Abstract
Clostridium septicum is a highly pathogenic microbe that causes gas gangrene in humans and is the principal cause of spontaneous gas gangrene in patients with gastrointestinal maladies, including adenocarcinoma of the colon. Despite modern approaches to manage C. septicum infections, morbidity and mortality remain high (>50%). At present, no objective in vivo data exists supporting the current antibiotic treatment recommendations for C. septicum infection. Utilizing our established murine model of clostridial myonecrosis, we investigated the efficacy of standard antibiotics for anaerobic Gram positive soft-tissue infections (penicillin, clindamycin, tetracycline, vancomycin) in treating C. septicum gas gangrene. Following intramuscular challenge with 1×106 colony forming units of C. septicum, antibiotics were administered by intraperitoneal injection every four hours for a total of four doses. At 30 hours, all animals in all treatment groups survived the C. septicum challenge, compared to no survivors in the untreated controls (100% mortality by 10 hours). However, by 60 hours, mice treated with vancomycin exhibited 40% mortality, with no mortality observed in any other antibiotic treatment group. Microbroth dilution MIC analyses for three strains of C. septicum also demonstrated high susceptibility to penicillin, clindamycin and tetracycline, but considerably lower susceptibility to vancomycin. These studies corroborate that penicillin, clindamycin and tetracycline are suitable alternatives for the treatment of C. septicum infection in humans.
Keywords: Clostridium septicum, in vivo efficacy, antibiotic treatment
1. Introduction
Clostridium septicum is a Gram-positive, anaerobic spore-forming pathogen that produces fatal infections in both healthy humans and animals [1]. In humans, C. septicum causes gas gangrene in normal hosts following deep penetrating or crushing injuries after trauma. C. septicum is also recognized as the principal cause of ‘spontaneous’ or ‘atraumatic’ gas gangrene in patients suffering from gastrointestinal maladies, including adenocarcinoma of the colon [2]. Due to the clinical challenges posed by spontaneous C. septicum infections, the mortality rate remains high (> 60%) [2;3].
Current strategies for managing C. septicum infections include surgical debridement, intensive care support, hyperbaric oxygen treatment, and in severe circumstances amputation of the infected limbs. Antimicrobial guidelines provided by the Infectious Disease Society of America (IDSA) suggest high dose IV penicillin in combination with intravenous clindamycin for treating C. septicum and other clostridium-related gas gangrene infections [4]. Chloramphenicol, metronidazole and vancomycin are also suggested as alternative treatments for patients allergic to penicillin [5]. The IDSA Guideline recommends vancomycin and piperacillin/tazobactam as empiric treatment of necrotizing infections until a definitive etiologic diagnosis is established. Still, these recommendations are largely based on antibiotic susceptibility results [5], and recent clinical trials on antibiotic efficacy have excluded patients with necrotizing infections.
In 1952, Dr. Henry Eagle described a ‘paradoxical effect’ between the in vitro susceptibilities of antibiotics and their efficacy in resolving infections in vivo. Eagle showed that mice infected intramuscularly with Streptococcus pyogenes failed to respond to high doses of penicillin, despite the pathogen’s known sensitivity to the drug [6]. Later studies by Stevens et al further demonstrated that in vitro susceptibilities infrequently correlated with in vivo efficacy in treating soft-tissue infections caused by toxin-producing Gram-positive pathogens such as Clostridium perfringens, Group A streptococcus and Staphylococcus aureus [7–9]. Specifically, Stevens et al showed that cell wall active agents such as penicillin, cephalosporins and vancomycin did not significantly improve the survival of animals challenged with these toxin-producing pathogens when compared to untreated controls [8]. This phenomenon is now referred to as the “Eagle effect” and has been described in several bacteria including Escherichia coli, Haemophilus influenza and several Staphylococcus, Mycobacterium and Proteus species [10;11].
Thus, currently there is no objective in vivo data to support the current antibiotic treatment recommendations for C. septicum infection. This study was designed to test the efficacy of standard antibiotics used to treat anaerobic Gram-positive soft-tissue infections (penicillin, clindamycin, erythromycin and vancomycin) to resolve C. septicum infection utilizing our established model of clostridial myonecrosis [7;12;13]. To the best of our knowledge, this is the first report of empirical data describing the in vivo effects of antibiotics in treating active C. septicum infection. Understanding the in vivo capacity of antibiotics is critical when considering treatment options for patients suffering from C. septicum infections.
