Supplemental Digital Content is available in the text.
Abstract
Background:
Severe trauma often results in the transection of major peripheral nerves. The RANGER Registry is an ongoing observational study on the use and outcomes of processed nerve allografts (PNAs; Avance Nerve Graft, AxoGen, Inc., Alachua, Fla.). Here, we report on motor recovery outcomes for nerve injuries repaired acutely or in a delayed fashion with PNA and comparisons to historical controls in the literature.
Methods:
The RANGER database was queried for mixed and motor nerve injuries in the upper extremities, head, and neck area having completed greater than 1 year of follow-up. All subjects with sufficient assessments to evaluate functional outcomes were included. Meaningful recovery was defined as ≥M3 on the Medical Research Council scale. Demographics, outcomes, and covariate analysis were performed to further characterize this subgroup.
Results:
The subgroup included 20 subjects with 22 nerve repairs. The mean ± SD (minimum–maximum) age was 38 ± 19 (16–77) years. The median repair time was 9 (0–133) days. The mean graft length was 33 ± 17 (10–70) mm with a mean follow-up of 779 ± 480 (371–2,423) days. Meaningful motor recovery was observed in 73%. Subgroup analysis showed no differences between gap lengths or mechanism of injury. There were no related adverse events.
Conclusions:
PNAs were safe and provided functional motor recovery in mixed and motor nerve repairs. Outcomes compare favorably to historical controls for nerve autograft and exceed those for hollow tube conduit. PNA may be considered as an option when reconstructing major peripheral nerve injuries.
BACKGROUND
Severe trauma to the upper extremities often results in the transection of mixed and motor peripheral nerves. Without surgical management, these transection injuries result in functional loss that can seriously affect the patient’s overall quality of life. To regain function, transected nerves must be reconstructed to permit axonal regeneration into distal target muscle and terminal receptors in the skin. Injuries that cannot be repaired primarily without tension require a bridging material to address the gap.1,2 Nerve autografts, historically selected for this purpose, involve the sacrifice of healthy sensory nerves from donor sites such as the sural nerve, or the lateral or medial antebrachial cutaneous nerves.3,4 Although not a guarantee of recovery, positive outcomes in the 40%–80% range are documented in the literature.5–11 However, limitations of their use have also been reported, including increased complexity of the repair procedure due to the addition of a secondary surgical site, permanent donor-site morbidity, and potential postoperative complications and pain.12,13 Another limitation of the nerve autograft is the finite amount of donor tissue available to the surgeon. This can become problematic for the reconstruction of severe gap injuries where multiple strands of nerve autograft must be cabled together to fit the larger caliber found in mixed nerves of the upper extremity. This places a higher demand on an already limited supply of graft material. For these reasons, identifying alternative repair modalities is of interest to the reconstructive surgeon.
One such alternative is the use of commercially available processed nerve allograft (PNA; Avance Nerve Graft, AxoGen, Alachua, Fla.). These grafts, composed of donated human peripheral nerve tissue, are chemically decellularized, enzymatically treated to remove growth-inhibiting chondroitin sulfate proteoglycans, and terminally sterilized. Cellular debris is removed while preserving both laminin activity and the 3-dimensional basal lamina scaffold of the native nerve tissue. The resultant structure is composed of bundles of endoneurial tubes comprised extracellular matrix proteins that include collagen, laminin, fibronectin, and glycosaminoglycans.14,15 Species-specific PNA analogs prepared with this method demonstrate effective repair of peripheral nerve discontinuities in animal studies by supporting axonal regeneration.14–19
Clinical data demonstrate that PNA is safe and results in positive functional outcomes for the reconstruction of nerve gaps up to 70 mm in length.20–27 However, a majority of these studies report primarily sensory outcomes. The RANGER Registry study was initiated in 2007 to collect data on the utilization, safety, and functional outcomes from the use of PNA for the reconstruction of peripheral nerve defects. Data from all patients repaired with PNA at participating institutions are included in the registry. This has resulted in a comprehensive database of PNA repairs in a variety of nerves and regions of the body, including the head, neck, and upper and lower extremities. Here, we use the RANGER database to specifically examine motor outcomes for mixed and motor nerve injuries repaired acutely or in a delayed fashion with PNA and report our findings with comparisons to historical controls in the literature.
