ABSTRACT
Objective:
To determine the impact of adherence to long-term oxygen therapy (LTOT) on quality of life, dyspnea, and exercise capacity in patients with COPD and exertional hypoxemia followed for one year.
Methods:
Patients experiencing severe hypoxemia during a six-minute walk test (6MWT) performed while breathing room air but not at rest were included in the study. At baseline and after one year of follow-up, all patients were assessed for comorbidities, body composition, SpO2, and dyspnea, as well as for anxiety and depression, having also undergone spirometry, arterial blood gas analysis, and the 6MWT with supplemental oxygen. The Saint George’s Respiratory Questionnaire (SGRQ) was used in order to assess quality of life, and the Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity (BODE) index was calculated. The frequency of exacerbations and the mortality rate were noted. Treatment nonadherence was defined as LTOT use for < 12 h per day or no LTOT use during exercise.
Results:
A total of 60 patients with COPD and exertional hypoxemia were included in the study. Of those, 10 died and 11 experienced severe hypoxemia during follow-up, 39 patients therefore being included in the final analysis. Of those, only 18 (46.1%) were adherent to LTOT, showing better SGRQ scores, higher SpO2 values, and lower PaCO2 values than did nonadherent patients. In all patients, SaO2, the six-minute walk distance, and the BODE index worsened after one year. There were no differences between the proportions of adherence to LTOT at 3 and 12 months of follow-up.
Conclusions:
Quality of life appears to be lower in patients with COPD and exertional hypoxemia who do not adhere to LTOT than in those who do. In addition, LTOT appears to have a beneficial effect on COPD symptoms (as assessed by SGRQ scores). (Brazilian Registry of Clinical Trials - ReBEC; identification number RBR-9b4v63 [http://www.ensaiosclinicos.gov.br])
Keywords: Respiratory insufficiency; Pulmonary disease, chronic obstructive; Patient compliance; Hypoxia; Oxygen inhalation therapy
RESUMO
Objetivo:
Determinar o impacto da adesão à oxigenoterapia de longa duração (OLD) na qualidade de vida, dispneia e capacidade de exercício em pacientes com DPOC e hipoxemia decorrente do esforço acompanhados durante um ano.
Métodos:
Foram incluídos no estudo pacientes que apresentaram hipoxemia grave durante um teste de caminhada de seis minutos (TC6) realizado enquanto respiravam ar ambiente, mas não em repouso. No início e após um ano de acompanhamento, todos os pacientes foram avaliados quanto a comorbidades, composição corporal, SpO2 e dispneia, bem como quanto a ansiedade e depressão, além de terem sido submetidos a espirometria, gasometria arterial e TC6 com oxigênio suplementar. O Saint George’s Respiratory Questionnaire (SGRQ) foi usado para avaliar a qualidade de vida, e o índice Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity (BODE; índice de massa corporal, obstrução do fluxo aéreo, dispneia e capacidade de exercício) foi calculado. A frequência de exacerbações e a taxa de mortalidade foram registradas. Usar OLD durante < 12 h por dia ou não usar OLD durante o exercício caracterizaram não adesão ao tratamento.
Resultados:
Foram incluídos no estudo 60 pacientes com DPOC e hipoxemia decorrente do esforço. Destes, 10 morreram e 11 apresentaram hipoxemia grave durante o acompanhamento; portanto, foram incluídos na análise final 39 pacientes. Destes, apenas 18 (46,1%) aderiram à OLD, apresentando melhor pontuação no SGRQ, maior SpO2 e menor PaCO2 do que os pacientes que não aderiram à OLD. Em todos os pacientes, a SaO2, a distância percorrida no TC6 e o índice BODE pioraram após um ano. Não houve diferenças entre as proporções de adesão à OLD aos 3 e 12 meses de acompanhamento.
Conclusões:
A qualidade de vida parece ser menor em pacientes com DPOC e hipoxemia decorrente do esforço que não aderem à OLD do que naqueles que o fazem. Além disso, a OLD parece ter efeito benéfico nos sintomas da DPOC (avaliados pela pontuação obtida no SGRQ). (Registro Brasileiro de Ensaios Clínicos - ReBEC; número de identificação RBR- 9b4v63 [http://www.ensaiosclinicos.gov.br])
Descritores: Insuficiência respiratória, Doença pulmonar obstrutiva crônica, Cooperação do paciente, Hipóxia, Oxigenoterapia
INTRODUCTION
Patients with COPD constitute the largest homogeneous group of patients who have arterial hypoxemia, 1 , 2 accounting for 67.8-81.6% of all patients on long-term oxygen therapy (LTOT). 3 The use of LTOT improves quality of life and respiratory symptoms, as well as reducing the risk of mortality. 4 , 5 In some patients, however, hypoxemia occurs only during activities of daily living. 6 The mechanisms involved in exertional hypoxemia are associated with ventilation/perfusion mismatch, decreased diffusion capacity, and increased pulmonary shunt. 6 As a result, exercise tolerance and quality of life are reduced in such patients. 6
The effectiveness of LTOT in patients with exertional hypoxemia has yet to be established. Although one study has shown that the use of LTOT during pulmonary rehabilitation improves the quality of life of patients with exercise-induced hypoxemia, 7 other studies have shown that the use of LTOT has no beneficial effect on COPD patients with exertional hypoxemia undergoing physical training. 6 , 8 , 9 In addition, in patients with moderate resting or exercise-induced hypoxemia, LTOT has been shown to have no beneficial effect on the time to death or first hospitalization. 10 There is no consensus regarding the use of LTOT in such patients. 6 , 11
Few studies have examined the impact of adherence to LTOT on clinical outcomes in such patients. 10 Therefore, the objective of the present study was to determine the impact of adherence to LTOT on quality of life, dyspnea, and exercise capacity in patients with COPD and exertional hypoxemia followed for one year.