2. Materials and Methods
2.1. Bacterial strains, cultivation and inoculum preparation
The American Type Culture Collection (ATCC) strain 11424 of C. septicum, and two strains collected from patients diagnosed with C. septicum at the VA Medical Center in Boise, ID, were used throughout the study. A Bactron II anaerobic chamber (Sheldon Manufacturing, Cornelius, OR) was utilized to maintain an anaerobic environment for C. septicum growth and manipulation. Stock cultures were streaked on a sheep’s blood agar plate and a single isolated colony was used to inoculate 10 mL of a defined liquid medium culture containing 20 g/L proteose peptone, 5 g/L yeast extract, 5 g/L NaCl, 5 g/L glucose, 1 g/L Na2HPO4, 1.4 g/L KH2PO4, 20 mg/L MgSO4, 10 mg/L MnSO4, 6 mg/L FeSO4, 6 mg/L ZnSO4, 1.2 mg/L Ca dpantothenate, 1.3 mg/L nicotinic acid, 1.2 mg/L thiamine, 1.2 mg/L riboflavin, 1.2 mg/L pyridoxamine HCl, 0.5 g/L L-cysteine and 0.1 g/L L-tryptophane. For in vivo intramuscular challenge studies, cultures were grown anaerobically overnight at 37°C. The following morning, 1% of the overnight culture was used to inoculate 100 mL of fresh, pre-reduced defined liquid media. This culture was grown to an OD600 of approximately 1.8 (late-stationary phase culture). Organisms were then collected by centrifugation (5000 x g, 15 min, 4°C), washed twice and resuspended in pre-chilled saline. A working stock of ~7.0 × 107 colony forming units (CFU)/mL was prepared based on a previously determined relationship between CFU and OD600. Bacterial concentrations of these preparations were confirmed by plating serially diluted samples in duplicate on sheep’s blood agar plates. Plates were incubated anaerobically at 37°C and colonies counted the following day.
2.2. Determination of Minimum Inhibitory Concentrations (MIC)
The antibiotics sodium penicillin G and tetracycline hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO), and clindamycin phosphate and vancomycin hydrochloride from Pharmacia (Stockholm, Sweden). The MIC of these antibiotics were determined for the C. septicum strains by microbroth dilution assay according to the Clinical & Laboratory Standards Institute (CLSI) guidelines for such testing in anaerobes [14]. In brief, 200 μL of a stationary overnight culture was used to inoculate 20 mL of pre-reduced defined liquid media. Cultures were grown anaerobically for approximately 2 hrs at 37°C until a turbidity equal to the 0.5 McFarland standard (OD630 of 0.300; ~2 × 106 CFU/mL) was achieved. The prepared C. septicum (50 μL) was then added to duplicate wells of a 96-well plate containing 50 μL of 2-fold serially diluted (2 – 2,000 ng/mL) penicillin, tetracycline, clindamycin or vancomycin. Plates were incubated anaerobically at 37°C for 48 hrs and growth (turbidity) was assessed by a microplate reader (OD630). MICs were defined as the lowest antibiotic concentration that inhibited measurable bacterial growth (i.e., OD630 equal to the negative control). MICs were performed 3 times in triplicate.
2.3. Experimental C. septicum infection
All animal experiments were approved by the Boise, ID Veterans Affairs Medical Center’s Institutional Animal Care and Use Committee and adhered to the National Institutes of Health guide for the care and use of laboratory animals. Adult female Swiss Webster mice were injected intramuscularly in the right upper thigh with 105 – 107 colony forming units of washed log-phase ATCC 11424 C. septicum organisms in 100 μL of normal saline. This range of C. septicum inoculum was determined to be optimal and was based on previous work by our group [15]. Pilot studies determined that a dose of ~ 5.0 × 106 colony forming units was required to generate a 100% lethal infection with this strain. Next, groups of animals (N = 10) inoculated with C. septicum received antibiotic treatments 2 hr after bacterial challenge and treatment was continued every 4 hr for an additional 3 doses (i.e., 6hr, 10hr and 14hr post-inoculation). Antibiotic treatments were delivered intraperitoneally in 300 μL of saline and included either: 1) clindamycin phosphate (86 mg/kg), 2) sodium penicillin G (98 mg/kg), 3) tetracycline hydrochloride (21 mg/kg), or 4) vancomycin hydrochloride (20 mg/kg). Animals receiving sterile saline served the as the negative treatment control. Animals were then observed every 4 hr for the next 100 hr, and the condition of the animals, local cutaneous findings, cliinical course of infection and time to death were recorded.