MATERIALS AND METHODS
Study Design
The RANGER registry is an ongoing observational study that collects data on the use and outcomes of PNA in peripheral nerve repair.28–30 All patients treated with PNA at participating sites were open to be in the study. Multiple efforts were taken to identify eligible subjects for the registry, including tissue utilization record review and internal hospital audits. Nerve repairs were performed by experienced plastic or orthopedic hand surgeons. Study centers followed their own standard of care for subject treatment, rehabilitation regime, and follow-up measures.
Data were collected in an observational manner on standardized paper or electronic case report forms from the charts of participating subjects and were organized into a centralized study database for analysis. Data were collected and reported to the extent available in the medical records. Preoperative, operative, postoperative follow-up and physical therapy notes were the main sources of information. Data collected included general subject demographics, details of the nerve injury, concomitant injury, the nerve repair(s) performed, concomitant treatments, follow-up evaluations performed, and the corresponding outcomes. Additionally, information on adverse events or complications occurring intra/postoperatively was collected.
This database was queried for nerve repairs that met the following criteria: mixed and motor nerve injuries in the upper extremity or head and neck region up to 70 mm; repaired within 6 months of initial injury; documented loss of function, completed at least 1 year (365 days) of follow-up assessments; and reporting relevant motor function assessments sufficient for outcome evaluation using the Medical Research Council (MRC) scale for motor function.
Clinical Evaluation
Follow-up assessments utilized throughout the sites included a variety of quantitative measures for motor function such as range of motion and grip/pinch strength. For facial nerve evaluation, facial weakness in the relevant region was examined and compared with the uninvolved side of the face. Although not all sites completed the same battery of assessments following repair, relevant outcomes were reported for all repairs. Table 1 summarizes the specific functional assessments conducted in this cohort.
Table 1.
Functional Assessments for Specific Nerve Repairs
Functional recovery was evaluated by comparing the results of final assessment with the preoperative assessments and earlier follow-up assessments. Electromyogram and nerve conduction study results were also documented in some of the subjects to provide additional information on reinnervation status. The Mackinnon modification of the MRC grading system was used for evaluation of motor recovery.1 To ensure consistency with a majority of the relevant literature, meaningful functional recovery was defined to be M3 or greater on the MRC scale, and a higher threshold of desired recovery was defined as M4 or greater.1,5–11
Statistical Methods
Data were gathered and compiled into a centralized study database. Descriptive statistics were used to analyze the demographics, baseline characteristics, data quality, and trends of clinical outcomes. Continuous parameters (eg, functional scores), N, mean, and the SD were recorded. All information was reported as the mean ± SD (range) unless stated otherwise. Analysis of variance or Mann–Whitney test was performed to test difference of age, preoperative interval, and follow-up length between subgroups. Fisher’s exact test was used to test differences in rate of meaningful recovery. Statistical significance was set at a probability of type I error less than 0.05 (P < 0.05).
RESULTS
A total of 191 mixed/motor nerve repairs in upper extremity, head, and neck were performed in 10 participating centers during years 2008–2015. Of these repairs, 40 reported quantitative assessments of motor function, among which 22 repairs in 20 subjects met the study cohort criteria for time to repair after injury, gap, and length of follow-up. The remaining repairs were excluded: 129 repairs reported only safety data with insufficient follow-up and 22 reported only subjective assessments of the outcome of the repair.
A review of the preoperative assessments of the cohort (n = 22) confirmed a MRC scale of M0 for pertinent motor function in 21 of the mixed/motor nerve injury cases. In the remaining repair, complete high-median nerve laceration (25-mm gap), the preoperative function was recorded as “motor and sensory weakness distal to lesion” with early follow-up visit (102 days postoperative) reporting progressive Tinel’s 7 cm from repair with documented loss of range of motion. In general, all the subjects in the cohort presented as loss of function corresponding to specific nerve injury.