METHODS
Patients
We evaluated 159 COPD patients referred to the Oxygen Therapy Outpatient Clinic of the São Paulo State University Botucatu School of Medicine, in the city of Botucatu, Brazil, in the period between November of 2011 and June of 2012. The inclusion criteria were as follows: having been diagnosed with COPD in accordance with the Global Initiative for Chronic Obstructive Lung Disease criteria 12 and having exertional hypoxemia.
At baseline (and while breathing room air), patients experiencing severe arterial hypoxemia during exercise but not at rest were classified as having exertional hypoxemia. To confirm the presence of a PaO2 > 59 mmHg 2 , 13 at rest and on room air, we performed arterial blood gas analysis. All patients performed a six-minute walk test (6MWT) while breathing room air, the presence of exertional hypoxemia being confirmed by an SpO2 of < 87% during the test. All patients with exertional hypoxemia received a prescription for oxygen supplementation at a flow rate of 0.5 L/min for at least 12 h per day for one year, to be used when performing activities of daily living (including walking) and during sleep. 11 The exclusion criteria were as follows: severe hypoxemia at rest (PaO2 ≤ 55 mmHg), other respiratory diseases, polycythemia, cor pulmonale, cancer, and active smoking. Clinically unstable patients (medication changes, disease exacerbations, or hospital admissions in the preceding 6 weeks) and those who presented with severe arterial hypoxemia at rest (a PaO2 of < 55 mmHg) during the follow-up period were also excluded. Patients were evaluated at baseline and every 4 months in order to determine the frequency of exacerbations and adherence to LTOT. Exacerbations were confirmed by changes in maintenance therapy or the need to prescribe oral corticosteroids, antibiotics, or both. Cause-of-death data were obtained by reviewing patient medical records. The study was approved by the Research Ethics Committee of the Botucatu School of Medicine University Hospital (Protocol no. 4020-2011), and all participating patients gave written informed consent.
Spirometry, arterial blood gas analysis, and pulse oximetry
Pre- and post-bronchodilator spirometry tests were performed with a Koko spirometer (Ferraris Respiratory, Louisville, CO, USA), in accordance with the American Thoracic Society criteria. 14 Disease severity was determined on the basis of criteria established by the Brazilian Thoracic Association and the Global Initiative for Chronic Obstructive Lung Disease. 2 , 15 Values of FEV1 and FVC were expressed as percentages of the predicted values. Radial artery puncture was used in order to collect blood samples for arterial blood gas analysis, PaO2, PaCO2, and SaO2 being measured with a blood gas analyzer (Stat Profile 5 Plus; Nova Biomedical, Waltham, MA, USA) and SpO2 being measured with an Onyx 9500™ pulse oximeter (Nonin Medical, Inc., Plymouth, MN, USA). The aforementioned parameters were assessed with the patients at rest and breathing room air.
Body composition
Body weight and height were measured with participants standing barefoot and wearing light clothing. The body mass index (BMI) was calculated by the formula weight/height2 (kg/m2). Body composition was assessed by bioelectrical impedance analysis (BIA 101A; RJL Systems, Inc., Clinton Township, MI, USA). Resistance was measured on the right side of the body in the supine position. Fat-free mass (FFM, in kg) was calculated by a group-specific regression equation developed by Kyle et al. 16 The FFM index (FFMI = FFM/height2) was also calculated. FFM depletion was defined as an FFMI of < 15 kg/m2 for women and an FFMI of < 16 kg/m2 for men. 17
Exercise capacity
The 6MWT was performed in accordance with the American Thoracic Society guidelines. 18 Patients were instructed to walk, attempting to cover as much ground as possible within 6 min. The walk was timed by a research assistant, and standardized verbal encouragement was given. Following a rest period of at least 30 min, each subject performed a second 6MWT in the same manner as the first. Patient SpO2 was monitored throughout the test. Before and after the test, data were obtained for SpO2, heart rate, respiratory rate, and blood pressure. The six-minute walk distance (6MWD) was expressed in meters.