2.4. PCR Screening for Vancomycin Resistance Gene
C. septicum plasmid and chromosomal DNA was isolated by a guanidium thiocyanate– based method. Briefly, cells were exposed to 5 mol/L guanidium thiocyanate, 100 mmol/L EDTA, and 0.5% (vol/vol) SDS; cellular debris was precipitated; and chloroform–isoamyl alcohol (24:1) was added to separate the phases. After centrifugation, the upper phase was transferred to a fresh 1.5-mL microfuge tube, and DNA was precipitated with 0.6 vol of ice cold isopropanol. Resultant DNA pellets were air dried, resuspended in 100 mL of TE buffer, and stored at 4°C. Screening for the presence of the vancomycin resistance cassette was performed using the primers vanF-5’ ACAGCACGTCCTATAAATAACTGT 3’ and vanR-5’ GTACAGCTATTTTTGGAGCTATTCC 3’, which amplified a 450-bp fragment of the vancomycin resistance gene. The PCR reaction was performed in a final volume of 25 μL containing 12.5 μL of (2X) DreamTaq Green PCR Master Mix (ThermoFisher, Eugene, OR), 10 μM final concentration of each primer, 100 ng of each DNA template plus enough water to bring the total volume to 25 μL. Thermocycling was carried out in a GeneAmp 9700 PCR System thermal cycler (Applied Biosystems, Foster City, CA) with an initial denaturation of 45 sec, followed by 30 cycles at 94°C for 10 sec, 55.5°C for 30 sec and 72°C for 60 sec, followed by a final extension of 72°C for 5 min.
3. Results
3.1. Minimum Inhibitory Concentrations-
The minimum inhibitory concentrations for penicillin, clindamycin, tetracycline and vancomycin were determined by microbroth dilution for the ATCC 11424 standard strain and two clinical isolate strains of C. septicum. All strains displayed high sensitivity to penicillin, clindamycin and tetracycline (0.008 – 0.064 μg/mL). In contrast, a considerably lower susceptibility to vancomycin was observed by all three strains of C. septicum, with MIC values ranging between 0.500 – 1.000 μg/mL. There are no established breakpoints for C. septicum relative to vancomycin, but these values are in the sensitive range for other gram positive pathogens such as C. difficile and methicillin resistant Staphylococcus aureus (MRSA).
3.2. C. septicum Myonecrosis and Survival Studie
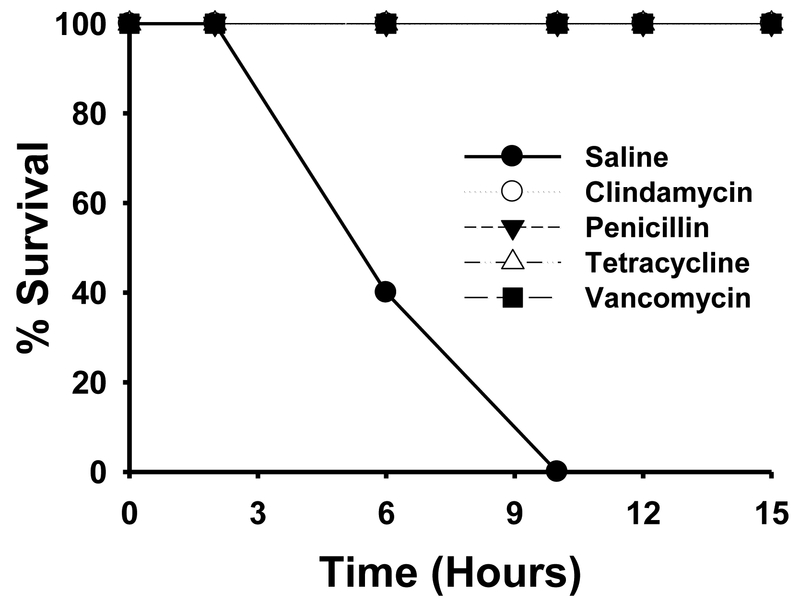
Mice infected intramuscularly with ~ 5.0 × 106 colony forming units of ATCC 11424 C. septicum rapidly succumbed to infection, and 100% of the animals died by 10 hr post-inoculation (Figure 1). By comparison, all animals receiving penicillin, clindamycin, tetracycline or vancomycin treatment exhibited 100% survival during the first 10 hr post-infection (Figure 1). In fact, none of the animals receiving antibiotics displayed any signs of illness throughout the course of antibiotic treatment (~15 hr post-inoculation) (Figure 1).