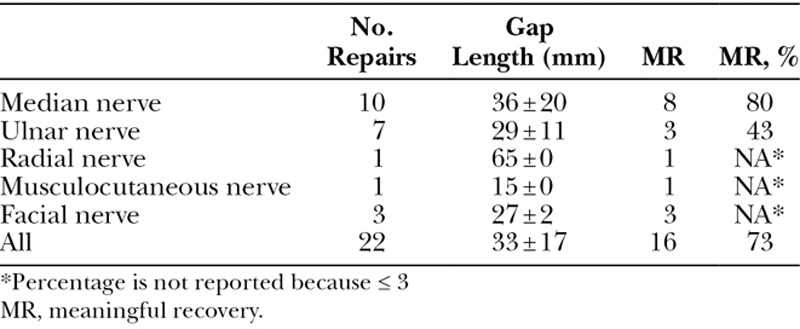
All repairs were completed in a tensionless fashion via epineural suture using size-matched PNA according to product instructions for use. Figure 1 shows an example of a mixed nerve reconstruction in an ulnar nerve injury using PNA. Subjects in this cohort had a mean age of 38 ± 19 (16–77) years. The mean graft length was 33 ± 17 (10–70) mm. The median preoperative interval was 9 days with a range from 0 to 133 days. Subjects were followed for an average of 779 ± 480 days (371–2,423 days) to determine the level of meaningful motor recovery (M3 or greater on the MRC scale). No significant differences were found in subject age, preoperative interval, or follow-up length among subgroups (P > 0.05, analysis of variance test). Meaningful motor recovery was reported in 73% of repairs in the cohort. When looking at higher thresholds of recovery, 50% of repairs reported M4 or greater on the MRC scale. The cohort was also divided and analyzed in 3 subgroups based on gap length: 10–25, 26–49, and 50 mm or greater. Meaningful recovery of motor function was reported in 80%, 63%, and 75% of repairs in these subgroups, respectively. Table 2 reports demographic and outcomes data for all nerve repairs by gap length. The difference in rate of meaningful recovery between gap length subgroups was not significant (P = 0.61, Fisher’s exact test). When repairs were grouped by mechanism of injury, 7 of 11 laceration repairs (64%) and 4 of 6 complex repairs (67%) reported meaningful recovery (P = 1, Fisher’s exact test). The outcome difference was not statistically significant when comparing less severe injuries like lacerations to high-energy/impact nerve injuries from explosive blasts, gunshots, and avulsion. No difference in age distribution (P = 0.55, Mann–Whitney test) or subject comorbidities, such as hypertension and smoking status (P = 0.57 and P = 0.07, respectively, Fisher’s exact test), between outcome groups (meaningful recovery versus nonmeaningful recovery) was detected. Figure 2 and Supplementary Digital Content 1 show an example of recovery of motor function in a subject with an ulnar nerve injury. (See video, Supplemental Digital Content 1, which displays motor functional outcomes at 16 months postulnar nerve reconstruction with PNA. This subject presented to the clinic in a delayed fashion with a low ulnar nerve palsy after accidental laceration to the left forearm with a pocket knife, http://links.lww.com/PRSGO/B12.) Figure 3 shows the distribution of MRC scale within this cohort. There were no reported adverse events related to the PNA.
Fig. 1.
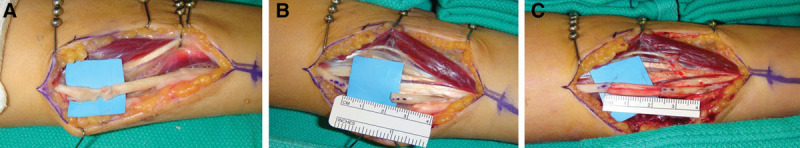
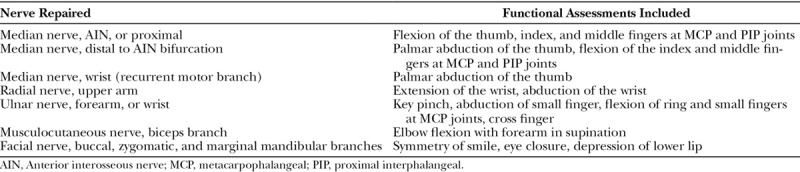
Injury and repair of an ulnar nerve with PNA. A, 16-year-old girl presented to the clinic in a delayed fashion with a low ulnar nerve palsy after accidental laceration to the left forearm with a pocket knife. Surgical exploration 41 days after injury, the flexor carpi ulnaris and ulnar neurovascular bundle was nearly completely transected and encased in tremendous amount of scar. B, Neurolysis of the ulnar nerve and resection to healthy fascicles resulting in a gap length of 23 mm. C, Following repair using two 3–4 mm diameter PNA in a grouped fascicular fashion.
Table 2.