Adherence
Adherence to treatment was assessed by asking questions regarding the oxygen flow rate and the number of hours of LTOT use per day. The information thus obtained was confirmed by comparing it to the prescription data on patient medical records. Treatment nonadherence was defined as LTOT use for < 12 h per day or no LTOT use during exercise. To improve the use of LTOT, adherence education was provided at each medical visit. In addition, nurse home visits were provided in order to reinforce the importance of and assess adherence to LTOT.
Quality of life, the Baseline Dyspnea Index, the Hospital Anxiety and Depression Scale, the Charlson comorbidity index, and the Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity index
The Brazilian Portuguese version of the Saint George’s Respiratory Questionnaire (SGRQ) 19 was used in order to evaluate quality of life. Dyspnea was assessed by the Brazilian Portuguese version of the modified Medical Research Council scale, as well as by the Baseline Dyspnea Index (BDI). 20 , 21 The Hospital Anxiety and Depression Scale was used in order to evaluate anxiety and depression. 22 Comorbidities were quantified by the Charlson comorbidity index. 23 The Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity (BODE) index was calculated by using the model of Celli et al. 24 All questionnaires were administered at baseline and after a one-year follow-up period.
Statistical analysis
For a power of 80% and a type I error of 0.05, the required sample size to detect a mean difference in quality of life (4.0 ± 4.5%) or dyspnea (1.0 ± 2.0) at the end of the follow-up period was calculated to be 34. Data were expressed as mean ± standard deviation or median (interquartile range). The Student’s t-test or the Mann-Whitney test was used in order to compare two independent groups according to their distribution. A paired t-test or the Wilcoxon test was used in order to compare repeated measures. The chi-square test was used in order to compare proportions. Multiple linear regression analysis was used in order to evaluate factors associated with the 6MWD and dyspnea after one year. A value of p < 0.05 was defined as statistically significant. All analyses were performed with the Stata statistical software package, version 10.0 (StataCorp LP, College Station, TX, USA).
RESULTS
A flow chart of inclusion and exclusion of patients is presented in Figure 1. A total of 60 patients with COPD and exertional hypoxemia were included in the study. Of those, 10 died during the study period. The causes of death were pulmonary complications of COPD, in 6 patients, and cardiovascular disease, in 4. In addition, 11 patients experienced severe hypoxemia during follow-up and were excluded from the analysis, 39 patients therefore being included in the final analysis. The BODE index and the BMI were lower in the patients who died than in those who survived (2.0 [1.0-3.0] vs. 1.0 [0.0-2.0], p = 0.005 and 17.8 [16.4-27.6] vs. 23.7 [19.5-30.0], p = 0.028, respectively), as was adherence to LTOT.
Figure 1. Flow chart of inclusion and exclusion of patients. LTOT: long-term oxygen therapy.
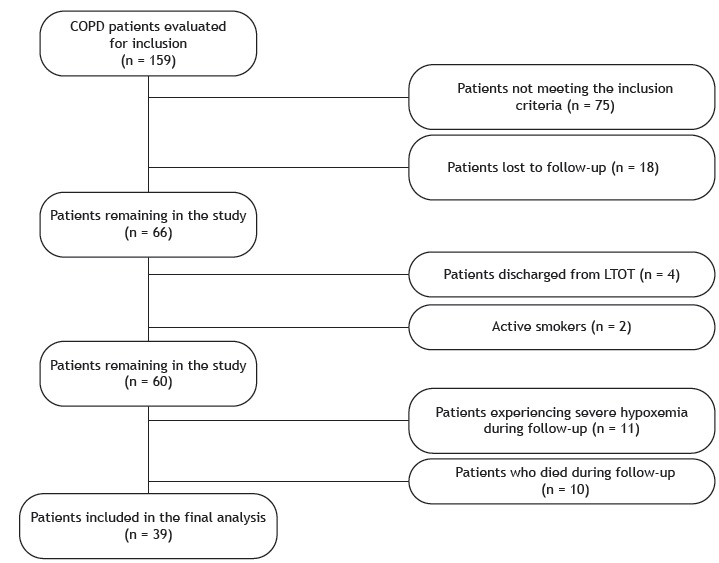
The characteristics of the 39 patients at baseline and after one year of follow-up are presented in Table 1. All 39 were using long-acting beta-adrenoceptor agonists, which were used in combination with long-acting anticholinergics in 15 and in combination with inhaled corticosteroids in 15. In all patients, the 6MWD and the BODE index worsened after one year, whereas SGRQ symptoms domain scores showed significant improvement. The proportion of patients with a lower FFMI at baseline was 12.8%. Adherence to LTOT was low during the study period (46.1%). Nonadherent patients reported using oxygen supplementation for 8.0 ± 1.2 h per day. In comparison with adherent patients, nonadherent patients showed significantly lower resting SpO2 (91.1 ± 2.5 vs. 93.3 ± 2.3; p = 0.011), higher PaCO2 (39.5 [35.8-47.3] vs. 35.7 [34.2-39.4]; p = 0.044), and worse scores for the SGRQ domains of symptoms (41.5 ± 19.6 vs. 27.6 ± 18.7; p = 0.039), activity (72.9 [66.2-79.7] vs. 60.4 [45.9-70.3]; p = 0.014), and impacts (39.5 ± 15.1 vs. 27.8 ± 18.3; p = 0.047), as well as significantly worse total SGRQ scores (49.9 ± 11.9 vs. 36.4 ± 17.7; p = 0.012), after one year (Table 2).
