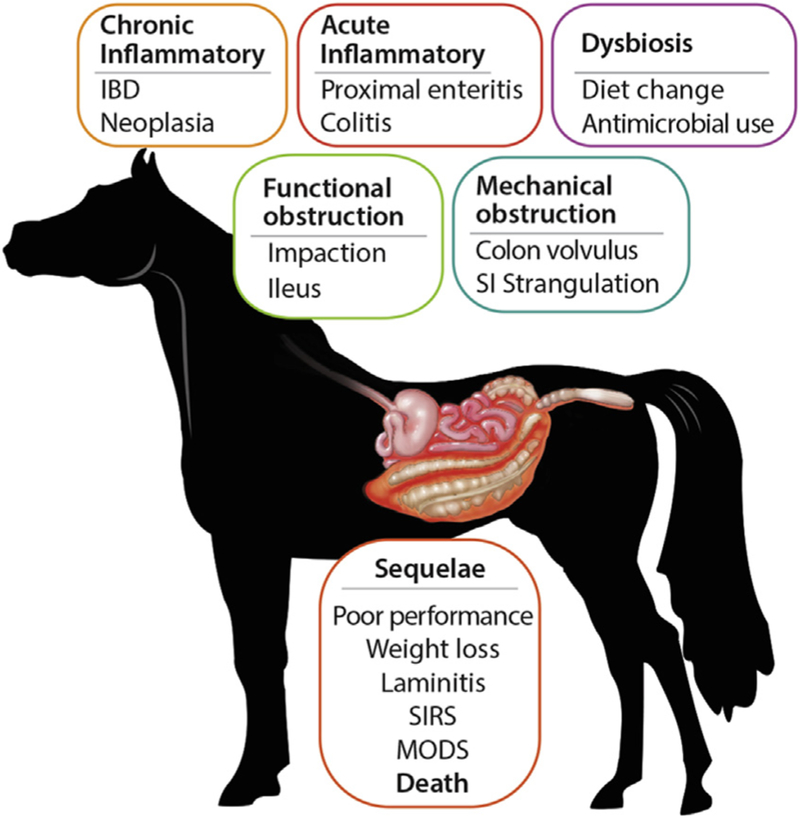
Altered intestinal permeability (leaky gut) in horses. Specific causes of leaky gut in horses range from alterations in the microbiota, acute and chronic inflammatory disease, and both mechanical and functional intestinal obstructions. These conditions alter permeability in several ways including disruption of normal blood flow to the intestine, increased production of proinflammatory cytokines, and disruption of the normal cell junctions, among others. Sequelae to leaky gut may include weight loss and poor performance in mild cases and SIRS, MODS, or death in severe cases. IBD, inflammatory bowel diseases; MODS, multiple organ dysfunction syndrome; SI, small intestinal; SIRS, systemic inflammatory response syndrome.
2. Intestinal Barrier and Permeability
Intestinal permeability is determined by the interaction of several components including an unstirred water layer that forms a diffusion barrier in combination with mucus. In addition, mucus protects the villi from physical friction and bacterial adhesion [4,5]. Other barrier components include phospholipids within the mucosal surface, epithelial factors including tight junctions, the intestinal immune system including lymphocytes and the gut microbiota [4,6–8]. The gut microbiota, a complex community of microorganisms that inhabits the intestine, varies with diet, age, and environment, and influences normal physiology and susceptibility to disease through its metabolic activities and host interactions [8]. A full review of the microbiota and its interactions with intestinal barrier function is outside the scope of this article and the interested reader is directed to several recent reviews [8–12].
One of the most important and widely studied intestinal barrier components is the intestinal epithelium. The intestinal epithelium is composed of a single layer of cells and is the largest of the body’s mucosal surfaces [13]. These epithelial cells are polarized, contain apical and basolateral membranes, and are responsible for creating a physical barrier, transporting nutrients, and protecting the underlying tissues [14]. The epithelial layer of the large intestine (colon) is folded into invaginated crypts of Lieberkühn that contain undifferentiated stem cells and are supported by the lamina propria (Fig. 2) [15]. The small intestinal epithelium is composed of villi that extend into the lumen and are lined by differentiated, post-mitotic cell types, and the crypts of Lieberkühn that contain Paneth cells and undifferentiated stem cells [16]. The stem cells are responsible for creating new epithelium every 5–7 days [17,18]. Enteroendocrine, goblet and Paneth cells are the specialized, secretory epithelial cells that maintain the digestive or barrier function of the epithelium via hormone, mucin, and antimicrobial peptide secretion, respectively [13]. When healthy, the epithelial barrier is impermeable to toxins, pathogens, and antigens while maintaining a selective permeability for the transport and absorption of nutrients, ions, and water (Fig. 2). Selective permeability occurs via the following two pathways: the paracellular and transcellular pathway [19]. The transcellular pathway, predominately mediated by transport channels located on the apical membrane, involves the transport of nutrients including sugars, amino acids, and fatty acids across the cell. The paracellular pathway, associated with passage of molecules in the space between adjacent cells, is regulated by an apical junctional complex (AJC) made up of adherens junctions and tight junctions (Fig. 3). Adherens junctions, along with desmosomes, provide strong connective bonds between epithelial cells. Cell to cell contact at the adherens junction is mediated by adhesion molecule complexes made up of protein families including the cadherins and catenins. Tight junctions consist of four unique families of transmembrane proteins including occludin, claudins, junctional adhesion molecules, and tricellulin, and are considered one of the principal determinants of mucosal permeability (Fig. 3) [20,21]. These transmembrane proteins interact with their partners on the opposing plasma membrane and provide a mechanical link between epithelial cells while establishing a diffusion barrier [22]. If disrupted, permeation of potentially noxious molecules and organisms from the intestinal lumen can result in a cascade of events including immune activation and inflammation, eventually triggering the development of intestinal and systemic diseases (Fig. 4).
Fig. 2.
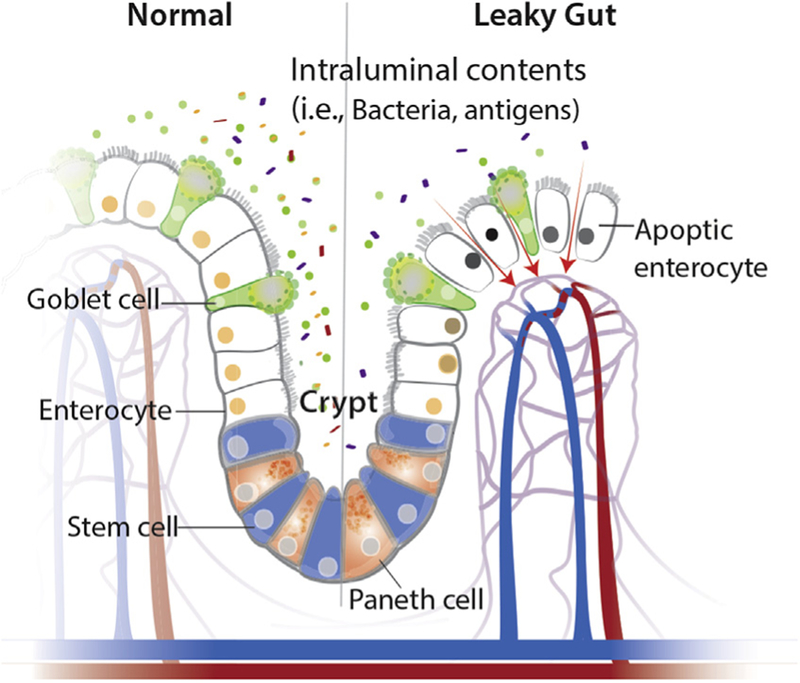
Components of the mucosal barrier in health and disease. The normal intestinal barrier is made up of a single layer of epithelial cells, with normal cell death (apoptosis) and turnover every 5–7 days. Undifferentiated stem cells are located at the crypt base and interspersed between post-mitotic Paneth cells. When intestinal permeability is altered, the junctions between the cells are disrupted and luminal contents can enter the surrounding tissues as well as systemic circulation. As a result, cells may undergo increased apoptosis and decreased barrier function.
Fig. 3.
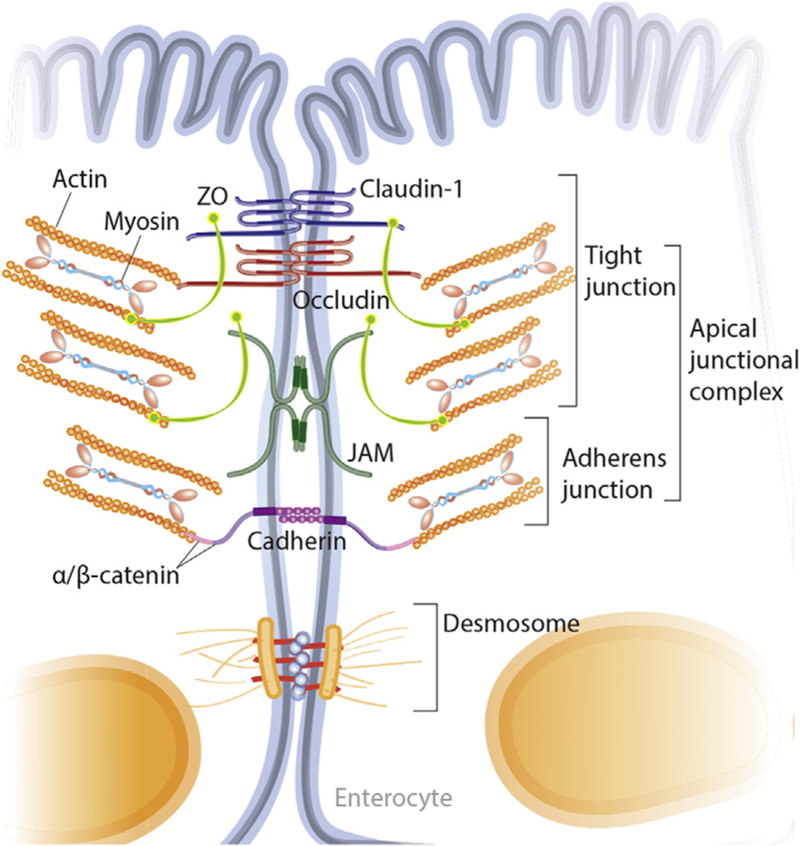
Detailed diagram of the intercellular junctions between intestinal epithelial cells. The intercellular junctions of intestinal epithelial cells are composed of several complexes including TJs, AJs, and desmosomes. The AJs and TJs together form the AJC. TJs are located at the apical end of the epithelial cells and are made up of multiple transmembrane proteins including occludins, claudins and JAM. Scaffolding proteins such as ZO in turn anchor the membrane proteins to the actin cytoskeleton. Simply, permeability is regulated at the TJ through actin and myosin contractility. Cell to cell contact at the AJ is mediated by adhesion molecule complexes made up of protein families including the cadherins and catenins. AJs, along with desmosomes, provide strong connective bonds between epithelial cells. AJ, adherens junction; AJC, apical junctional complex; JAM, junctional adhesion molecules; TJs, tight junctions; ZO, zonula occludens.