Summary of Mixed and Motor Nerve Repair Outcomes by Gap Length
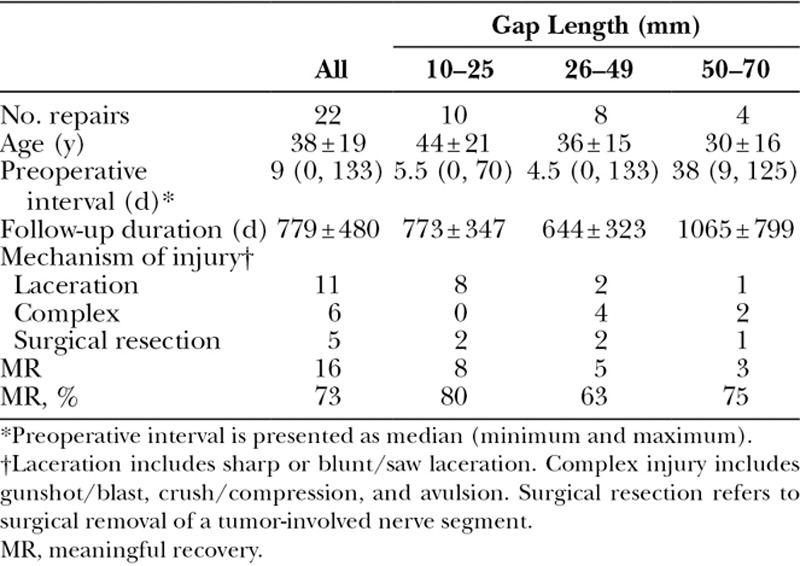
Fig. 2.
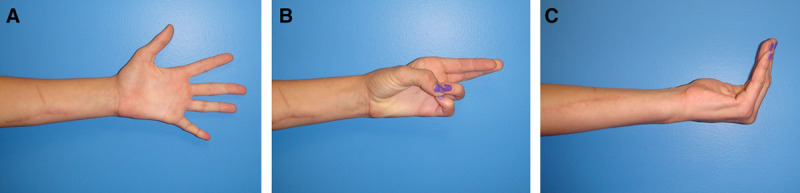
Recovery of motor function in subject with the ulnar nerve injury 481 days after repair. A, Recovery of finger abduction. B, Reinnervation of the hypothenar muscles allowing the fifth digit opposition with the thumb. C, Recovery of finger metacarpal-phalangeal joint flexion and interphalangeal joint extension of the small and ring fingers.
Video Graphic 1.
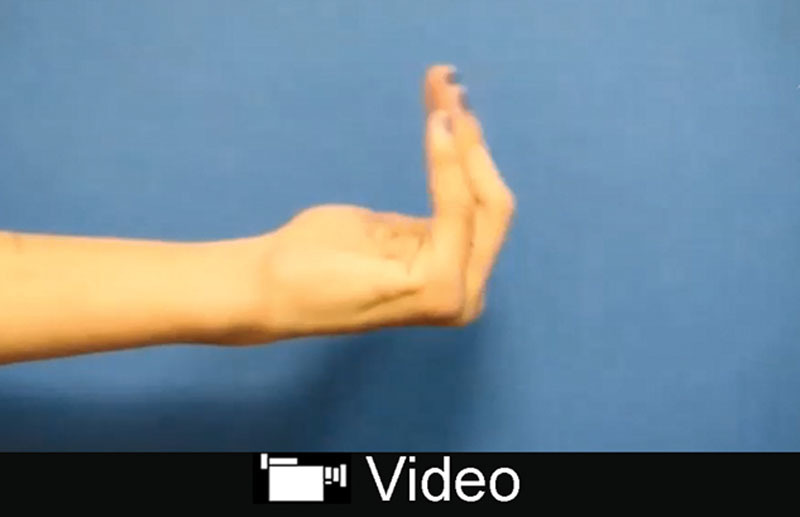
. See video, Supplemental Digital Content 1, which displays motor functional outcomes at 16 months postulnar nerve reconstruction with PNA. This subject presented to the clinic in a delayed fashion with a low ulnar nerve palsy after accidental laceration to the left forearm with a pocket knife, http://links.lww.com/PRSGO/B12.
Fig. 3.
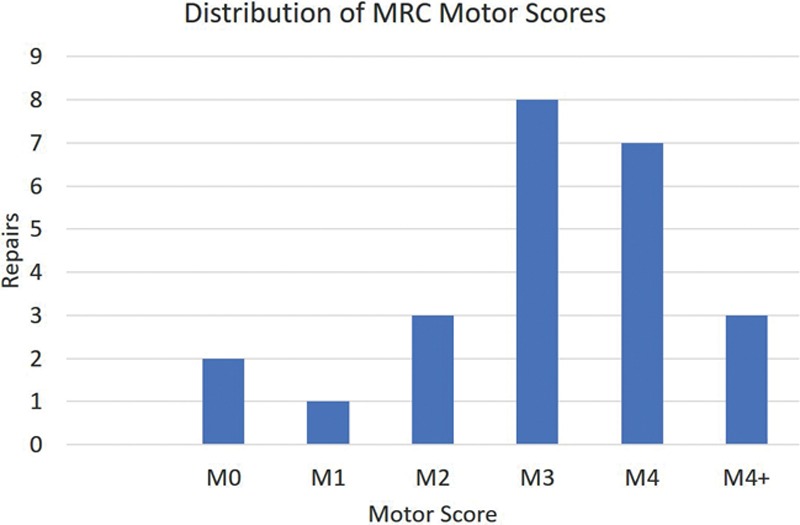
Distribution of MRC motor scores.