Table 1. General characteristics of patients with COPD and exercise-induced hypoxemia at baseline and after one year of follow-up (N = 39).a .
| Characteristic | Baseline | One year later | p |
|---|---|---|---|
| Men, % | 41.1 | ||
| Age, yearsb | 69.0 (62.0-77) | ||
| BMI, kg/m2b | 23.7 (19.5-30.0) | 26.5 (21.7-30.8) | 0.674 |
| FFM, kgb | 45.6 (41.1-51.6) | 44.3 (38.5-51.8) | 0.544 |
| FFMI, kgb | 18.0 (16.8-20.0) | 18.0 (16.3-19.8) | 0.642 |
| Charlson comorbidity index | 3.2 ± 1.1 | 3.2 ± 1.1 | 1.000 |
| BODE indexb | 1.0 (0.0-2.0) | 1.5 (0.0-2.0) | 0.031 |
| SpO2, % | 92.1 ± 2.5 | 92.3 ± 2.6 | 0.885 |
| PaO2, mmHg | 67.0 ± 5.4 | 63.6 ±7.9 | 0.128 |
| PaCO2, mmHg | 38.1 ± 6.0 | 38.6 ± 8.5 | 0.633 |
| FVC, L | 2.23 ± 0.78 | 2.11 ± 0.73 | 0.177 |
| FEV1, L | 1.13 ± 0.49 | 1.07 ± 0.40 | 0.056 |
| FEV1/FVC, Lb | 0.50 (0.41-0.58) | 0.53 (0.41-0.58) | 0.754 |
| SGRQ symptoms domain score, % | 53.0 ± 19.8 | 34.5 ± 20.4 | < 0.001 |
| SGRQ activity domain score, % | 63.6 ± 20.5 | 65.2 ± 19.4 | 0.695 |
| SGRQ impacts domain score, % | 34.6 ± 18.7 | 33.5 ± 17.7 | 0.707 |
| Total SGRQ score, % | 46.7 ± 16.5 | 43.3 ± 16.5 | 0.232 |
| Anxiety, HADS | 4.3 ± 4.4 | 2.8 ± 2.1 | 0.065 |
| Depression, HADS | 3.3 ± 3.7 | 4.3 ± 3.8 | 0.170 |
| mMRC scaleb | 2.0 (1.0-2.5) | 2.0 (1.0-2.5) | 0.612 |
| BDI | 5.5 ± 2.7 | 5.2 ± 2.7 | 0.601 |
| 6MWD, mb | 336.0 (264.0-390.0) | 306.0 (210.0-355.0) | < 0.001 |
BMI: body mass index; FFM: fat-free mass; FFMI: fat-free mass index; BODE: Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity; SGRQ: Saint George’s Respiratory Questionnaire; HADS: Hospital Anxiety and Depression Scale; mMRC: modified Medical Research Council; BDI: Baseline Dyspnea Index; and 6MWD: six-minute walk distance. aValues expressed as mean ± SD, except where otherwise indicated. bValues expressed as median (interquartile range). p < 0.05 (Student’s t-test or Mann-Whitney test).
Table 2. Comparison between adherent and nonadherent patients with COPD and exertional hypoxemia.a .