Fig. 4.
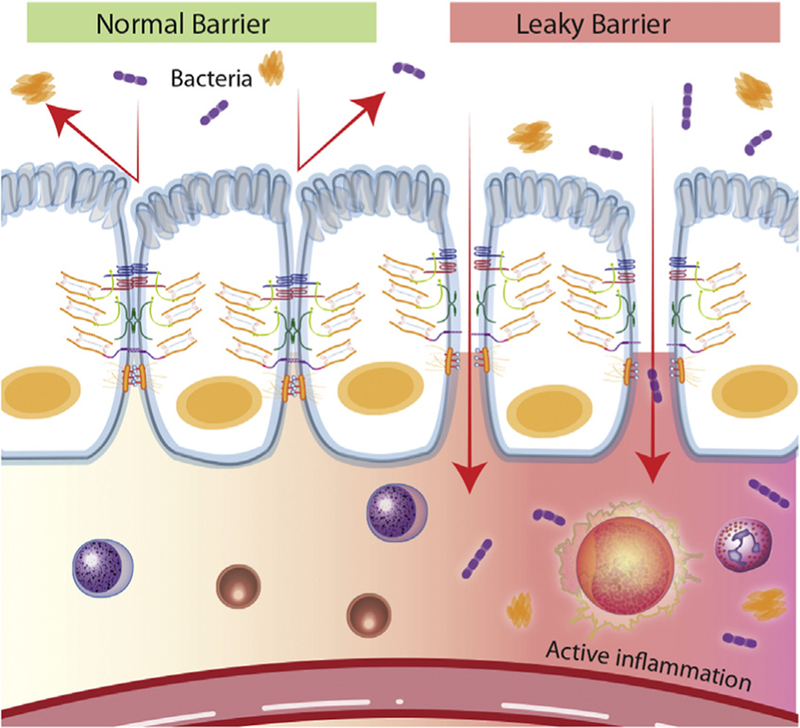
Normal versus impaired barrier function. The role of the intestinal epithelium is to provide a physical barrier against luminal contents such as bacteria. There are several important components of the barrier including tight junctions, adherens junctions, and desmosomes. Potentially harmful molecules cannot normally penetrate the barrier; however, when the barrier becomes compromised at any contact point, the passage of noxious molecules can occur and result in both an inflammatory and immune response. Disruption of the intestinal barrier may result in the development of local and systemic disease.
3. Assessment of Intestinal Permeability
There are a variety of methods to assess intestinal permeability in both human and animal models clinically and experimentally. Clinically, in horses, used methods of assessing intestinal permeability in vivo include urine or serum recovery of enterally delivered radio-labeled markers including 51Cr-ethylene diaminetetra-acetate (51Cr-EDTA) [23], 99mTc-diethylene triaminopenta-acetate (99mTc-DPTA) [24], and poorly absorbed sugars such as D-xylose [25,26] and sucrose [27]. After the administration of 51Cr-EDTA via nasogastric tube, the urine of ponies was collected to assess the effects of gastrointestinal motility chemically altered by atropine and bethanechol, as well as the effect of nematode infection on intestinal permeability [23]. There was no difference in recovery after the administration of motility altering medications; however, significant increases in 51Cr-EDTA urine recovery were present when ponies had an experimental cyathostome infection compared with control. 51Cr-ethylene diaminetetra-acetate was well tolerated and was shown to be a useful marker for the assessment of intestinal permeability in horses. Radioactivity of urine and blood technetium (99mTc) was also used to assess intestinal permeability after the administration of excessive carbohydrates in ponies, and was increased transiently after the administration [28]. This study concluded that excessive carbohydrate administration could directly cause a change in measured 99mTc, supporting an abnormal intestinal barrier. The measurement of fluorescent probes such as fluorescein isothiocyanate (FITC)-dextrans [29] has also been used to assess intestinal permeability in vivo. To assess abnormal gastric permeability, sucrose has been used in many species including dogs and humans [30,31]. Earlier studies in dogs also quantified the concentrations of several other sugars including rhamnose, 3-O-methyl-D-glucose, and lactulose to assess permeability [32]. In horses, significant increases in serum sucrose concentration were recorded following nasogastric intubation of table sugar in horses with moderate to severe gastric ulceration, suggesting the possibility of this test both as a screening tool and diagnostic aid for clinicians [27]. The main advantage of these techniques is that they can be tested in vivo. Other factors must be taken into consideration when evaluating these testing methods including molecule absorption and metabolism, gastrointestinal motility, concurrent medication administration, as well as blood volume and blood flow to the intestine.
Experimentally, the Ussing chamber provides an ex vivo method for the measurement of electrolyte, nutrient, and drug transport across intestinal epithelial tissues [33]. The use of the chamber has been documented in a variety of species including humans [34], rodents [35], horses [36], swine, and poultry [37]. While tissue is mounted in the Ussing chamber, a variety of measurements can be collected including transepithelial potential difference and transepithelial electrical resistance (TER) indicating tissue viability and barrier integrity, as well as flux of FITC-dextran, FITC-lipopolysaccharide (LPS), or mannitol to measure tight junction damage and leakage across the mucosa [37]. Measurements of TER and permeability in the chamber are calculated based on the serosal surface area, as the surface area of the mucosa varies based on species and the intestinal section under study [38]. Mucosal surface area is amplified by villus projections, which change in shape and height throughout the small intestine. The colon has no villi. When TER data are corrected for differences in surface area, the permeability of the small intestine and colon are similar [38]. The Ussing chamber, therefore, provides opportunities to measure changes in both paracellular and transcellular transport pathways in addition to the ability to manipulate the tissue restitution process through various reagent treatments. In vitro, TER and permeability have been assessed in human colonic epithelial cell (CACO-2) monolayers using a trans-well system [39]. In vitro scratch assays are also used to study cell migration and intestinal “healing” in a variety of cell lines including IPEC-J2 [40,41].
4. Causes of Increased Intestinal Permeability (All Species)
4.1. Introduction
As discussed, intestinal permeability is regulated by the physical barrier created by epithelial cells and junctional complexes (Figs. 3 and 4), other factors such as immune cells and cytokines, and exogenous influences like intestinal pathogens, alterations in intestinal blood flow, and environmental factors such as temperature [21]. With multiple factors contributing to intestinal permeability and barrier function, an understanding of these complex interactions in both health and disease remains critical to the future development of therapeutics aimed at modulating intestinal barrier function.
4.2. Stress-Induced Barrier Dysfunction
A variety of stressors (physiologic, pharmacological, psychologic, and others) affect the intestinal barrier and have been studied using human and animal models [42]. Early models of acute stress in rats first demonstrated that physical restraint was enough to alter normal gastrointestinal function. Using an Ussing chamber, jejunal tissue from rats subjected to several hours of physical restraint was shown to have increased ion transport, increased tissue conductance, and increases in mannitol and Cr-EDTA flux consistent with altered intestinal permeability [43]. In addition, rats had increased plasma corticosterone compared with normal controls, consistent with a stress response. The effect of other physical “stressors,” including exercise, has been studied in a variety of species including humans and dogs. Humans who participated in moderate exercise had decreases in jejunal absorption of sodium, chloride, potassium, and water [44]. Alaskan sled dogs undergoing sustained strenuous exercise demonstrated altered small intestinal permeability, with an increased lactulose:rhamnose ratio in both serum and urine [45]. The mechanisms of stress-altered permeability are likely related to changes in tissue perfusion and altered gastrointestinal motility; however, the effects seem to be intensity-dependent [46]. Exercise has been shown to reduce splanchnic blood flow [47] in an effort to preferentially provide enough blood to working tissues including the skeletal muscles and skin, leaving the gastrointestinal tract in a state of relative hypoperfusion. Ponies undergoing maximal exertion on a treadmill were found to have significant vasoconstriction of the renal and splanchnic vascular beds and resultant decreases in blood flow to the spleen, pancreas, small intestine, and colon, among other organs [48]. Shunting of the blood from visceral tissues to more active tissues during exercise can therefore result in varying degrees of intestinal damage including alterations in permeability [49,50]. Similar damage is reported in animal models of heat stress [29,42,51,52]. Following periods of extreme hyperthermia, blood is distributed preferentially to the periphery to help maximize heat dissipation, leaving the gastrointestinal tract in a state of hypoperfusion and ultimately leading to alterations in normal permeability. These alterations, secondary to heat damage, lead to increased concentrations of endotoxin and reactive oxygen species (ROS) [52], promoting significant damage to the intestinal mucosa by disrupting cellular membranes and the tight junctions [42]. This damage allows for the continued influx of endotoxins, primarily LPS, into systemic circulation, stimulating the production of proinflammatory cytokines and other immune responses [42]. Local and systemic inflammation can occur due to the increase flood of inflammatory cells. These responses can lead to systemic inflammatory response syndrome (SIRS) and ultimately multiple-organ failure [53,54]. Clinically, veterinary patients who suffer from heat stress have been shown to experience clinical signs that range in severity from mildly decreased feed intake and milk production (reported in dairy cows) to more severe consequences including altered immune responses [55] and death [56,57].