Most of the repairs in the cohort were performed on median nerve (n = 10) and ulnar nerve (n = 7). Although our current dataset shows that median nerve trended better recovery than ulnar nerves after PNA repair (80% versus 43%), the difference did not reach significant level, possibly because of limited sample size (Table 3; P = 0.16, Fisher’s exact test). The gap length in these 2 subgroups was not significantly different (P = 0.73, Mann–Whitney test). Other repairs included radial nerve (n = 1), musculocutaneous nerve (n = 1), facial nerve buccal branch (n = 1), marginal mandibular branch (n = 1), and zygomatic branch (n = 1). The radial nerve repair in the upper arm, 684 days of follow-up, reported return of wrist and finger extension. The musculocutaneous nerve case reported loss of function preoperatively. Signs of early reinnervation into the biceps muscle were observed via electromyogram study 6 months postoperative, and 5/5 elbow flexion was documented at final follow-up (428 days after nerve repair). Segmental resection of the 3 branches of facial nerve was performed in a patient because of parotid tumor involvement. These branches were repaired with PNA at the time of resection. Recorded recovery of the marginal mandibular nerve included depression of lower lip. Buccal and zygomatic nerve recovery was rated as 4/5 versus the other at 4+/5 at 1,422 days after repair. It is noted that although there is crossover between the zygomatic and buccal nerve innervation zones, the goal was to assess the usefulness of the graft. Because both nerves were injured and reconstructed for this functional area, an evaluation of the innervation zone for this patient confirmed loss of function and subsequent return of function to near normal, thus confirming regeneration through the grafts.
Table 3.
Summary of Outcomes by Nerve
Our cohort also included 4 repairs to nerve gap injuries from 50 to 70 mm. The mechanisms of these injuries include 3 gunshot/blast wounds, 1 avulsion, 1 laceration, and 1 surgical resection. Three of the 4 repairs reported regain of motor function to M3 level or greater at their last follow-up visit, indicating the capability of PNA in supporting nerve regeneration in these long-gap repair cases (Table 4).
Table 4.
Outcomes of Long Nerve Gap (≥50 mm) Repairs using PNA

DISCUSSION
Clinical evidence on the use of commercially available PNA was first documented by Karabekmez et al.22 who reported 100% recovery in digital nerve repairs. These were followed by Guo et al.,21 Taras et al.,23 and Means et al.,25 also reporting similar outcomes with recovery of static 2-point discrimination. Cumulative outcomes from the RANGER registry demonstrate sensory and motor outcomes across multiple centers with meaningful recovery of between 77% and 89%, despite longer gap lengths.27–30 Zuniga20 showed comparable recovery rates in both short- and long-gap repairs in a series of trigeminal nerve reconstructions up to 70 mm. And Souza et al.26 reported on resolution of pain after resection and reconstruction in a case series of lower extremity nerves with symptomatic neuroma. The RANGER registry is designed to collect data on nerve repairs and outcomes through the body. In this study, we reviewed the return of motor function after mixed and motor nerve injury from the ongoing RANGER registry and demonstrated that PNA can be used to provide functional motor recovery. Return of motor function to M3 or higher on the MRC scale was reported for 73% of all repairs with no graft-related adverse experiences reported. This is in line with other reports in the literature that confirm the safety profile of PNAs.