Figure 1. Survival curves of mice infected intramuscularly with C. septicum from 0–15 hr.
Groups of Swiss Webster mice (N = 10) were inoculated in the right thigh muscle with approximately 5.0 × 106 colony forming units of washed, log phase C. septicum. At 2 hr, 6hr, 10hr and 14hr post-inoculation, animals were treated with either saline, clindamycin phosphate (86 mg/kg), 2) sodium penicillin G (98 mg/kg), 3) tetracycline hydrochloride (21 mg/kg), or 4) vancomycin hydrochloride (20 mg/kg). Observations were made and mortalities noted over the next 15 hr post-inoculation.
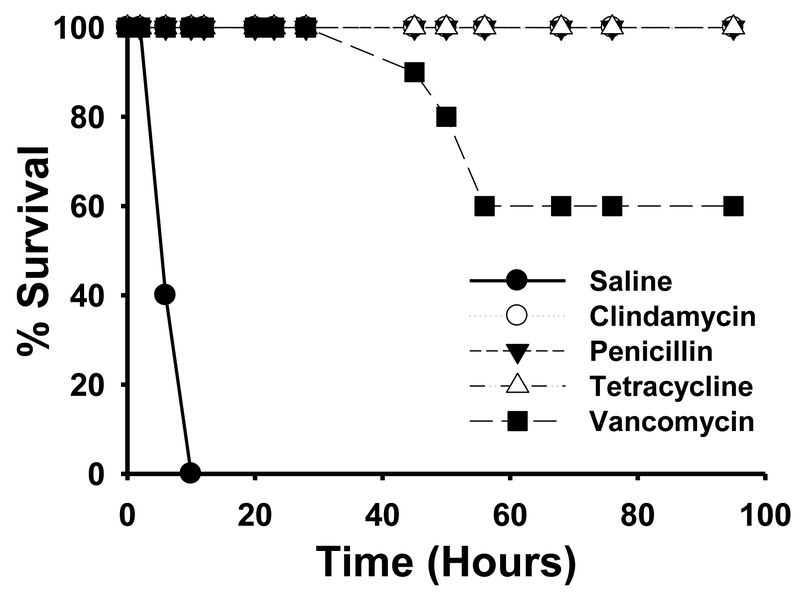
Following the final antibiotic treatment, all animals receiving penicillin, clindamycin and tetracycline remained asymptomatic and were 100% healthy throughout the course of the study (Figure 2). In contrast, mice receiving vancomycin began displaying signs of local infection (limb swelling, limping, discolored foot) and systemic infection (lethargy and ruffled fur) upon completion of the antibiotic regimen. By 30 hr post-antibiotic, vancomycin-treated animals began dying (Figure 2), and by 40 hr post-antibiotics (57 hr post-inoculation), 40% of the vancomycin treatment group succumbed to infection (Figure 2). The surviving mice gradually recovered over the next 48 hr and their condition fully recovered by 100 hr (Figure 2).
Figure 2. Survival curves of mice infected intramuscularly with C. septicum from 0–100 hr.