4.3. Gastrointestinal Ischemia-Reperfusion Injury
Interruption of the blood supply results in ischemic injury and the damage of metabolically active tissues including the intestine [58]. Prolonged ischemia alters membrane potential, disrupts normal ion distribution, impairs cytoskeletal organization of cells, and leads to increases in intracellular volume [59]. In addition, energy stores (ATP) become depleted, whereas the expression of proinflammatory cytokines, adhesion molecules, and bioactive agents such as endothelin and thromboxane A2 become accelerated [59,60]. These agents act as potent vasoconstrictors and may lead to further alterations in blood flow. Restoration of normal blood flow may then further augment tissue injury in excess of that from ischemia alone, termed reperfusion injury. Reperfusion of ischemic tissue results in the formation of toxic ROS. These ROS have high reactivity with other biological molecules, inducing oxidative stress and directly damage cellular membranes via lipid peroxidation [61]. Reactive oxygen species increase leukocyte activation, chemotaxis, and adherence resulting in a vicious cycle of cellular damage as the activated leukocytes continue to release harmful proteases, elastases, and additional ROS [60,61]. Ischemia-reperfusion injury occurs in a variety of conditions including shock, vascular surgery, strangulated bowel, trauma, intestinal volvulus, and intestinal transplantation, leaving both human and veterinary patients with a decreased chance of survival as the mucosal barrier is lost due to resultant tissue hypoxia, inflammation, and cellular infiltration [60,62]. Ischemia-reperfusion injury of the intestine is also associated with increased bacterial translocation into the systemic circulation, a contributing factor in the development of SIRS [63]. In horses, the survival rate for ischemic/strangulating lesions was lower than that for simple obstructions in a large retrospective study [64]. These lesions resulted in a higher rate of postoperative shock [65], likely due to barrier dysfunction and resultant endotoxemia. In horses, the intestinal damage found in natural obstructions is likely related to the severity and duration of ischemia and the subsequent reperfusion injury [66]. Intestinal ischemia is rarely preventable, and as a result, most research is aimed at targeting early detection and the recovery (postischemic) period, with efforts aimed at minimizing reperfusion injury and hastening epithelial repair.
4.4. Pathogen-Induced Barrier Dysfunction
The effects of pathogenic organisms on host intestinal epithelial cells are complex. These primary pathogen-host interactions may result in disturbances in the normal intestinal barrier, activation of the inflammatory cascade, and alterations of normal fluid and electrolyte secretion [67]. Enteric pathogens can bind to the cell surface and induce changes in the expression of tight junction proteins [21]. In addition, the production of toxins by pathogens can promote cellular damage through disruption of intracellular protein interactions, leading to increased cellular permeability, and ultimately trigger cell death [21]. While some pathogens primarily use one mechanism to alter host physiology, others, including Salmonella and Escherichia coli, are capable of altering the cellular functions of the intestinal epithelium through multiple mechanisms [67]. Although a detailed review of specific intestinal pathogens affecting both human and veterinary patients is outside the scope of this review article, several previous reports document important pathogens and their role in altering intestinal permeability including Clostridium, E. coli, Bacteroides, Vibrio, Lawsonia, and mycotoxin producing fungi such as Fusarium sp. [68–73].
Mycotoxin ingestion may be an inciting cause of leaky gut in pasture grazing animals. Pasture grasses, hay, and grain can all support the growth of various types of fungi, depending on the climate and season. Mycotoxins are secondary metabolites produced by fungi that when ingested or inhaled could result in adverse effects including gastrointestinal disease (vomiting and/or diarrhea) and alterations in growth and immune function in both humans and animals [73,74]. Common mycotoxins include aflatoxins, ergot alkaloids, fumonisins, ochratoxin and tricho- thecenes [75]. Deoxynivalenol, a mycotoxin of the trichothecenes family, has been shown to alter intestinal permeability and decrease claudin expression in porcine models [76]. A species sensitivity to mycotoxin exposure is documented, and research in horses is variable [74,77,78]. As horses ingest a variety of feed sources that could become contaminated with mycotoxins, there is a need for research in this field and its potential impact on intestinal health.
4.5. Altered Permeability in Inflammatory Bowel Disease
Inflammatory bowel diseases affect both human and veterinary patients and are associated with gastrointestinal dysfunction due to infiltration of the mucosa, submucosa, or lamina propria with abnormal populations of immune cells. The two most common forms of chronic IBD in humans are CD and ulcerative colitis (UC) [79]. In dogs, the most frequently detected form of IBD is lymphocytic- plasmacytic enteritis [80]. Horses also suffer from several forms of inflammatory disease including eosinophilic enteritis, granulomatous enteritis, and lymphocytic- plasmacytic enteritis [81]. In advanced equine disease, the large intestine can be involved.
Although the definitive cause is not known, IBD is thought to result from inappropriate and ongoing activation of the mucosal immune system [82]. Genetic factors can contribute to the susceptibility of IBD in humans and studies have shown an increased prevalence of IBD among relatives of patients with CD and UC [83,84]. An association was also found between abnormal small intestinal permeability and a specific mutation in the NOD2 gene, which functions to modulate immune responses to intestinal bacteria, suggesting a genetic pathway in which an abnormal intestinal barrier could lead to chronic intestinal inflammation [85,86]. Other factors proposed in the pathogenesis of IBD include an initial breakdown of the epithelial barrier which can lead to a self-amplifying cycle of immune activation and cytokine release [79], and loss of tolerance to endogenous microflora and dietary antigens [82,87]. Since normal epithelial barrier function is determined in part by the integrity of the AJC, it was initially hypothesized that defects in this structure, made of tight junctions and adherens junctions, led to alterations in intestinal permeability seen in cases of IBD. Initial studies of inflamed epithelium from UC and CD cases, demonstrated reduced expression of the complex proteins, E-cadherin and α-catenin [88]. Additional studies showed the down-regulation of E-cadherin in UC cases, and the upregulation of P-cadherin in both CD and UC cases [89]. Similarly, in dogs with IBD compared with normal controls, the expression of the protein E-cadherin was lower in the villus epithelium, suggesting the role of this protein in the pathogenesis of IBD in dogs [80]. Claudin expression was not significantly different in this canine study. In cases of mild to moderately active ‘CD, alterations in tight junction structure and barrier dysfunction were associated with downregulation of claudins-5 and −8 and an upregulation of claudin-2 [90]. Structurally, both UC and CD cases have abnormal architecture with decreases in the number and complexity of tight junction strands, glandular atrophy, and chronic epithelial damage [79,91]. To the author’s knowledge, no studies have been conducted investigating the expression of AJC proteins and their potential contribution in the pathogenesis of equine IBD.
Immune activation also contributes to altered barrier function in cases of IBD. Early studies demonstrated different cytokine secretion patterns in cases of UC and CD, which may determine the type of inflammatory process present [92]. The proinflammatory cytokines IFN-ϒ and TNF-α are both elevated in the mucosa of IBD patients [93]. These cytokines have been shown to decrease epithelial barrier function in several model epithelial cell lines [91,94,95]. In vitro treatment with IFN-γ and TNF-α led to redistribution of AJC transmembrane proteins, junction adhesion molecule 1, occludin, and claudin-1/4 in an epithelial cell line [93]. Another inflammatory cytokine, IL-13 was shown to be upregulated in patients with UC [96]. Upregulation of IL-13 led to increased epithelial cell apoptosis, conductance, and upregulation of the claudin-2 gene [96]. In dogs with small intestinal enteropathies, there was greater expression of IL-2, IL-5, IL-12, TNF-α, and TGF-β in duodenal mucosa when compared with control dogs [97]. In horses with large intestinal disease, a significant difference in TNF-α expression was found in diseased mucosa, suggesting a possible role for this cytokine in the pathogenesis of equine IBD [98]. Recent equine research found an involvement of proinflammatory T cells (T helper type 17) in active cases of IBD with greater expression of IL-17 in rectal biopsies [99]. This cytokine was also shown to be increased in human IBD patients [100]. In addition, TNF-α increases myosin light chain kinase (MLCK) phosphorylation [101], which may alter paracellular permeability through its association with actin and myosin (Fig. 3). Myosin light chain kinase expression and enzymatic activity are increased in cases of IBD and correlated with disease activity in one study [102]. It is possible that MLCK plays a role in the induction of intestinal barrier dysfunction in some cases of IBD.
A review of the literature demonstrates a variety of factors influencing the altered intestinal permeability seen in cases of IBD. The etiology is complex and likely involves the interaction between genetic, environmental, and immunological influences. What remains unclear is whether the changes observed within the tight junctions in cases of IBD are causal leading to further barrier dysfunction and abnormal immune responses, or the alterations in the function of tight junctions are related to the inflammation itself [101]. Regardless, further research is indicated in this complicated, multifactorial group of diseases that plagues both humans and veterinary species.