Outcomes from subgroup analyses demonstrated consistent recovery across multiple variables such as gap length and mechanism of injury, indicating that PNA performed reproducibly to at least a level of M3. Although limited by relatively small sample size and heterogeneity in gap length, meaningful recovery of complex injuries was comparable to laceration repairs, suggesting a potential clinical benefit of PNA for nerve injuries complicated by significant adjacent tissue damage. These outcomes are consistent with historical data of similar injury type. In a study of nerve lesions caused by blast, gunshot, and shrapnel repaired with nerve autograft, 67% of injuries reported meaningful motor recovery.31 When looking at specific mixed nerve types, median nerve repairs recovered better than ulnar nerves in our cohort. These outcomes were expected and consistent with previous reports using nerve autograft.32
Reconstructive plastic surgeons are often challenged with how best to manage a variety of nerve injuries. This cohort also included facial nerve reconstructions with nerve allograft. Of the 22 nerves in the cohort, 3 included a marginal mandibular, zygomatic, and a buccal nerve. The authors believe inclusion of these nerve repairs was warranted as data on recovery of pure motor nerves are very limited in the literature for both allograft and autograft repairs and, because of their low incidence rates, are difficult to incorporate as a single clinical study. In this case, all 3 nerves reported meaningful recovery. If these were excluded from the cohort, meaningful recovery for the remaining group is 68%. These outcomes are consistent with historical data of similar nerve type.
Reconstruction of long nerve defects remains a clinical challenge to surgeons. Although considered as “gold standard,” nerve autografting cannot guarantee satisfactory functional outcome in long-gap nerve repairs. Furthermore, when presented with long large caliber nerve injuries, the availability of sufficient nerve autograft may be limited. Our cohort includes 4 repairs with nerve gaps of 50–70 mm with an average follow-up of 3 years. Three of 4 repairs achieved meaningful recovery. The outcome is comparable to shorter gap repairs and suggests the usefulness of PNA in long-gap nerve repairs. Additional collection of long-term follow-up from subjects with long-gap injuries in the registry will provide further insight into the factors that affect recovery and utility of PNA as compared to autograft.
Limitations of our study include its observational study design, inherent challenges of MRC motor scale, lack of standardized assessments to reproducibly evaluate motor recovery, a relatively small, heterogeneous dataset due to the challenges of obtaining long-term follow-up, and the vulnerability to selection bias and lack of blinding. Although measures were taken to ensure that all eligible subjects were included and only quantitative follow-up assessments were used in this study, potential bias could not be entirely eliminated.
We compared our findings with those previously reported for both nerve autograft and hollow tube conduits. Most existing data are derived from retrospective studies, meta-analysis reviews, and case series reports with few multicenter randomized controlled studies describing functional motor recovery after nerve reconstruction. As was found in the registry, this is largely due to the challenges of this traumatic patient population. The variety of presented nerve injury levels and patterns, need for emergent treatment, lack of patient compliance, and high attrition rates make preoperative informed consent and managing a well-controlled study an arduous and long-term endeavor. Kim et al.5–7 retrospectively showed 67%–80% of mixed nerve injuries demonstrated meaningful motor and sensory recovery using their grading system. In a meta-analysis, Brushart33 reports meaningful motor outcomes of M3 or better in approximately 84% of median nerves and 73% of ulnar nerves repaired with autograft. Frykman and Gramyk8 found 80% of median nerves and 60% of ulnar nerves repaired with autograft had meaningful recovery. He et al.32 showed satisfactory motor recovery in 50% of nerve graft repairs as compared to 74% of direct repairs. In a large meta-analysis of median and ulnar nerve injuries across 23 studies, Ruijs et al.10 demonstrated 47% motor recovery of M4 or better with autograft repairs. In our study, 73% of PNA repairs achieved motor recovery of M3 or greater and 50% M4 or greater. This result is on par with published data that demonstrated M3 or greater recovery in 48%–84%9,11,33 and M4 or better in 21%–51%9–11 of autograft repairs for mixed and motor nerve injuries.
Manufactured tube conduits were introduced in the 1990s as an alternative to traditional coaptation. The indications quickly expanded to provide an alternative to nerve autograft. Reported clinical outcomes show hollow tube conduits to have limited clinical applications. Typically used for very short defects in sensory nerves, their use for longer gaps and larger nerves is not recommended.34–39 Wangensteen and Kallianien40 in a multicenter retrospective study on collagen conduits for sensory, mixed, and motor nerve repairs found only 43% of patients reported recovery. Although very limited, the use of manufactured conduits specifically in mixed nerve reconstruction has been reported. One study shows that when silicon tube conduits are used as coaptation aids for gaps under 5 mm in median and ulnar nerves, motor and sensory outcomes are equivalent to those for direct end-to-end repair.41 A study of the use of Neurolac nerve conduits (Polyganics, Groningen, The Netherlands) for the repair of 2–25 mm gaps in mixed nerve finds that only 1 in 12 repairs (8%) achieved sensory recovery of S3 or greater on the Medical Research Council Scale for sensory function.42 In a more recent study comparing direct suture with collagen conduits in gaps 6 mm or less, motor recovery was significantly better for direct suture at 12 months but was comparable after 24 months.39 Although direct suture reported faster time to recovery, the utility of conduits at these short lengths can be justified as a coaptation aid in this application. Complication rates for conduits range from 8% to 35% with the most common adverse events being extrusion or fistulation of the conduit.40–47 Our study found that PNA returned a higher level of meaningful motor recovery when compared with the published literature for tube conduits, without their observed complications.