Groups of Swiss Webster mice (N = 10) were inoculated in the right thigh muscle with approximately 5.0 × 106 colony forming units of washed, log phase C. septicum. At 2 hr, 6hr, 10hr and 14hr post-inoculation, animals were treated with either saline, clindamycin phosphate (86 mg/kg), 2) sodium penicillin G (98 mg/kg), 3) tetracycline hydrochloride (21 mg/kg), or 4) vancomycin hydrochloride (20 mg/kg). Observations were made and mortalities noted over the next 100 hr post-inoculation.
3.3. Mechanism of Vancomycin Resistance in C. septicum
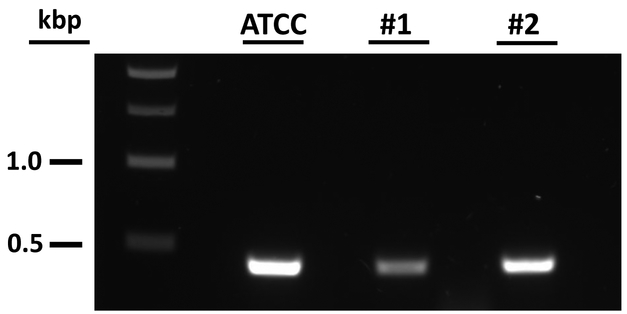
To further investigate the enigma of in vitro sensitivity to vancomycin and lack of efficacy in vivo, primers were designed to amplify a region of a vancomycin resistance-like cassette (VRC) recently identified on a 5 kb plasmid from the annotated genomic sequence of the C. septicum strain RMA 8861 (personal communication with Dr. Michael Mallozzi; University of Arizona). Interestingly, all three C. septicum isolates, including the ATCC 11424 strain used in this current study, revealed amplification of the VRC gene (Figure 3). Studies to further characterize the VRC and determine if this mechanism plays a role in pathogenesis in vivo are currently underway.
Figure 3. Detection of the vancomycin resistance cassette in multiple strains of C. septicum.
Gel electrophoresis showing the amplification of a region of the VRC gene in 3 out of 3 strains of C. septicum. Lane 1) DNA ladder; Lane 2) C. septicum ATCC 11424 strain; Lanes 3 and 4) C. septicum clinical isolate strains from our collection.
4. Discussion
Clostridium septicum is a motile pathogen commonly isolated from the intestinal flora of humans. In humans, C. septicum causes fatal traumatic and atraumatic myonecrotic gas gangrene infections. Spontaneous C. septicum infections are rare and commonly associated with neutropenia, diabetes mellitus and immunosuppression [16]. However, the most common predisposing factors for spontaneous C. septicum infections are hematological and colorectal malignancies. While the association involving C. septicum and neutropenia, both congenital and cyclic, is unexplained, the relationship between malignant tumors and C. septicum infection is better understood. In this setting, lesions such as carcinomas compromise the mucosal lining of the intestinal wall and provide a portal of entry to the bloodstream for C. septicum organisms residing in the colon. Once blood borne, these microaerophilic clostridia translocate to remote areas throughout the body where secondary infections may develop, including myonecrosis, aortitis and mycotic aneurysms, arthritis, endocarditis and endophthalmitis [17]. The ability of anaerobic clostridia to cause infection in well perfused tissues has been an enigma, yet those who work with C. septicum have observed that it, unlike its cousins Clostridium perfringens and Clostridium difficile, does not require strict anaerobic environments. Patients diagnosed with septic C. septicum infections should be evaluated for an underlying colonic malignancy. Due to difficulties associated with diagnosing and treating spontaneous C. septicum infections, the majority of patients succumb to this form of the disease despite aggressive medical treatments and management strategies.
In our current study, all three strains of C. septicum displayed strong sensitivity to penicillin, clindamycin and tetracycline in vitro (≥ 0.064 μg/mL), but less sensitivity to vancomycin (0.5 – 1.0 μg/mL). These results are similar to a study by Gabay et al who showed that out of the 33 strains of C. septicum screened, all were sensitive to penicillin and clindamycin (MIC < 0.125 μg/mL), whereas 90% displayed a minimum inhibitory concentration to vancomycin of 2 μg/mL [5]. A more recent Canadian survey by Leal et al further demonstrated that 100% of 19 C. septicum isolates were also sensitive to penicillin, 47% were resistant to clindamycin and 5% were resistant to both clindamycin and metronidazole [18]. In our current study, no clindamycin resistance was observed in any of the strains tested.