4.6. Microbiota, Diet, and Gastrointestinal Health
The intestinal tract is home to a vast, complex microbial ecosystem in all species. The resident microflora is often thought of as an organ system, in that it provides nourishment, regulates epithelial development, and plays a role in immune responses [103]. The gut microbial species composition is diverse and varies among individuals. Most host-microbiota interactions promote health; however, under certain conditions, such as environmental alterations, normally symbiotic organisms in the gastrointestinal tract have pathogenic potential, resulting in immune system activation and inflammatory disease [104,105]. A wide range of microorganisms have been suggested as causative agents of IBD; however, no single pathogenic agent has been routinely isolated in clinical cases [106]. It is likely that a dysbiosis arises in these conditions in which a decrease of beneficial bacteria and their metabolic byproducts occurs along with an increase in detrimental microbial populations and their toxic metabolites, leading to an altered luminal environment [106]. In work predominantly based on animal models, certain symbiotic microorganisms have been shown to initiate gut inflammation and pathology when colonizing a genetically susceptible host (e.g., Helicobacter and segmented filamentous bacteria). In other cases, specific resident microflora can expand following antibiotic use, clearing competing symbiotic organisms, and promoting disease (e.g., Clostridium difficile) [104]. In cases of canine dysbiosis, compositional changes in the small intestinal microbiota have been suggested [107].
In horses, the diverse bacterial population of the gastrointestinal tract has been characterized using fecal samples and varies greatly among the different intestinal compartments [108,109]. With such diversity, it is thought that many of the risk factors for gastrointestinal diseases are related to disruption of the intestinal microbiota and function [110]. Increased risk of colic has been associated with changes in management and nutrition including high amounts of concentrate, forage digestibility, and changes in diet [111–113]. Similarly, alterations in the horse intestinal microbiota have been shown in relation to diet change [114], dietary starch source and concentration [115], systemic antimicrobial usage [116], and administration of excessive carbohydrates [117]. Therefore, it is presumed that alterations in the microbiome may be detrimental to the horse, resulting in abnormal intestinal permeability and subsequent diarrhea, endotoxemia, and laminitis [117,118]. Indeed, disruption of fecal microbiota populations has been associated with incidence of colic [119,120] and colitis [121]. In particular, Fusobacterium were shown to be abundant in horses with colitis compared with healthy horses, and this genus has been associated with CD and appendicitis in humans [121].
It is well-recognized that the proportion of complex carbohydrates or fiber (typically found in the diet as long-stem forages) to more simple carbohydrates (such as starches and sugars), as well as abrupt changes in diet, cause fluctuations in the populations of microbes within the hindgut of the horse [109,122–124]. As such, different proportions of fermentative by-products, such as short chain fatty acids (SCFAs, also known as volatile fatty acids [VFAs]), methane, and hydrogen are produced depending on dietary carbohydrate composition. Higher proportions of starch and sugar intake result in higher production of hydrogen, lactate, and propionate, whereas higher intakes of fiber result in increased acetate production [122,125].
Starch intake can be quite high as concentrates are often fed to athletic horses to meet energetic demands. Potter et al [126] showed an upper limit to preileal starch digestion, such that starch intake over 3 g per kg body weight results in a “spillover” of undigested starch to the large intestine. Starch reaching the hindgut increases total VFA production, lactic acid production and results in a drop in pH and acidosis [127]. Acidosis may damage the epithelium and alter permeability, as hyperpermeability has been reported in both cattle and swine mucosa with acidosis [128,129]. Therefore, both microbial composition and dietary composition affect intestinal health. Further research is needed both in healthy and disease states to determine the critical role that the microbiota and diet may play in the progression of disease.
5. Alternative Therapeutic Opportunities in Leaky Gut
Conventional therapies, including the use of systemic antimicrobials and surgical resection of nonviable intestinal tissue remain the cornerstone to successful treatment of several causes of leaky gut. Leaky gut and altered intestinal permeability result from a variety of factors including physical stressors, immune dysfunction, and disruption of normal gut homeostasis. As a result, research regarding manipulation of the intestinal barrier often focuses on therapies aimed at the regulation of host immune and inflammatory responses along with epithelial barrier function. The use of alternative therapies including SCFAs, amino acids, nutrients, and probiotics/prebiotics has been investigated in natural and induced models of altered intestinal permeability in several species. Limited research has been performed with many of these immunomodulatory therapies in the horse, leaving a critical gap in our knowledge as clinicians.
5.1. Short Chain Fatty Acids
The three major SCFAs produced during bacterial carbohydrate fermentation are acetate, propionate, and butyrate. They are readily absorbed and metabolized into energy [130]. Butyrate is the preferred substrate for enterocytes and several studies have shown that SCFAs have anti-inflammatory properties [131]. In an experimental model of 5-fluorouracil-induced mucositis in mice, butyrate treatment improved intestinal permeability and minimized intestinal damage [132]. In a dextran sulfate sodium (DSS)-induced colitis model, mice receiving sodium butyrate in the diet had reduced mucosal inflammation, improvement in diarrhea and an improved inflammatory profile in local lymph nodes [130]. In piglets with acetic acid-induced colitis, butyrate supplementation (in the form of tributyrin) decreased colonic lymphocyte infiltration, decreased expression of cell death marker caspase-3, and increased expression of tight junction protein claudin-1 in the colonic mucosa, suggesting that butyrate may alleviate injury by decreasing cellular apoptosis and improving tight junction formation [133].
The success of butyrate treatment in vitro has yielded variable results. Using an in vitro medium, butyrate and propionate treatment was shown to improve intestinal cell proliferation in human biopsy samples [134]. Sodium butyrate (4 mM) was able to increase wound healing in porcine small intestinal epithelial cells and enhance the expression of tight junction proteins occludin and zonula-occluden protein-1 [40]. A dose-dependent effect of butyrate was demonstrated in vitro using caco-2 cells, in which low concentrations (2 mM) promoted intestinal barrier function, whereas excessive butyrate induced cell apoptosis and increases in inulin permeability [39]. In an equine in vitro model of oxidant-injured right dorsal colon, butyrate treatment (20 mM) did not influence mucosal restitution [135].
Endogenous butyrate production is another potential therapeutic mechanism for improving gut health. In the poultry industry, where antimicrobial growth promoters are now banned, there is significant interest in the role of intestinal butyrate production for pathogen control and for optimizing the intestinal barrier. Increasing butyrate production is a function of both the microbial population (butyric acid producing bacteria) and the diet. The Ruminococcaceae and Lachnospiraceae are major butyrate producing families, although some clusters of Clostridium are also important [136]. Providing these bacteria to the animal directly are difficult because they are strict anaerobes, while prebiotics that stabilize these bacterial families have promise. Prebiotics and probiotics are described further below.
Given the beneficial effects of butyrate and other SCFA noted in a variety of animal models, the use of these therapies warrants consideration in the treatment of equine gastrointestinal disease; however, further research is needed at this time.
5.2. Amino Acids
Amino acids are essential substrates required for protein, nitric oxide, and polyamine synthesis, and serve as a major fuel for the small intestinal mucosa [131,137]. Several studies support the potential therapeutic benefit of amino acids including glutamine, arginine, lysine, threonine, and others in gut-related disease [137].
Arginine, a conditionally essential, versatile amino acid, serves as a precursor for protein synthesis and other important molecules including nitric oxide, urea, and creatine [138]. Arginine has been shown in vitro to improve barrier function in caco-2 cells injured with a commonly used immunomodulatory medication (methotrexate) [139]. In several animal models of intestinal disease, intestinal permeability was maintained by arginine administration [131]. Oral arginine supplementation decreased intestinal mucosal injury following lipopolysaccharide treatment in rats [140]. Recent work demonstrated the safety of arginine supplementation in healthy rats, pigs, and gestating sheep [141] and previous research demonstrated tolerance to long-term supplementation in neonatal and pregnant pigs [142]. Limited studies in horses have demonstrated the successful absorption of arginine in normal mares; however, arginine supplementation altered the absorption of other amino acids [143]. Research is warranted to determine if arginine supplementation may benefit horses with gastrointestinal disease.
Glutamine, a nonessential amino acid with several important functions, accounts for the majority of energy generated in the small intestine along with glutamate and aspartate [131,144]. Glutamine is considered “conditionally essential” in times of stress and disease [145]. Depleted levels of glutamine have been associated with an impaired stress response in human lymphocytes in vitro [146]. Glutamine supplementation was found to decrease jejunal atrophy in weaned pigs and improved growth performance [147]. In weaned piglets, supplementation of glutamine increased the expression of genes necessary for cell growth, and reduced expression of genes that may promote oxidative stress in the small intestine when compared with age-matched controls [148]. Intravenous supplementation of glutamine attenuated the degree of intestinal damage seen in experimental ischemia/reperfusion in rats with reductions in leukocyte infiltration, improved histologic damage scores, and decreased apoptosis [149]. In a DSS-induced colitis model, mice fed glutamine had decreased expression of chemokine receptors and had less colonic T-cell infiltration compared with controls thereby decreasing inflammatory mediators in treated individuals [150].
In horses, glutamine was shown to increase the efficiency of intestinal restitution in an in vitro model of colitis [135]. This is similar to other in vitro experiments demonstrating the role glutamine may play in preventing intestinal cell damage [139,151]. Previous work showed that plasma glutamine concentrations could be increased in the short-term when administered orally to healthy horses [152]. A more recent feed trial in young horses showed no adverse effects of a supplemental product containing L-glutamine [153]. At this time, no research has been performed looking at the effects of glutamine supplementation in horses with gastrointestinal disease, and additional research is necessary to determine an appropriate dose and potential efficacy in diseased gut.