We demonstrate that PNAs were safe and provided functional motor recovery for mixed and motor nerve in short- and long-gap repairs. Outcomes are comparable to historical controls from available literature for nerve autograft and exceed that of nerve conduit. The overall success rate of motor recovery from this study supports the use of PNA as part of the treatment algorithm for mixed and motor nerve injuries. The RANGER registry is currently ongoing, and future reports using these data may provide additional clinical evidence for the expanding role of PNA in mixed and motor nerve repairs.
ACKNOWLEDGMENTS
The authors would like to acknowledge Donghang Zheng, MD, PhD, and Steven L. Bramer, PhD, of First-Stop Consulting for their contributions to data management and data analysis of the study.
Statement of Conformity: This investigation and the RANGER registry were performed in accordance with our institutional review boards and guidelines of the Helsinki Declaration of 1975.
Supplementary Material
Footnotes
Published online 13 March 2019.
Clinical Trial Registration Information: Trial registry name: Registry of Avance Nerve Graft Evaluating Utilization and Outcomes for the Reconstruction of Peripheral Nerve Discontinuities (RANGER). Identification Number: NCT01526681, http://clinicaltrials.gov/ct2/show/NCT01526681?term=axogen&rank=4.
Disclosure: Drs. Safa, Weber, Niacaris, and Buncke are research advisors for AxoGen Corporation. None of the other authors has any financial disclosures. The RANGER study is sponsored through a research grant to participating study centers by AxoGen Corporation.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerve. 19881st ed New York, NY: Thieme Medical Publishers. [Google Scholar]
- 2.Lundborg G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling. 20042nd ed Philadelphia, PA: Churchill Livingstone. [Google Scholar]
- 3.Berger A, Millesi H. Nerve grafting. Clin Orthop Relat Res. 1978;133:49–55. [PubMed] [Google Scholar]
- 4.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Han K, Tiel RL, et al. Surgical outcomes of 654 ulnar nerve lesions. J Neurosurg. 2003;98:993–1004. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Kam AC, Chandika P, et al. Surgical management and outcomes in patients with median nerve lesions. J Neurosurg. 2001;95:584–594. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Kam AC, Chandika P, et al. Surgical management and outcome in patients with radial nerve lesions. J Neurosurg. 2001;95:573–583. [DOI] [PubMed] [Google Scholar]
- 8.Frykman G, Gramyk K. Gelberman R. Results of nerve grafting. In: Operative Nerve Repair and Reconstruction. 1991:Philadelphia, PA: JB Lippincott; 553–568. [Google Scholar]
- 9.Vastamäki M, Kallio PK, Solonen KA. The results of secondary microsurgical repair of ulnar nerve injury. J Hand Surg Br. 1993;18:323–326. [DOI] [PubMed] [Google Scholar]
- 10.Ruijs AC, Jaquet JB, Kalmijn S, et al. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484–494; discussion 495. [DOI] [PubMed] [Google Scholar]
- 11.Kallio PK, Vastamäki M. An analysis of the results of late reconstruction of 132 median nerves. J Hand Surg Br. 1993;18:97–105. [DOI] [PubMed] [Google Scholar]
- 12.Ehretsman RL, Novak CB, Mackinnon SE. Subjective recovery of nerve graft donor site. Ann Plast Surg. 1999;43:606–612. [DOI] [PubMed] [Google Scholar]
- 13.IJpma FF, Nicolai JP, Meek MF. Sural nerve donor-site morbidity: thirty-four years of follow-up. Ann Plast Surg. 2006;57:391–395. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. [DOI] [PubMed] [Google Scholar]
- 15.Graham JB, Xue QS, Neubauer D, et al. A chondroitinase-treated, decellularized nerve allograft compares favorably to the cellular isograft in rat peripheral nerve repair. J Neurodegen and Regen. 2009;2:19–29. [Google Scholar]
- 16.Johnson PJ, Newton P, Hunter DA, et al. Nerve endoneurial microstructure facilitates uniform distribution of regenerative fibers: a post hoc comparison of midgraft nerve fiber densities. J Reconstr Microsurg. 2011;27:83–90. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore AM, MacEwan M, Santosa KB, et al. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang P, Kilic A, Konopka G, et al. Histologic and functional outcomes of nerve defects treated with acellular allograft versus cabled autograft in a rat model. Microsurgery. 2013;33:460–467. [DOI] [PubMed] [Google Scholar]