| Variable | Patients who adhered to LTOT | Patients who did not adhere to LTOT | p |
|---|---|---|---|
| (n = 18) | (n = 21) | ||
| Men, % | 33.4 | 28.3 | |
| Age, years | 69.1 ± 10.0 | 71.5 ± 8.7 | 0.433 |
| BMI, kg/m2 | 23.2 ± 8.4 | 25.5 ± 6.0 | 0.684 |
| FFM, kg | 45.8 ± 9.3 | 45.0 ± 8.7 | 0.787 |
| FFMI, kg | 17.5 ± 2.1 | 18.1 ± 2.6 | 0.459 |
| Borg scaleb | 0.0 (0.0-1.7) | 0.0 (0.0-0.0) | 0.420 |
| BODE index | 1.1 ± 0.9 | 1.4 ± 1.1 | 0.081 |
| SpO2, % | 93.3 ± 2.3 | 91.1 ± 2.5 | 0.011 |
| PaO2, mmHg | 64.4 ± 6.9 | 62,7 ± 9.1 | 0.536 |
| PaCO2, mmHgb | 35.7 (34.2-39.4) | 39.5 (35.8-47.3) | 0.044 |
| SaO2, %b | 93.3 (90.6-94.3) | 91.2 (90.6-94.1) | 0.509 |
| FVC, Lb | 1.8 (1.4-2.6) | 2.1 (1.5-2.4) | 0.609 |
| FEV1, Lb | 0.9 (0.7-1.0) | 1.0 (0.8-1.3) | 0.083 |
| FEV1/FVC, L | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.072 |
| SGRQ symptoms domain score, % | 27.6 ± 18.7 | 41.5 ± 19.6 | 0.039 |
| SGRQ activity domain score, %b | 60.4 (45.9-70.3) | 72.9 (66.2-79.7) | 0.014 |
| SGRQ impacts domain score, % | 39.5 ± 15.1 | 27.8 ± 18.3 | 0.047 |
| Total SGRQ score, % | 36.4 ± 17.7 | 49.9 ± 11.9 | 0.012 |
| Anxiety, HADS | 3.1 ± 2.6 | 2.4 ± 1.7 | 0.373 |
| Depression, HADSb | 3.5 (1.0-5.0) | 3.0 (0.0-7.0) | 0.981 |
| mMRC scale | 1.9 ± 0.7 | 1.6 ±1.2 | 0.384 |
| BDIb | 5.0 (4.0-5.0) | 6.0 (3.0-8.0) | 0.392 |
| 6MWD, m | 309.6 ± 90.3 | 266.5 ± 97.1 | 0.190 |
BMI: body mass index; FFM: fat-free mass; FFMI: fat-free mass index; BODE: Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity; SGRQ: Saint George’s Respiratory Questionnaire; HADS: Hospital Anxiety and Depression Scale; mMRC: modified Medical Research Council; BDI: Baseline Dyspnea Index; and 6MWD: six-minute walk distance. aValues expressed as mean ± SD, except where otherwise indicated. bValues expressed as median (interquartile range). p < 0.05 (Student’s t-test or Mann-Whitney test).
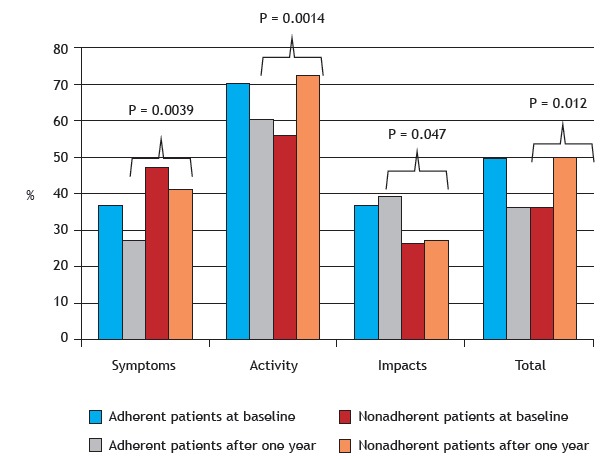
The scores for the SGRQ domain of symptoms improved significantly after one year of follow-up in the nonadherent group (47.3 ± 18.7 vs. 41.5 ± 19.6; p = 0.005) and in the adherent group (36.8 ± 16.8 vs. 27.6 ± 18.7; p = 0.001). With regard to the scores for the remaining SGRQ domains and total SGRQ scores, there were no significant differences (Figure 2).
Figure 2. Saint George’s Respiratory Questionnaire (SGRQ) domain scores for adherent and nonadherent patients at baseline and after one year of follow-up. p < 0.05 (paired t-test or Wilcoxon test).
Of the 39 patients in the sample, 16 (41%) were diagnosed with COPD exacerbation, and 26% required hospitalization, the need for hospitalization being unrelated to adherence to LTOT (p = 1.00). The multiple linear regression model showed a negative association between the BDI and SGRQ symptoms domain scores after one year (p = 0.04; Table 3).
Table 3. Multiple linear regression analysis of factors associated with the Saint George’s Respiratory Questionnaire symptoms domain after one year of follow-up.
| Dependent variable | Variable | Coefficient | (95% CI) | p |
|---|---|---|---|---|
| Symptoms after one year (R2 = 0.23) | Baseline SpO2, mmHg | 0.01 | −2.62 to 2.65 | 0.99 |
| BDI | −2.85 | −5.59 to −0.107 | 0.04 | |
| Adherence after one year, yes | −0.86 | −18.40 to 16.77 | 0.91 |
BDI: Baseline Dyspnea Index.
DISCUSSION
The objective of the present study was to determine the impact of adherence to LTOT on quality of life, dyspnea, and exercise capacity in patients with COPD and exertional hypoxemia followed for one year. The main finding of our study is that quality of life is worse in patients who do not adhere to LTOT than in those who do. Adherence to LTOT contributes to improving quality of life by improving symptoms. In comparison with the COPD patients who did not adhere to LTOT in the present study, those who did had better quality of life after one year of follow-up. Nevertheless, respiratory symptoms were found to have improved in the group of nonadherent patients, despite the fact that they did not use LTOT for as long as recommended. The mean duration of LTOT use in our study was quite similar to that in a trial conducted by the Long-Term Oxygen Treatment Trial Research Group (i.e., 10.4 h/day). That trial included 148 patients receiving LTOT during exercise and sleep. 10
In a study analyzing 191 patients with severe hypoxemia, only 52.4% adhered to LTOT as prescribed, 25 a finding that is consistent with those of the present study. The authors of the aforementioned study reported 51 adverse events attributed to the use of oxygen supplementation, 23 patients having reported tripping on the device. 25 Adverse events can contribute to poor adherence to treatment. 10 In a qualitative study of 27 COPD patients receiving LTOT, 26 most (59.2%) reported nonadherence to LTOT, which was reported to be difficult to use during physical activity and was associated with social stigma and fear of side effects.