5.3. Other Nutrients
Zinc is a trace element which functions in cellular turnover, regulation, and repair and is a key component of several enzyme systems [1,154]. Several in vivo animal models have demonstrated the potential for zinc to enhance intestinal function in disease. Zinc supplementation helped maintain the stability of the intestinal microflora and diversity of coliforms in treated pigs when compared with control pigs for 2 weeks post weaning [155]. In experimental colitis, zinc supplemented rats had less diarrhea and less weight loss, but no effect on macroscopic inflammation [156]. In a similar model, rats supplemented with zinc had a significant reduction in opened tight junctions when compared with untreated rats with colitis, but no amelioration of clinical disease [157]. A study in broiler chickens supplemented with zinc and challenged with Salmonella, demonstrated improved intestinal morphology following infection, lowered plasma endotoxin levels, and enhanced expression of claudin-1 and occludin from ileal mucosa when compared with controls [158]. Following a challenge with enterotoxigenic E. coli, piglets supplemented with zinc oxide had reduced expression of inflammatory immune response genes [159]. In humans with quiescent CD, zinc supplementation resulted in improved alterations in intestinal permeability [160]. Serum zinc concentrations following oral supplementation of two different zinc compounds has been investigated in ponies [161], but to date no studies have been performed assessing zinc supplementation in horses with gastrointestinal disease.
Selenium and vitamin E may prove beneficial in improving intestinal permeability. The effects of selenium and vitamin E supplementation were studied on heat-stressed pigs and shown to reduce oxidative stress and improve intestinal permeability when fed in high concentrations [162]. Further data and trials are needed to rationalize the use of vitamin E and selenium for gastrointestinal diseases in horses and other species.
5.4. Probiotics, Prebiotics, and Synbiotics
Probiotics are living microorganisms that when supplemented in certain numbers could offer a beneficial effect to the host. This is in contrast to a prebiotic, which is typically a nondigestible food ingredient that may benefit the host intestinal microflora by stimulating growth and activity of a few selected organisms [163]. Prebiotics often include oligosaccharides that can resist normally produced digestive enzymes, but remain susceptible to fermentation by the colonic microflora [148]. The combination of a prebiotic and probiotic, termed a synbiotic, may offer synergistic therapy [164]. An extensive review of these therapies is outside the scope of this paper, however, the authors direct readers to several published reviews [164–168].
The potential benefits of probiotic use are diverse and may include immune system activation and modulation, enhanced mucosal barrier function, competitive exclusion of pathogens, and decreased risk of infection through production of antimicrobial substances including lactic and acetic acids [131,166]. Probiotics have been used in the treatment and prevention of IBDs, diarrhea, irritable bowel syndrome, and gastroenteritis, among others [169]. Although several organisms have been studied, commonly used species include Bifidobacterium, Lactobacillus, and Saccharomyces [169].
In two murine models of colitis, treatment with Bifidobacterium bifidum improved histologic scores and decreased several inflammatory markers [170].
Mice with DSS-induced colitis had improved survival and barrier function following treatment with heat-killed Lactobacillus brevis compared with control mice [171]. Using a similar model, Lactobacillus rhamnosus was shown to increase the expression of the junctional complex component zonula-occludin-1 and improve intestinal permeability in treated mice [172]. A recent study demonstrated that Lactobacillus fed to broiler chickens subjected to heat stress improved average daily gain [173] when compared with controls.
When Saccharomyces boulardii was added as an adjunctive therapy to mesalamine, an anti-inflammatory medication used in the management of CD, the rate of clinical relapse decreased compared with mesalamine alone [174] suggesting that this organism may be useful in improving the effectiveness of commonly used medications for IBD management.
Similar to other species, probiotics commonly used in large animals include the bacterial genera Lactobacillus, Enterococcus, Bifidobacterium, and Streptococcus, and the yeast Saccharomyces [110]. In horses, there is limited research regarding the use of probiotics and the results are variable [110,166]. Most studies investigating the use of probiotics in horses have studied the effect of Saccharomyces cerevisiae administration to improve hindgut fermentation and diet digestibility in healthy horses. Unfortunately, results are mixed [175–177]. In horses fed a high starch diet, Lactobacillus acidophilius alone or in combination with several other species of bacteria including B. bifidum and Enterococcus faecium had limited effects on nutrient digestibility and did not reduce the risk of acidosis (determined from fecal pH and fecal VFA concentration) compared with controls [178].
There is limited research evaluating probiotic use in horses with gastrointestinal disease [166]. Horses with acute enterocolitis administered S. boulardii had a shorter duration of diarrhea than those fed a placebo [179]. However, there was no significant difference in duration of hospitalization or outcome in the two groups. Treatment with S. boulardii was also evaluated in horses with antimicrobial-associated enterocolitis [180]. Although the organism could be successfully cultured from more than half of the horses receiving it, there were no statistically significant differences in any of the outcome measures. In other large animal species, probiotics have been used to help control Salmonella infection [181–183]. In horses, postoperative colic patients treated with two different probiotic products (containing combinations of organisms including Lactobacillus sp., Streptococcus sp., and Bifidobacterium sp.) had no difference in Salmonella shedding compared with placebo-treated horses [184]. Of note, no adverse effects were noted when probiotic administration exceeded the manufacturers recommended dose [184]. Horses treated with a multistrain probiotic had no difference in Salmonella shedding compared with control horses in a later trial [185].
Prebiotics that have been used in poultry to increase butyrate production include xylo-oligosaccharides (XOS) and other oligosaccharides. Work in poultry showed that XOS supplementation increased the conversion of lactate to butyrate [186]. In the horse, fructooligosaccharides (FOS) were shown to increase fecal butyrate concentrations [187], whereas Gürbüz et al [188] reported no effect of FOS on fecal pH, VFA composition, or immune status. Short-chain FOS supplementation mitigated an increase in lactate concentration following an abrupt introduction of barley (starch overload) [189].
Given the variability of results and the diversity of the equine microflora, additional studies are needed before excluding any potential beneficial effects of probiotics or prebiotics in horses with gastrointestinal disease.
6. Conclusion
There are many causes of altered intestinal permeability, and impairments in barrier function can have devastating consequences on the health of an individual (Fig. 1). To better understand the effects of leaky gut, clinicians and scientists must recognize the multiple factors that influence barrier function including the host immune response, barrier permeability, resident microflora, and others. A variety of tools are available in vitro, ex vivo, and in vivo to test intestinal permeability, and each offers unique advantages and disadvantages.
As our knowledge and understanding of normal and altered barrier function continues to expand, the ability to manipulate and modify intestinal permeability through the use of novel therapeutics remains promising. Most therapeutic research remains centered around experimental models of disease in rodents with limited work in the larger species, arguably narrowing the utility of these findings in horses on a clinical level. As reviewed above, alterations in intestinal permeability can be complex and multifactorial, offering researchers and clinicians several avenues to aid in disease recognition and diagnosis, investigate pathogenesis, and develop therapies to improve outcome and hopefully, in the future, prevent these diseases from occurring. With regards to the horse, additional research is critically needed and should use models of both healthy horses and those suffering from gastrointestinal disease. Several of the therapies discussed above, including SCFAs such as butyrate, essential nutrients including zinc and probiotics/prebiotics may offer horses similar benefits to those demonstrated in other species, ultimately improving the health and well-being of these animals. Before these therapies can be used clinically; however, studies on efficacy and safety are warranted and remain crucial to successful treatment of these complicated, often life-altering, conditions.
Acknowledgments
The authors wish to acknowledge Kenneth J. Kopp, DVM for his assistance in editing this review article, Lance H. Baumgard, PhD, Iowa State University, for providing his expertise and reviewing the final paper, and Alice Mac-Gregor Harvey, NC State College of Veterinary Medicine, for the graphical illustrations in this text. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Preparation of this paper was supported by Kemin Industries (Kemin Animal Nutrition - Equine Division).