- 20.Zuniga JR. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft–a case series. J Oral Maxillofac Surg. 2015;73:734–744. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Chen G, Tian G, et al. Sensory recovery following decellularized nerve allograft transplantation for digital nerve repair. J Plast Surg Hand Surg. 2013;47:451–453. [DOI] [PubMed] [Google Scholar]
- 22.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taras JS, Amin N, Patel N, et al. Allograft reconstruction for digital nerve loss. J Hand Surg Am. 2013;38:1965–1971. [DOI] [PubMed] [Google Scholar]
- 24.Fleming ME, Bharmal H, Valerio I. Regenerative medicine applications in combat casualty care. Regen Med. 2014;9:179–190. [DOI] [PubMed] [Google Scholar]
- 25.Means KR, Jr, Rinker BD, Higgins JP, et al. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y). 2016;11:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza JM, Purnell CA, Cheesborough JE, et al. Successful treatment of foot and ankle neuroma pain with processed nerve allografts. Foot Ankle Int. 2016;37:1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaacs J, Safa B. A preliminary assessment of the utility of large-caliber processed nerve allografts for the repair of upper extremity nerve injuries. Hand (N Y). 2017;12:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. [DOI] [PubMed] [Google Scholar]
- 29.Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340–2349. [DOI] [PubMed] [Google Scholar]
- 30.Rinker BD, Ingari JV, Greenberg JA, et al. Outcomes of short-gap sensory nerve injuries reconstructed with processed nerve allografts from a multicenter registry study. J Reconstr Microsurg. 2015;31:384–390. [DOI] [PubMed] [Google Scholar]
- 31.Secer HI, Daneyemez M, Gonul E, et al. Surgical repair of ulnar nerve lesions caused by gunshot and shrapnel: results in 407 lesions. J Neurosurg. 2007;107:776–783. [DOI] [PubMed] [Google Scholar]
- 32.He B, Zhu Z, Zhu Q, et al. Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res. 2014;9:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brushart TM. Nerve Repair. 2011New York, NY: Oxford University Press, Inc. [Google Scholar]
- 34.Agnew SP, Dumanian GA. Technical use of synthetic conduits for nerve repair. J Hand Surg Am. 2010;35:838–841. [DOI] [PubMed] [Google Scholar]
- 35.Chiu DT. Autogenous venous nerve conduits. A review. Hand Clin. 1999;15:667–71, ix. [PubMed] [Google Scholar]
- 36.Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. [DOI] [PubMed] [Google Scholar]
- 37.Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg. 1990;6:117–121. [DOI] [PubMed] [Google Scholar]
- 38.Isaacs J. Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am. 2010;35:491–497; quiz 498. [DOI] [PubMed] [Google Scholar]
- 39.Boeckstyns ME, Sørensen AI, Viñeta JF, et al. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. J Hand Surg Am. 2013;38:2405–2411. [DOI] [PubMed] [Google Scholar]
- 40.Wangensteen KJ, Kalliainen LK. Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand (N Y). 2010;5:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundborg G, Rosén B, Dahlin L, et al. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg Br. 2004;29:100–107. [DOI] [PubMed] [Google Scholar]
- 42.Chiriac S, Facca S, Diaconu M, et al. Experience of using the bioresorbable copolyester poly(DL-lactide-ε-caprolactone) nerve conduit guide Neurolac™ for nerve repair in peripheral nerve defects: report on a series of 28 lesions. J Hand Surg Eur Vol. 2012;37:342–349. [DOI] [PubMed] [Google Scholar]
- 43.Navissano M, Malan F, Carnino R, et al. Neurotube for facial nerve repair. Microsurgery. 2005;25:268–271. [DOI] [PubMed] [Google Scholar]
- 44.Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am. 2005;30:513–518. [DOI] [PubMed] [Google Scholar]
- 45.Rinker B, Liau JY. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg Am. 2011;36:775–781. [DOI] [PubMed] [Google Scholar]
- 46.Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg Am. 2011;36:1441–1446. [DOI] [PubMed] [Google Scholar]
- 47.Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–1045; discussion 1046. [DOI] [PubMed] [Google Scholar]