Previous studies 4 , 5 , 27 , 28 have shown the beneficial effects of LTOT on mortality, quality of life, and respiratory symptoms in COPD patients with severe hypoxemia. The use of LTOT might also be beneficial for patients with exertional hypoxemia, improving their symptoms, dyspnea, functional capacity, and disease progression. Analysis of data from the National Emphysema Treatment Trial, a longitudinal study conducted in the United States, showed that 33.8% of 1,215 patients with emphysema were using LTOT despite having no severe hypoxemia. 6 In comparison with the patients who were not using LTOT, those who were had worse dyspnea, lower quality of life, and lower functional capacity, exercise-induced desaturation being more common in those using LTOT. 6
According to international guidelines, LTOT should not be used in patients with exertional hypoxemia. 6 - 8 , 11 , 19 , 29 , 30 In a recently published study, 10 patients with moderate resting or exercise-induced hypoxemia were followed for six years and no differences were found between those using LTOT and those not using it regarding quality of life, lung function, or the 6MWD. In addition, the BODE index was lower in the patients using LTOT than in those not using it (p = 0.007). 10 However, the fact that the study included a heterogeneous sample of patients might have affected the results.
In the present study, quality of life (as assessed by SGRQ symptoms domain scores) was found to have improved after one year (53.0 ± 19.8 vs. 34.5 ± 20.4; p < 0.001). Eaton et al. 7 divided 41 COPD patients with exertional hypoxemia into two groups on the basis of LTOT use during physical exercise performed three times a week for 4 months. The group of patients using LTOT showed significant improvement in quality of life. 7 Garrod et al. 8 reported that patients participating in an 8-week exercise training program and receiving supplemental oxygen showed significant improvement in the 6MWD (169 ± 112 m vs. 269 ± 124 m; p = 0.001) and in Chronic Respiratory Disease Questionnaire scores (24.0 vs. 17.4; p < 0.001) when compared with those who participated in the program but did not receive supplemental oxygen. 8 The finding of a higher mean 6MWD is consistent with those of Morakami et al., 31 who compared COPD patients in Brazil and Austria and found that mean walking time and movement intensity were greater in the former than in the latter.
Wadell et al. 9 found that the use of LTOT did not improve quality of life or dyspnea in COPD patients with exercise-induced hypoxemia. The authors evaluated 20 patients with COPD and exercise-induced hypoxemia. The participants were randomized to undergo exercise training while breathing room air or receiving supplemental oxygen for 30 min three times per week for 8 weeks. 9 Unlike our study, in which LTOT use was found to improve dyspnea, the study by Wadell et al. included no other periods of LTOT use during the day. 9
Although our results showed no significant changes in dyspnea scores, multiple linear regression analysis showed that a higher BDI translated to worse SGRQ symptoms domain scores after one year of follow-up. This result is consistent with those obtained by Ferrari et al., 32 who followed 90 COPD patients for three years and found that dyspnea perception was positively associated with impaired quality of life at the end of the follow-up period.
Reduced functional capacity is a marker of severity and mortality in patients with COPD and exercise-induced hypoxemia. 33 In the present study, the 6MWD decreased significantly after one year of follow-up. Takigawa et al. 34 reported that COPD patients with a ≥ 6% decrease in SpO2 during the 6MWT were at an increased risk of death. Casanova et al. 35 reported that COPD patients with a ≥ 4% decrease in SpO2 or an SpO2 of < 90% during the 6MWT had a relative risk of death of 2.63 when compared with those without those changes.