References
- [1].Michielan A, D’Inca R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fasano A Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012;42:71–8. [DOI] [PubMed] [Google Scholar]
- [3].USDA APHIS Veterinary Services Centers for Epidemiology and Animal Health. Part. I: baseline reference of equine health and management CO: Fort Collins; 2005. [Google Scholar]
- [4].Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 2003;18:479–97. [DOI] [PubMed] [Google Scholar]
- [5].Korjamo T, Heikkinen AT, Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci 2009; 98:4469–79. [DOI] [PubMed] [Google Scholar]
- [6].Smithson KW, Millar DB, Jacobs LR, Gray GM. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science 1981;214:1241–4. [DOI] [PubMed] [Google Scholar]
- [7].Laissue JA, Chappuis BB, Muller C, Reubi JC, Gebbers JO. The intestinal immune system and its relation to disease. Dig Dis 1993; 11:298–312. [DOI] [PubMed] [Google Scholar]
- [8].Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caricilli AM, Castoldi A, Camara NO. Intestinal barrier: a gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol 2014;5:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The micro-biome: stress, health and disease. Mamm Genome 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- [11].Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology 2014;146:1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J Gastroenterol 2014;20:16489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14:141–53. [DOI] [PubMed] [Google Scholar]
- [14].Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 1995;16:351–80. [DOI] [PubMed] [Google Scholar]
- [15].Abdul Khalek FJ, Gallicano GI, Mishra L. Colon cancer stem cells. Gastrointest Cancer Res 2010;(Suppl 1):S16–23. [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 1974;141:537–61. [DOI] [PubMed] [Google Scholar]
- [17].Gonzalez LM. The mother of a gut cell: intestinal epithelial stem cells. Equine Vet Education 2015;27:559–60. [Google Scholar]
- [18].Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol 2003;15:763–70. [DOI] [PubMed] [Google Scholar]
- [19].Suzuki T Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013;70:631–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- [21].Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 2009; 124:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010;177: 512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Escala J, Gatherer ME, Voute L, Love S. Application of the 51Cr- EDTA urinary recovery test for assessment of intestinal permeability in the horse. Res Vet Sci 2006;80:181–5. [DOI] [PubMed] [Google Scholar]
- [24].Weiss DJ, Evanson OA, Green BT, Brown DR. In vitro evaluation of intraluminal factors that may alter intestinal permeability in ponies with carbohydrate-induced laminitis. Am J Vet Res 2000; 61:858–61. [DOI] [PubMed] [Google Scholar]
- [25].Ferrante PL, Freeman DE, Ramberg CF, Kronfeld DS. Kinetic analysis of D-xylose absorption after its intragastric administration to mares deprived of food. Am J Vet Res 1993;54:2110–4. [PubMed] [Google Scholar]
- [26].Freeman DE. In vitro concentrative accumulation of D-xylose by jejunum from horses and rabbits. Am J Vet Res 1993;54: 965–9. [PubMed] [Google Scholar]
- [27].Hewetson M, Cohen ND, Love S, Buddington RK, Holmes W, Innocent GT, et al. Sucrose concentration in blood: a new method for assessment of gastric permeability in horses with gastric ulceration. J Vet Intern Med 2006;20:388–94. [DOI] [PubMed] [Google Scholar]
- [28].Weiss DJ, Evanson OA, MacLeay J, Brown DR. Transient alteration in intestinal permeability to technetium Tc99m diethylene-triaminopentaacetate during the prodromal stages of alimentary laminitis in ponies. Am J Vet Res 1998;59:1431–4. [PubMed] [Google Scholar]
- [29].Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol (1985) 2002;92:1750–61. discussion 49. [DOI] [PubMed] [Google Scholar]
- [30].Meddings JB, Kirk D, Olson ME. Noninvasive detection of nonsteroidal anti-inflammatory drug-induced gastropathy in dogs. Am J Vet Res 1995;56:977–81. [PubMed] [Google Scholar]
- [31].Sutherland LR, Verhoef M, Wallace JL, Van Rosendaal G, Crutcher R, Meddings JB. A simple, non-invasive marker of gastric damage: sucrose permeability. Lancet 1994;343:998–1000. [DOI] [PubMed] [Google Scholar]
- [32].Sorensen SH, Proud FJ, Rutgers HC, Markwell P, Adam A, Batt RM. A blood test for intestinal permeability and function: a new tool for the diagnosis of chronic intestinal disease in dogs. Clin Chim Acta 1997;264:103–15. [DOI] [PubMed] [Google Scholar]
- [33].Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 2009;296:G1151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moore SA, Nighot P, Reyes C, Rawat M, McKee J, Lemon D, et al. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg 2016;51:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol 2010;299: G449–56. [DOI] [PubMed] [Google Scholar]
- [36].Davis JL, Little D, Blikslager AT, Papich MG. Mucosal permeability of water-soluble drugs in the equine jejunum: a preliminary investigation. J Vet Pharmacol Ther 2006;29:379–85. [DOI] [PubMed] [Google Scholar]
- [37].Neirinckx E, Vervaet C, Michiels J, De Smet S, Van den Broeck W, Remon JP, et al. Feasibility of the Ussing chamber technique for the determination of in vitro jejunal permeability of passively absorbed compounds in different animal species. J Vet Pharmacol Ther 2011;34:290–7. [DOI] [PubMed] [Google Scholar]
- [38].Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 2007;87: 545–64. [DOI] [PubMed] [Google Scholar]
- [39].Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res 2007;61:37–41. [DOI] [PubMed] [Google Scholar]
- [40].Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci 2012;90(Suppl 4):266–8. [DOI] [PubMed] [Google Scholar]
- [41].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2:329–33. [DOI] [PubMed] [Google Scholar]
- [42].Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 2009;87(14 Suppl):E101–8. [DOI] [PubMed] [Google Scholar]
- [43].Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol 1994;267(5 Pt 1):G794–9. [DOI] [PubMed] [Google Scholar]
- [44].Barclay GR, Turnberg LA. Effect of moderate exercise on salt and water transport in the human jejunum. Gut 1988;29:816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Davis MS, Willard MD, Williamson KK, Steiner JM, Williams DA. Sustained strenuous exercise increases intestinal permeability in racing Alaskan sled dogs. J Vet Intern Med 2005;19:34–9. [DOI] [PubMed] [Google Scholar]
- [46].de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med 2014;44(Suppl 1):S79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut 1987;28:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Manohar M Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol 1986;377:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].van Wijck K, Lenaerts K, van Loon LJ, Peters WH, Buurman WA, Dejong CH. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One 2011;6:e22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marchbank T, Davison G, Oakes JR, Ghatei MA, Patterson M, Moyer MP, et al. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol 2011;300:G477–84. [DOI] [PubMed] [Google Scholar]
- [51].Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 2013;8: e70215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 2001;280:H509–21. [DOI] [PubMed] [Google Scholar]
- [53].Simon HB. Hyperthermia and heatstroke. Hosp Pract (Off Ed) 1994; 29:65–8. [DOI] [PubMed] [Google Scholar]
- [54].Knochel JP. Heat stroke and related heat stress disorders. Dis Mon 1989;35:301–77. [PubMed] [Google Scholar]
- [55].Das R, Sailo L, Verma N, Bharti P, Saikia J, Imtiwati, et al. Impact of heat stress on health and performance of dairy animals: a review. Vet World 2016;9:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dey S, Dwivedi SK, Malik P, Panisup AS, Tandon SN, Singh BK. Mortality associated with heat stress in donkeys in India. Vet Rec 2010;166:143–4. [DOI] [PubMed] [Google Scholar]
- [57].D’Allaire S, Drolet R, Brodeur D. Sow mortality associated with high ambient temperatures. Can Vet J 1996;37:237–9. [PMC free article] [PubMed] [Google Scholar]
- [58].Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 2004;49:1359–77. [DOI] [PubMed] [Google Scholar]
- [59].Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000;190:255–66. [DOI] [PubMed] [Google Scholar]
- [60].Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001;94:1133–8. [DOI] [PubMed] [Google Scholar]
- [61].Toyokuni S Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int 1999;49:91–102. [DOI] [PubMed] [Google Scholar]
- [62].Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res 2015;166:12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kong SE, Blennerhassett LR, Heel KA, McCauley RD, Hall JC. Ischaemia-reperfusion injury to the intestine. Aust N Z J Surg 1998; 68:554–61. [DOI] [PubMed] [Google Scholar]
- [64].Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 1: short-term survival following a single laparotomy. Equine Vet J 2005;37: 296–302. [DOI] [PubMed] [Google Scholar]
- [65].Mair TS, Smith LJ. Survival and complication rates in 300 horses undergoing surgical treatment of colic. Part 2: short-term complications. Equine Vet J 2005;37:303–9. [DOI] [PubMed] [Google Scholar]
- [66].Snyder JR. The pathophysiology of intestinal damage: effects of luminal distention and ischemia. Vet Clin North Am Equine Pract 1989;5:247–70. [DOI] [PubMed] [Google Scholar]
- [67].Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 2003;52:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol 1995;268(2 Pt 1):G374–9. [DOI] [PubMed] [Google Scholar]
- [69].Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem 1997;272:26652–8. [DOI] [PubMed] [Google Scholar]
- [70].Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol 2000;2:11–7. [DOI] [PubMed] [Google Scholar]
- [71].Eichner M, Protze J, Piontek A, Krause G, Piontek J. Targeting and alteration of tight junctions by bacteria and their virulence factors such as Clostridium perfringens enterotoxin. Pflugers Arch 2017; 469:77–90. [DOI] [PubMed] [Google Scholar]
- [72].Page AE, Slovis NM, Horohov DW. Lawsonia intracellularis and equine proliferative enteropathy. Vet Clin North Am Equine Pract 2014;30:641–58. [DOI] [PubMed] [Google Scholar]