We also demonstrated that in vivo, early treatment with multiple doses of penicillin, clindamycin and tetracycline fully protected mice from fatal C. septicum infections in this model. These data support the current treatment recommendations of intravenous penicillin and clindamycin for necrotizing C. septicum (and other clostridial) infections of the skin, fascia and muscle [4]. Interestingly, only 60% of the mice receiving vancomycin survived the C. septicum challenge. Further investigation determined that the C. septicum ATCC 11424 strain harbored a plasmid containing a vancomycin resistance cassette, likely explaining the inability of vancomycin to completely resolve infections in mice challenged with C. septicum.
Vancomycin is a tricyclic glycopeptide that inhibits bacterial cell wall synthesis. It is typically prescribed to treat complicated, and often life-threatening, infections caused by Gram-positive bacteria that are unresponsive to other antibiotics. As a whole, Clostridium species are commonly susceptible to glycopeptide antibiotics such as vancomycin. However, a study by Mory et al demonstrated that several isolates of Clostridium innocuum displayed intermediate to strong resistance to vancomycin, suggesting to the authors that low-level vancomycin resistance is intrinsic to this species [19]. Decreased susceptibility to vancomycin has also been described in several clinical isolates of Clostridium difficile, including ~40% of the epidemic NAP1 strains tested [20].
The pathogenesis of C. septicum infections is mediated by several exotoxins, including a lethal and necrotizing toxin (alpha-toxin), DNase (beta-toxin), hyaluronidase (gamma-toxin) and thiol-activated septicolysin (delta-toxin) [1]. The alpha-toxin, a pore forming cytolysin, is considered the major virulence factor and is essential for the symptoms associated with infection [21]. Several studies by our group and others have showed that sub-inhibitory concentrations of some antimicrobial agents can actually increase and prolong the production of potent exotoxins in several Gram-positive pathogens including C. perfringens, Group A streptococcus, S. aureus and C. difficile [22;23]. While such studies have never been performed in C. septicum, the ability of antibiotics to induce toxin production in Gram-positive pathogens should be considered when prescribing antibiotics like vancomycin to treat severe C. septicum infections.
5. Conclusion
In conclusion, C. septicum can cause rapidly fatal infections in humans, especially those who may be suffering from underlying malignancies of the colon. The data collected here is the first report of empirical evidence describing the in vivo efficacy of antibiotics in treating C. septicum soft-tissue infections. Our study supports the use of penicillin, clindamycin and tetracycline in treating severe cases of C. septicum myonecrosis. However, caution should be used when considering vancomycin as an alternative to treating these infections.
Table 1.
Minimum inhibitory concentrations for the C. septicum standard ATCC 11242 and two clinical isolate strains. MICs were calculated by standard broth microdilutions for such anaerobes in defined clostridial culture media.
| ATCC 11424 | 0.031 | 0.031 | 0.031 | 0.500 |
| Clinical Isolate #1 | 0.016 | 0.031 | 0.064 | 1.000 |
| Clinical Isolate #2 | 0.008 | 0.016 | 0.032 | 1.000 |
Highlights.
C. septicum displays mild resistance to vancomycin in vitro.
Penicillin, clindamycin and tetracycline protect mice during C. septicum infection
Vancomycin is only semi-protective against C. septicum infections.
First in vivo evidence for antibiotic treatment of C. septicum infections.
Data corroborates the current IDSA guidelines for treating such infections.
Funding:
This material is based upon work supported in part by the U.S. Department of Veterans Affairs, Office of Research and Development Biomedical Laboratory Research Program, The Gut Check Foundation and the NIH Grant No. P20GM109007 (National Institute of General Medical Sciences).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research article has not been published or being considered for publication with another journal. However, Portions of the information provided herein were presented at the biennial Anaerobe Society of the Americas Conference in Nashville, TN, July 2016.