Exacerbations can potentiate chronic inflammation and, consequently, have a detrimental effect on symptoms, quality of life, and exercise capacity, as well as accelerating the functional deterioration of COPD patients. 35 - 37 With regard to the frequency of exacerbations in our sample of COPD patients, nearly 50% experienced at least one exacerbation during follow-up. Of those, 26 required hospitalization, the need for which was unrelated to adherence to LTOT. In another study conducted at our institution, 32 90 COPD patients were followed over a three-year period. Of those, 75% experienced at least one exacerbation, which was classified as severe in 26.6%. 32 In a recent study, no significant differences were found between patients with COPD and moderate hypoxemia using LTOT and patients with COPD and moderate hypoxemia not using LTOT in terms of disease exacerbation and hospitalization. 10 In a study conducted by Marino et al., 38 47 COPD patients were followed for 6 months, and 27.6% experienced at least one exacerbation during the study period. In the present study, the mortality rate was 15%. In a study involving 78 COPD patients receiving LTOT for one year, the mortality rate was 15.4%. 39
Nutritional depletion is a risk factor for clinical worsening in COPD patients receiving LTOT. 37 In the present study, there were no significant changes in the prevalence of nutritional depletion during follow-up. However, the BMI was found to be lower in the patients who experienced worsening hypoxemia or died than in those with transient hypoxemia. In a study evaluating 128 COPD patients using LTOT and followed for three years, the number of deaths was higher among those with a BMI of < 25 kg/m2 and a higher number of comorbidities. 39
Our study has some limitations. First, given that patient adherence to LTOT was self-reported, it is impossible to determine actual adherence. Nevertheless, in a recent study conducted by the Long-Term Oxygen Treatment Trial Research Group, 10 adherence to LTOT was also self-reported, and adherence education was provided at each medical visit in order to improve LTOT use, as was done in the present study. Unfortunately, there is currently no other method for accurately determining patient adherence to LTOT. Second, there was no true control group (i.e., a group of patients receiving no supplemental oxygen). Second, by including a group without oxygen (true control group) will probably add more significant alterations on several parameters of disease progression and quality of life. If there had been one, we might have observed significant changes in several disease progression and quality of life parameters. However, although the changes observed in the group of patients who did not adhere to LTOT were not as marked as they might have been in a true control group, quality of life was found to have improved in the group of patients who adhered to LTOT. Therefore, our findings are relevant.
In conclusion, quality of life appears to be lower in patients with COPD and exercise-induced hypoxemia who do not adhere to LTOT than in those who do. In addition, LTOT appears to have a beneficial effect on COPD symptoms (as assessed by SGRQ scores) in patients with COPD and exercise-induced hypoxemia. Further studies investigating LTOT use during exercise are needed in order to provide a better understanding of patient clinical response to LTOT.
Study carried out in the Disciplina de Pneumologia, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista - UNESP - Botucatu (SP) Brasil.
Financial support: Carolina Bonfanti Mesquita is the recipient of a grant from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education).
REFERENCES
- 1.Costa CH, Rufino R, Lapa E Silva JR. Células inflamatórias e seus mediadores na patogênese da DPOC. Rev Assoc Med Bras (1992) 2009;55(3):347–354. doi: 10.1590/S0104-42302009000300031. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary, updated 2015. Bethesda: GOLD; 2015. [Google Scholar]
- 3.Coleta KD, Lima DF, Tanni SE, Silveira LV, Godoy I, Godoy I. Gender and health status response to long-term oxygen therapy in COPD patients [Article in Spanish] Arch. Bronconeumol. 2011;47(8):382–388. doi: 10.1016/j.arbres.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Nocturnal Oxygen Therapy Trial Group Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 5.Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–686. [PubMed] [Google Scholar]
- 6.Drummond MB, Blackford AL, Benditt JO, Make BJ, Sciurba FC, McCormack MC. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients retrospective analysis of the National Emphysema Treatment Trial. Chest. 2008;134(3):497–506. doi: 10.1378/chest.08-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton T, Garret JE, Young P, Fergusson W, Kolbe J, Rudkin S, Whyte K. Ambulatory oxygen improves quality of life of COPD patients a randomised controlled study. Eur Respir J. 2002;20(2):306–312. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- 8.Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Pt 1Am J Respir Crit Care Med. 2000;162(4):1335–1341. doi: 10.1164/ajrccm.162.4.9912029. [DOI] [PubMed] [Google Scholar]
- 9.Wadell K, Henriksson-Larsén K, Lundgren R. Physical training with and without oxygen in patients with chronic obstructive pulmonary disease and exercise-induced hypoxaemia. J Rehabil Med. 2001;33(5):200–205. doi: 10.1080/165019701750419581. [DOI] [PubMed] [Google Scholar]
- 10.Long-Term Oxygen Treatment Trial Research Group.Albert RK.Au DH.Blackford AL.Casaburi R.Cooper JA Jr A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitrouska I, Tzanakis N, Siafakas NM. Oxygen therapy in chronic obstructive pulmonary disease. Eur Respir Mon. 2006;38:302–312. doi: 10.1183/1025448x.00038018. [DOI] [Google Scholar]