- [73].Molds WB. Mycotoxins and their effect on horses 2003 [updated 01 2005] Available at: http://www.omafra.gov.on.ca/english/livestock/horses/facts/info_mycotoxin.htm. [accessed 21.12.16]
- [74].Pestka JJ, Smolinski AT. Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev 2005;8: 39–69. [DOI] [PubMed] [Google Scholar]
- [75].Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev 2003;16: 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pinton P, Nougayrede JP, Del Rio JC, Moreno C, Marin DE, Ferrier L, et al. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol Appl Pharmacol 2009;237:41–8. [DOI] [PubMed] [Google Scholar]
- [77].Raymond SL, Smith TK, Swamy HV. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry, and hematology of horses, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J Anim Sci 2003;81:2123–30. [DOI] [PubMed] [Google Scholar]
- [78].Johnson PJ, Casteel SW, Messer NT. Effect of feeding deoxynivalenol (vomitoxin)-contaminated barley to horses. J Vet Diagn Invest 1997;9:219–21. [DOI] [PubMed] [Google Scholar]
- [79].Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 2004;84:282–91. [DOI] [PubMed] [Google Scholar]
- [80].Ohta H, Sunden Y, Yokoyama N, Osuga T, Lim SY, Tamura Y, et al. Expression of apical junction complex proteins in duodenal mucosa of dogs with inflammatory bowel disease. Am J Vet Res 2014; 75:746–51. [DOI] [PubMed] [Google Scholar]
- [81].Kalck KA. Inflammatory bowel disease in horses. Vet Clin North Am Equine Pract 2009;25:303–15. [DOI] [PubMed] [Google Scholar]
- [82].Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347: 417–29. [DOI] [PubMed] [Google Scholar]
- [83].Satsangi J, Jewell DP, Rosenberg WM, Bell JI. Genetics of inflammatory bowel disease. Gut 1994;35:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Satsangi J, Parkes M, Jewell DP, Bell JI. Genetics of inflammatory bowel disease. Clin Sci (Lond) 1998;94:473–8. [DOI] [PubMed] [Google Scholar]
- [85].Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci 2012;1258:159–65. [DOI] [PubMed] [Google Scholar]
- [86].Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut 2006;55:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- [88].Karayiannakis AJ, Syrigos KN, Efstathiou J, Valizadeh A, Noda M, Playford RJ, et al. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J Pathol 1998; 185:413–8. [DOI] [PubMed] [Google Scholar]
- [89].Jankowski JA, Bedford FK, Boulton RA, Cruickshank N, Hall C, Elder J, et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn’s colitis. Lab Invest 1998;78:1155–67. [PubMed] [Google Scholar]
- [90].Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007;56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci 2006;1072: 242–52. [DOI] [PubMed] [Google Scholar]
- [92].Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996;157:1261–70. [PubMed] [Google Scholar]
- [93].Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003;171:6164–72. [DOI] [PubMed] [Google Scholar]
- [94].Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci 1999;112(Pt 1):137–46. [DOI] [PubMed] [Google Scholar]
- [95].Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1989;83:724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129:550–64. [DOI] [PubMed] [Google Scholar]
- [97].German AJ, Helps CR, Hall EJ, Day MJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci 2000;45:7–17. [DOI] [PubMed] [Google Scholar]
- [98].Davidson AJ, Edwards GB, Proudman CJ, Cripps PJ, Matthews JB. Cytokine mRNA expression pattern in horses with large intestinal disease. Res Vet Sci 2002;72:177–85. [DOI] [PubMed] [Google Scholar]
- [99].Olofsson KM, Hjertner B, Fossum C, Press CM, Lindberg R. Expression of T helper type 17 (Th17)-associated cytokines and toll-like receptor 4 and their correlation with Foxp3 positive cells in rectal biopsies of horses with clinical signs of inflammatory bowel disease. Vet J 2015;206:97–104. [DOI] [PubMed] [Google Scholar]
- [100].Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 2016; 22:3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006;86:191–201. [DOI] [PubMed] [Google Scholar]
- [103].Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science 2005;308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol 2011; 23:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol 2017;312:G171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol 2011;17:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Suchodolski JS. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J Anim Sci 2011;89: 1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Costa MC, Silva G, Ramos RV, Staempfli HR, Arroyo LG, Kim P, et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. Vet J 2015; 205:74–80. [DOI] [PubMed] [Google Scholar]
- [109].Julliand V, Grimm P. HORSE SPECIES SYMPOSIUM: the microbiome of the horse hindgut: history and current knowledge. J Anim Sci 2016;94:2262–74. [DOI] [PubMed] [Google Scholar]
- [110].Coverdale JA. HORSE SPECIES SYMPOSIUM: can the microbiome of the horse be altered to improve digestion? J Anim Sci 2016;94: 2275–81. [DOI] [PubMed] [Google Scholar]
- [111].Hudson JM, Cohen ND, Gibbs PG, Thompson JA. Feeding practices associated with colic in horses. J Am Vet Med Assoc 2001;219: 1419–25. [DOI] [PubMed] [Google Scholar]
- [112].Archer DC, Proudman CJ. Epidemiological clues to preventing colic. Vet J 2006;172:29–39. [DOI] [PubMed] [Google Scholar]
- [113].Tinker MK, White NA, Lessard P, Thatcher CD, Pelzer KD, Davis B, et al. Prospective study of equine colic risk factors. Equine Vet J 1997;29:454–8. [DOI] [PubMed] [Google Scholar]
- [114].Daly K, Proudman CJ, Duncan SH, Flint HJ, Dyer J, Shirazi- Beechey SP. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br J Nutr 2012;107:989–95. [DOI] [PubMed] [Google Scholar]
- [115].Harlow BE, Lawrence LM, Hayes SH, Crum A, Flythe MD. Effect of dietary starch source and concentration on equine fecal microbiota. PLoS One 2016;11:e0154037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Costa MC, Stampfli HR, Arroyo LG, Allen-Vercoe E, Gomes RG, Weese JS. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet Res 2015;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Milinovich GJ, Burrell PC, Pollitt CC, Klieve AV, Blackall LL, Ouwerkerk D, et al. Microbial ecology of the equine hindgut during oligofructose-induced laminitis. ISME J 2008;2:1089–100. [DOI] [PubMed] [Google Scholar]
- [118].Sprouse RF, Garner HE, Green EM. Plasma endotoxin levels in horses subjected to carbohydrate induced laminitis. Equine Vet J 1987;19:25–8. [DOI] [PubMed] [Google Scholar]
- [119].Weese JS, Holcombe SJ, Embertson RM, Kurtz KA, Roessner HA, Jalali M, et al. Changes in the faecal microbiota of mares precede the development of post partum colic. Equine Vet J 2015;47:641–9. [DOI] [PubMed] [Google Scholar]
- [120].Venable EB, Kelley MS, Raub R. Assessment of equine fecal microbial profiles during and after a colic episode using pyrosequencing. J Equine Vet Sci 2013;33:347–8. [Google Scholar]
- [121].Costa MC, Arroyo LG, Allen-Vercoe E, Stampfli HR, Kim PT, Sturgeon A, et al. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS One 2012;7:e41484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Julliand V, de Fombelle A, Drogoul C, Jacotot E. Feeding and microbial disorders in horses: part 3—effects of three hay:grain ratios on microbial profile and activities. J Equine Vet Sci 2001;21:543–6. [Google Scholar]
- [123].Fernandes KA, Kittelmann S, Rogers CW, Gee EK, Bolwell CF, Bermingham EN, et al. Faecal microbiota of forage-fed horses in New Zealand and the population dynamics of microbial communities following dietary change. PLoS One 2014;9:e112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].de Fombelle A, Julliand V, Drogoul C, Jacotot E. Feeding and microbial disorders in horses: 1-effects of an abrupt incorporation of two levels of barley in a hay diet on microbial profile and activities. J Equine Vet Sci 2001;21:439–45. [Google Scholar]
- [125].Hintz HF, Argenzio RA, Schryver HF. Digestion coefficients, blood glucose levels and molar percentage of volatile acids in intestinal fluid of ponies fed varying forage-grain ratios. J Anim Sci 1971;33: 992–5. [DOI] [PubMed] [Google Scholar]
- [126].Potter GD, Arnold FF, Householder DD, Hansen DH, Brown KM. Digestion of starch in the small or large intestine of the equine. Pferdeheilkunde 1992:107–11. Sonderheft. [Google Scholar]
- [127].Biddle AS, Black SJ, Blanchard JL. An in vitro model of the horse gut microbiome enables identification of lactate-utilizing bacteria that differentially respond to starch induction. PLoS One 2013;8: e77599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Khafipour E, Krause DO, Plaizier JC. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysac-charide and triggers inflammation. J Dairy Sci 2009;92:1060–70. [DOI] [PubMed] [Google Scholar]
- [129].Salzman AL, Wang H, Wollert PS, Vandermeer TJ, Compton CC, Denenberg AG, et al. Endotoxin-induced ileal mucosal hyperpermeability in pigs: role of tissue acidosis. Am J Physiol 1994; 266(4 Pt 1):G633–46. [DOI] [PubMed] [Google Scholar]
- [130].Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM, et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 2012;23:430–6. [DOI] [PubMed] [Google Scholar]
- [131].Andrade ME, Araujo RS, de Barros PA, Soares AD, Abrantes FA, Generoso Sde V, et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr 2015;34: 1080–7. [DOI] [PubMed] [Google Scholar]
- [132].Ferreira TM, Leonel AJ, Melo MA, Santos RR, Cara DC, Cardoso VN, et al. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-fluorouracil administration. Lipids 2012;47:669–78. [DOI] [PubMed] [Google Scholar]
- [133].Hou Y, Wang L, Yi D, Ding B, Chen X, Wang Q, et al. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br J Nutr 2014;111:1748–58. [DOI] [PubMed] [Google Scholar]
- [134].Scheppach W, Bartram P, Richter A, Richter F, Liepold H, Dusel G, et al. Effect of short-chain fatty acids on the human colonic mucosa in vitro. JPEN J Parenter Enteral Nutr 1992;16:43–8. [DOI] [PubMed] [Google Scholar]
- [135].Rotting AK, Freeman DE, Constable PD, Eurell JA, Wallig MA. Effects of phenylbutazone, indomethacin, prostaglandin E2, butyrate, and glutamine on restitution of oxidant-injured right dorsal colon of horses in vitro. Am J Vet Res 2004;65:1589–95. [DOI] [PubMed] [Google Scholar]