Competing Interests: None
Ethical Approval: Not required
References
- [1].Smith LDS. Clostridium sordellii In: Smith LDS, editor. The pathogenic anaerobic bacteria.Springfield, IL, Charles C. Thomas Publishing, 1975: p. 291–8. [Google Scholar]
- [2].Srivastava I, Aldape MJ, Bryant AE, Stevens DL. Spontaneous C. septicum gas gangrene: A literature review. Anaerobe 2017. December;48:165–71. [DOI] [PubMed] [Google Scholar]
- [3].Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer - a reminder. World J Surg Oncol 2009. October 6;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014. July 15;59(2):e10–e52. [DOI] [PubMed] [Google Scholar]
- [5].Gabay EL, Rolfe RD, Finegold SM. Susceptibility of Clostridium septicum to 23 antimicrobial agents. Antimicrob Agents Chemother 1981. December;20(6):852–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eagle H Experimental approach to the problem of treatment failure with penicillin. I. Group A streptococcal infection in mice. Am J Med 1952. October;13(4):389–99. [DOI] [PubMed] [Google Scholar]
- [7].Stevens DL, Maier KA, Laine BM, Mitten JE. Comparison of clindamycin, rifampin, tetracycline, metronidazole, and penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. J Infect Dis 1987. February;155(2):220–8. [DOI] [PubMed] [Google Scholar]
- [8].Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis 1988. July;158(1):23–8. [DOI] [PubMed] [Google Scholar]
- [9].Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 2007. January 15;195(2):202–11. [DOI] [PubMed] [Google Scholar]
- [10].Yourassowsky E, Vander Linden MP, Lismont MJ, Schoutens E. Qualitative study of paradoxical zone phenomenon of penicillins against 17 bacterial species of clinical importance. Chemotherapy 1978;24(2):92–6. [DOI] [PubMed] [Google Scholar]
- [11].Wu ML, Tan J, Dick T. Eagle Effect in Nonreplicating Persister Mycobacteria. Antimicrob Agents Chemother 2015. December;59(12):7786–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aldape MJ, Bayer CR, Bryant AE, Stevens DL. A novel murine model of Clostridium sordellii myonecrosis: Insights into the pathogenesis of disease. Anaerobe 2016. April;38:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ballard J, Bryant A, Stevens D, Tweten RK. Purification and characterization of the lethal toxin (alpha-toxin) of Clostridium septicum. Infect Immun 1992. March;60(3):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. Approved Standard, 6th ed. NCCLS document M11-A6. Wayne, PA: NCCLS; 2004. [Google Scholar]
- [15].Ballard J, Bryant A, Stevens D, Tweten RK. Purification and characterization of the lethal toxin (alpha-toxin) of Clostridium septicum. Infect Immun 1992;60(3):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jessamy K, Ojevwe FO, Ubagharaji E, et al. Clostridium septicum: An Unusual Link to a Lower Gastrointestinal Bleed. Case Rep Gastroenterol 2016. May;10(2):489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khalid M, Lazarus R, Bowler IC, Darby C. Clostridium septicum sepsis and its implications. BMJ Case Rep 2012. September 7;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leal J, Gregson DB, Ross T, Church DL, Laupland KB. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000–2006. J Infect 2008. September;57(3):198–203. [DOI] [PubMed] [Google Scholar]
- [19].Mory F, Lozniewski A, David V, Carlier JP, Dubreuil L, Leclercq R. Low-level vancomycin resistance in Clostridium innocuum. J Clin Microbiol 1998. June;36(6):1767–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tickler IA, Goering RV, Whitmore JD, Lynn AN, Persing DH, Tenover FC. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother 2014. July;58(7):4214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ballard J, Bryant A, Stevens D, Tweten RK. Purification and characterization of the lethal toxin (alpha-toxin) of Clostridium septicum. Infect Immun 1992. March;60(3):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 2007. January 15;195(2):202–11. [DOI] [PubMed] [Google Scholar]
- [23].Aldape MJ, Packham AE, Nute DW, Bryant AE, Stevens DL. The effects of ciprofloxacin on the expression and production of exotoxins by Clostridium difficile. J Med Microbiol 2013. February 21. [DOI] [PMC free article] [PubMed] [Google Scholar]