- 12.Fabbri LM, Luppi F, Beghé B, Rabe KF. Update in chronic obstructive pulmonary disease 2005. Am J Respir Crit Care Med. 2006;173(10):1056–1065. doi: 10.1164/rccm.2603005. [DOI] [PubMed] [Google Scholar]
- 13.Hardinge M, Suntharalingam J, Wilkinson T. British Thoracic Society Guideline update The British Thoracic Society Guidelines on home oxygen use in adults. Thorax. 2015;70(6):589–591. doi: 10.1136/thoraxjnl-2015-206918. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Standardization of spirometry--1987 update Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136(5):1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 15.Sociedade Brasileira de Pneumologia e Tisiologia II Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica. J Pneumol. 2004;30(Suppl 5):S1–S42. [Google Scholar]
- 16.Kyle UG, Pichard C, Rochat T, Slosman DO, Fitting JW, Thiebaud D. New bioelectrical impedance formula for patients with respiratory insufficiency comparison to dual-energy X-ray absorptiometry. Eur Respir J. 1998;12(4):960–966. doi: 10.1183/09031936.98.12040960. [DOI] [PubMed] [Google Scholar]
- 17.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi: 10.1093/ajcn/82.1.53. [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Souza TC, Jardim JR, Jones P. Validação do questionário do hospital Saint George na doença respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil. J Pneumol. 2000;26(3):119–128. doi: 10.1590/S0102-35862000000300004. [DOI] [Google Scholar]
- 20.Kovelis D, Segretti NO, Probst VS, Lareau SC, Brunetto AF, Pitta F. Validation of the Modified Pulmonary Functional Status and Dyspnea Questionnaire and the Medical Research Council scale for use in Brazilian patients with chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(12):1008–1018. doi: 10.1590/S1806-37132008001200005. [DOI] [PubMed] [Google Scholar]
- 21.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill B, Bradley JM, Heaney L, O'Neill C, MacMahon J. Short burst oxygen therapy in chronic obstructive pulmonary disease a patient survey and cost analysis. Int J Clin Pract. 2005;59(7):751–753. doi: 10.1111/j.1368-5031.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 26.Verduri A, Ballerin L, Simoni M, Cellini M, Vagnoni E, Roversi P. Poor adherence to guidelines for long-term oxygen therapy (LTOT) in two Italian university hospitals. Intern Emerg Med. 2014;9(3):319–324. doi: 10.1007/s11739-012-0898-2. [DOI] [PubMed] [Google Scholar]
- 27.Earnest MA. Explaining adherence to supplemental oxygen therapy. J Gen Intern Med. 2002;17(10):749–755. doi: 10.1046/j.1525-1497.2002.20218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaney JC, Jones K, Grathwohl K, Olivier KN. Implementation of an oxygen therapy clinic to manage users of long-term oxygen therapy. Chest. 2002;122(5):1661–1667. doi: 10.1378/chest.122.5.1661. [DOI] [PubMed] [Google Scholar]
- 29.Cano NJ, Pichard C, Roth H, Court-Fortuné I, Cynober L, Gérard-Boncompain M. C-reactive protein and body mass index predict outcome in end-stage respiratoryfailure. Chest. 2004;126(2):540–546. doi: 10.1378/chest.126.2.540. [DOI] [PubMed] [Google Scholar]
- 30.Rooyackers JM, Dekhuijzen PN, Van Herwaarden CL, Folgering HT. Training with supplemental oxygen in patients with COPD and hypoxaemia at peak exercise. Eur Respir J. 1997;10(6):1278–1284. doi: 10.1183/09031936.97.10061278. [DOI] [PubMed] [Google Scholar]
- 31.Morakami FK, Morita AA, Bisca GW, Felcar JM, Ribeiro M, Furlanetto KC, et al. Can the six-minute walk distance predict the occurrence of acute exacerbations of COPD in patients in Brazil. J Bras Pneumol. 2017;43(4):280–284. doi: 10.1590/S1806-37562016000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrari R, Tanni SE, Lucheta PA, Faganello MM, do Amaral RA, Godoy I. Gender differences in predictors of health status in patients with COPD. J Bras Pneumol. 2010;36(1):37–43. doi: 10.1590/S1806-37132010000100008. [DOI] [PubMed] [Google Scholar]
- 33.Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic pulmonary disease patients. Am J Respir Crit Care Med. 2003;168(9):1034–1042. doi: 10.1164/rccm.200212-1525OC. [DOI] [PubMed] [Google Scholar]
- 34.Takigawa N, Tada A, Soda R, Date H, Yamashita M, Endo S. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101(3):561–567. doi: 10.1016/j.rmed.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, Aguirre-Jaíme A. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134(4):746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 36.Rubin AS, Souza FJ, Hetzel JL, Moreira Jda S. Immediate bronchodilator response to formoterol in poorly reversible chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(6):373–379. doi: 10.1590/S1806-37132008000600007. [DOI] [PubMed] [Google Scholar]
- 37.Figueiredo AB, Silva SR, Filho, Lôbo RR, Moriguti JC. Exacerbação da doença pulmonar obstrutiva crônica. Medicina (Ribeirão Preto) 2010;43(3):223–230. doi: 10.11606/issn.2176-7262.v43i3p223-230. [DOI] [Google Scholar]
- 38.Marino DM, Marrara KT, Arcuri JF, Candolo C, Jamami M, Di Lorenzo VA. Determination of exacerbation predictors in patients with COPD in physical therapy - a longitudinal study. Braz J Phys Ther. 2014;18(2):127–136. doi: 10.1590/S1413-35552012005000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleta KD, Silveira LV, Lima DF, Rampinelli EA, Godoy I, Godoy I. Predictors of first-year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med. 2008;102(4):512–518. doi: 10.1016/j.rmed.2007.12.003. [DOI] [PubMed] [Google Scholar]