- [136].Onrust L, Ducatelle R, Van Driessche K, De Maesschalck C, Vermeulen K, Haesebrouck F, et al. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front Vet Sci 2015;2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang W, Qiao S, Li D. Amino acids and gut function. Amino Acids 2009;37:105–10. [DOI] [PubMed] [Google Scholar]
- [138].Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 1998;336(Pt 1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Beutheu S, Ghouzali I, Galas L, Dechelotte P, Coeffier M. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin Nutr 2013; 32:863–9. [DOI] [PubMed] [Google Scholar]
- [140].Sukhotnik I, Mogilner J, Krausz MM, Lurie M, Hirsh M, Coran AG, et al. Oral arginine reduces gut mucosal injury caused by lipo-polysaccharide endotoxemia in rat. J Surg Res 2004;122:256–62. [DOI] [PubMed] [Google Scholar]
- [141].Wu Z, Hou Y, Hu S, Bazer FW, Meininger CJ, McNeal CJ, et al. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 2016;48:1541–52. [DOI] [PubMed] [Google Scholar]
- [142].Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A, et al. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 2007;137(6 Suppl 2):1673s–80s. [DOI] [PubMed] [Google Scholar]
- [143].Kelley DE, Warren LK, Mortensen CJ. Orally supplemented L-arginine impairs amino acid absorption depending on dose in horses. J Anim Sci 2014;92:5560–6. [DOI] [PubMed] [Google Scholar]
- [144].Reeds PJ, Burrin DG. The gut and amino acid homeostasis. Nutrition 2000;16:666–8. [DOI] [PubMed] [Google Scholar]
- [145].Rao R, Samak G. Role of glutamine in protection of intestinal epithelial tight junctions. J Epithel Biol Pharmacol 2012;5(Suppl 1- M7):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Oehler R, Pusch E, Dungel P, Zellner M, Eliasen MM, Brabec M, et al. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr 2002;87(Suppl 1):S17–21. [DOI] [PubMed] [Google Scholar]
- [147].Wu G, Meier Sa, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 1996;126: 2578–84. [DOI] [PubMed] [Google Scholar]
- [148].Wang J, Chen L, Li P, Li X, Zhou H, Wang F, et al. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 2008;138:1025–32. [DOI] [PubMed] [Google Scholar]
- [149].Mondello S, Galuppo M, Mazzon E, Domenico I, Mondello P, Carmela A, et al. Glutamine treatment attenuates the development of ischaemia/reperfusion injury of the gut. Eur J Pharmacol 2010; 643:304–15. [DOI] [PubMed] [Google Scholar]
- [150].Hou YC, Wu JM, Wang MY, Wu MH, Chen KY, Yeh SL, et al. Glutamine supplementation attenuates expressions of adhesion molecules and chemokine receptors on T cells in a murine model of acute colitis. Mediators Inflamm 2014;2014:837107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chow A, Zhang R. Glutamine reduces heat shock-induced cell death in rat intestinal epithelial cells. J Nutr 1998;128:1296–301. [DOI] [PubMed] [Google Scholar]
- [152].Harris RC, Harris PA, Routledge NB, Naylor JR, Wilson AM. Plasma glutamine concentrations in the horse following feeding and oral glutamine supplementation. Equine Vet J Suppl 2006;36:637–42. [DOI] [PubMed] [Google Scholar]
- [153].Lindinger MI, Anderson SC. Seventy day safety assessment of an orally ingested, L-glutamine-containing oat and yeast supplement for horses. Regul Toxicol Pharmacol 2014;70:304–11. [DOI] [PubMed] [Google Scholar]
- [154].Maret W Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 2013;4:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Katouli M, Melin L, Jensen-Waern M, Wallgren P, Mollby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol 1999;87:564–73. [DOI] [PubMed] [Google Scholar]
- [156].Di Leo V, D’Inca R, Barollo M, Tropea A, Fries W, Mazzon E, et al. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis 2001;33:135–9. [DOI] [PubMed] [Google Scholar]
- [157].Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med 2002;139:311–5. [DOI] [PubMed] [Google Scholar]
- [158].Zhang B, Shao Y, Liu D, Yin P, Guo Y, Yuan J. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian Pathol 2012;41: 361–7. [DOI] [PubMed] [Google Scholar]
- [159].Sargeant HR, McDowall KJ, Miller HM, Shaw MA. Dietary zinc oxide affects the expression of genes associated with inflammation: transcriptome analysis in piglets challenged with ETEC K88. Vet Immunol Immunopathol 2010;137:120–9. [DOI] [PubMed] [Google Scholar]
- [160].Sturniolo GC, Di Leo V, Ferronato A, D’Odorico A, D’Inca R. Zinc supplementation tightens “leaky gut’’ in Crohn’s disease. Inflamm Bowel Dis 2001;7:94–8. [DOI] [PubMed] [Google Scholar]
- [161].Wichert B, Kreyenberg K, Kienzle E. Serum response after oral supplementation of different zinc compounds in horses. J Nutr 2002;132(6 Suppl 2):1769s–70s. [DOI] [PubMed] [Google Scholar]
- [162].Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp Physiol 2016;101:801–10. [DOI] [PubMed] [Google Scholar]
- [163].Weese JS. Probiotics, prebiotics, and synbiotics. J Equine Vet Sci 2002;22:357–60. [Google Scholar]
- [164].Wasilewski A, Zielinska M, Storr M, Fichna J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1674–82. [DOI] [PubMed] [Google Scholar]
- [165].Guandalini S Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 2011;45(Suppl):S149–53. [DOI] [PubMed] [Google Scholar]
- [166].Schoster A, Weese JS, Guardabassi L. Probiotic use in horses - what is the evidence for their clinical efficacy? J Vet Intern Med 2014;28: 1640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 2014;7:473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Barnes D, Yeh AM. Bugs and guts: practical applications of probiotics for gastrointestinal disorders in children. Nutr Clin Pract 2015;30:747–59. [DOI] [PubMed] [Google Scholar]
- [169].Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci 2013;9:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Philippe D, Heupel E, Blum-Sperisen S, Riedel CU. Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int J Food Microbiol 2011;149:45–9. [DOI] [PubMed] [Google Scholar]
- [171].Ueno N, Fujiya M, Segawa S, Nata T, Moriichi K, Tanabe H, et al. Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis 2011;17: 2235–50. [DOI] [PubMed] [Google Scholar]
- [172].Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci 2009;92:2400–8. [DOI] [PubMed] [Google Scholar]
- [173].Faseleh Jahromi M, Wesam Altaher Y, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, et al. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int J Biometeorol 2016;60: 1099–110. [DOI] [PubMed] [Google Scholar]
- [174].Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 2000;45:1462–4. [DOI] [PubMed] [Google Scholar]
- [175].Medina B, Girard ID, Jacotot E, Julliand V. Effect of a preparation of Saccharomyces cerevisiae on microbial profiles and fermentation patterns in the large intestine of horses fed a high fiber or a high starch diet. J Anim Sci 2002;80:2600–9. [DOI] [PubMed] [Google Scholar]
- [176].Jouany JP, Gobert J, Medina B, Bertin G, Julliand V. Effect of live yeast culture supplementation on apparent digestibility and rate of passage in horses fed a high-fiber or high-starch diet. J Anim Sci 2008;86:339–47. [DOI] [PubMed] [Google Scholar]
- [177].Mackenthun E, Coenen M, Vervuert I. Effects of Saccharomyces cerevisiae supplementation on apparent total tract digestibility of nutrients and fermentation profile in healthy horses. J Anim Physiol Anim Nutr (Berl) 2013;97(Suppl 1):115–20. [DOI] [PubMed] [Google Scholar]
- [178].Swyers KL, Burk AO, Hartsock TG, Ungerfeld EM, Shelton JL. Effects of direct-fed microbial supplementation on digestibility and fermentation end-products in horses fed low- and high-starch concentrates. J Anim Sci 2008;86:2596–608. [DOI] [PubMed] [Google Scholar]
- [179].Desrochers AM, Dolente BA, Roy MF, Boston R, Carlisle S. Efficacy of Saccharomyces boulardii for treatment of horses with acute enterocolitis. J Am Vet Med Assoc 2005;227:954–9. [DOI] [PubMed] [Google Scholar]
- [180].Boyle AG, Magdesian KG, Durando MM, Gallop R, Sigdel S. Saccharomyces boulardii viability and efficacy in horses with antimicrobial-induced diarrhoea. Vet Rec 2013;172:128. [DOI] [PubMed] [Google Scholar]
- [181].Casey PG, Gardiner GE, Casey G, Bradshaw B, Lawlor PG, Lynch PB, et al. A five-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 2007; 73:1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Vandeplas S, Dubois Dauphin R, Beckers Y, Thonart P, Thewis A. Salmonella in chicken: current and developing strategies to reduce contamination at farm level. J Food Prot 2010;73:774–85. [DOI] [PubMed] [Google Scholar]
- [183].Frizzo LS, Zbrun MV, Soto LP, Bertozzi E, Sequeira GJ, Marti LE, et al. Pathogen translocation and histopathological lesions in an experimental model of Salmonella Dublin infection in calves receiving lactic acid bacteria and lactose supplements. J Vet Sci 2012;13: 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Parraga ME, Spier SJ, Thurmond M, Hirsh D. A clinical trial of probiotic administration for prevention of Salmonella shedding in the postoperative period in horses with colic. J Vet Intern Med 1997;11:36–41. [DOI] [PubMed] [Google Scholar]
- [185].Kim LM, Morley PS, Traub-Dargatz JL, Salman MD, Gentry-Weeks C. Factors associated with Salmonella shedding among equine colic patients at a veterinary teaching hospital. J Am Vet Med Assoc 2001;218:740–8. [DOI] [PubMed] [Google Scholar]
- [186].De Maesschalck C, Eeckhaut V, Maertens L, De Lange L, Marchal L, Nezer C, et al. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol 2015;81: 5880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Berg EL, Fu CJ, Porter JH, Kerley MS. Fructooligosaccharide supplementation in the yearling horse: effects on fecal pH, microbial content, and volatile fatty acid concentrations. J Anim Sci 2005; 83(7):1549–53. [DOI] [PubMed] [Google Scholar]
- [188].Gürbüz E, Inal F, Ata S, Çitil O, Kav K, Kücükkaya F. Effects of supplemental fructo-oligosaccharide and mannan-oligosaccharide on nutrient digestibilities, volatile fatty acid concentrations, and immune function in horses. Turk J Vet Anim Sci 2010;34:39–44. [Google Scholar]
- [189].Respondek F, Goachet AG, Julliand V. Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. J Anim Sci 2008;86:316–23. [DOI] [PubMed] [Google Scholar]


