Abstract
Background:
The therapies for anterior chest wall keloids include surgical excision, postoperative radiotherapy, silicone taping stabilization, and steroid plaster. However, to date, there is no universally accepted combination treatment strategy for anterior chest wall keloids.
Methods:
All consecutive patients with single or multiple anterior chest wall keloids who underwent keloid excision, tension-reducing suturing, z-plasty, and postoperative radiotherapy in 2013–2016 in Nippon Medical School were included in this case series study. Only keloids that arose from small injuries such as folliculitis or acne were selected. The surgery was followed by tension-reducing self-management of the wounds with silicone tape and steroid plaster. The postsurgical radiotherapy modality was 18 Gy administered in 3 fractions over 3 days. The primary study outcome was keloid recurrence during the 24-month follow-up period. Recurrence was defined as the development of stiff and red lesions in even a small part of the scar that did not respond to 6 months of steroid plaster therapy.
Results:
In total, 141 patients with 141 lesions were enrolled. Of the 141 lesions, 15 (10.6%) recurred. All recurrences were successfully treated by steroid plaster and steroid injection. The recurrence patients did not differ from the nonrecurrence patients in terms of the size of the original keloid or gender distribution.
Conclusions:
Anterior chest wall keloids can be successfully treated by customized plans that involve appropriate surgical modalities (including z-plasty) followed by postoperative radiotherapy (18 Gy in 3 fractions over 3 days) and scar self-management with silicone tape and steroid plaster.
INTRODUCTION
Keloids are particularly prevalent on the anterior chest wall; our previous study showed that approximately 50% of all keloids develop on this part of the body.1 This is largely due to 2 reasons. First, folliculitis and acne, which are well known to trigger keloid development, are common on the anterior chest. Second, the anterior chest wall is a high-tension load area due to the frequent movements of the upper limbs by the pectoralis major muscle. These movements stretch the skin of the anterior chest wall horizontally. When this cyclical tension is imposed on anterior chest wounds, it exacerbates and prolongs the inflammation in the reticular dermis of the wound. The inability of wounds to progress through the inflammatory phase of wound healing in a timely manner is well known to be a cause of keloid development.2,3
The most widely used treatments for anterior chest wall keloids are surgical excision, postoperative radiotherapy, steroid injection, sheeting, pressure therapy, and laser therapy.4–6 However, although various treatment strategies for anterior chest wall keloids have been proposed, none have won widespread agreement or usage. We analyzed all small to moderately sized anterior chest wall keloid cases that were treated in our facility in 2013–2016, and show here that a combination of treatment strategy that we have developed is highly effective for these keloids.
METHODS
Ethics Statement
This case series study was performed after approval from the Ethics Committee of Nippon Medical School Hospital was obtained. The requirement to obtain patient consent was waived due to the retrospective nature of the study.
Patient Selection
All consecutive adult patients with anterior chest wall keloids who (1) underwent surgical treatment between 2013 and 2016 in the outpatient clinic of the keloid-/scar-specialist clinic in the Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School in Tokyo, Japan and (2) were followed up for at least 24 months were identified. All patients with single or multiple keloids that arose from folliculitis/acne, that could be treated by complete excision and z-plasty, and that were then treated with the postoperative radiation and postsurgical wound self-management protocol described below were selected from this group. Patients with keloids that were caused by major trauma or artificial injury, including thoracic surgery, were excluded. For the purposes of this study, keloid was defined as a continually growing elevated red scar, whereas hypertrophic scar was defined as a hard and mildly elevated but not continually growing scar. Patients with hypertrophic scars were excluded from the study. Patients with multiple keloids that were treated by conservative therapies, partial resection, or flap surgery were also excluded.
Surgical and Postoperative Radiation Treatment Protocol
All selected patients were treated with a treatment protocol that consisted of complete excision, z-plasty, postoperative adjuvant radiotherapy, and postsurgical wound self-management.
For the surgery, all patients were placed under general anesthesia. To reduce the risk of recurrence, subcutaneous/fascial tensile reduction sutures (Fig. 1) and z-plasties were employed. Thus, the keloid(s) were completely excised along with a minimal normal skin margin and all fatty tissues under the keloid. As a result, all tissues above the deep fascia of the pectoralis major muscle or periosteum of the sternum were removed. The wound edges were then undermined under the deep fascia. Subsequently, each deep fascia was sutured using 0 polydioxanone sutures (PDSII; Ethicon, Inc., Somerville, N.J.). The fibrous membrane in the fatty tissues, namely, the superficial fascia, was then sutured using 2-0 and 3-0 PDSII. This suturing protocol places minimal tension on the dermis and elevates the wound edges smoothly, thereby allowing the wound edges to attach naturally to each other.
Fig. 1.

Schematic depiction of the multiple layers of sutures used to close the wound after keloid excision. Because keloids develop from the reticular dermis, it is important to release the tension on the dermis after keloid excision. This can be largely achieved by applying deep sutures. Dermal sutures themselves do not reduce dermal tension. A, The red lines indicate the excision and undermine lines. B, The view after the fat tissues under the keloid were removed and the tissue above the muscles was undermined. C, The view after suturing the deep and superficial fascias, the dermis, and the superficial layer.
Z-plasties were then designed. The sides of each triangular flap ranged from 7 to 10 mm in length, and the pitch between each z-plasty was 2–4 cm depending on the total length of the wound. In our experience, this pitch yields the most satisfactory results (personal observations). After confirming that the triangular flaps were fully elevated and could be transposed with each other, dermal sutures using 4-0 PDSII were started. This was followed by superficial sutures with 6-0 polypropylene (Proline; Ethicon, Inc., Somerville, N.J.).
All patients underwent postoperative radiotherapy with a 4-MeV electron beam. A total radiation dose of 18 Gy were delivered in 3 fractions over 3 consecutive days. In general, surgery was performed on a Wednesday or Friday. When the operation was on Wednesday, the radiation was started on the next day, namely, on the following Thursday; the second and third treatments were conducted on Friday and Monday, respectively. When the operation was on Friday, the radiation was delivered on the following Monday, Tuesday, and Wednesday.
Depending on the patient’s schedule, the sutures were removed between postoperative days 7 and 14. After the sutures were removed, all patients were asked to employ round-the-clock taping fixation with silicone tape (Mepitac; Mölnlycke Health Care, Gothenburg, Sweden) for more than 6 months. It was explained to the patient that this approach would prevent external mechanical forces from being placed on the wound, thereby reducing the risk of the keloid recurring. The patients were asked to keep each tape on, including during bathing or showering, until it detached naturally from the wound. The patients were then required to attach a new tape.
Patient Follow-up and Additional Therapies
All patients were followed up for more than 24 months via visits to the outpatient clinic at 3, 6, and every 3–6 postoperative months thereafter. If even a small part of the postoperative scar exhibited stiffness with redness, the silicone tape was replaced with steroid plaster (Eclar Plaster; Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan) to reduce the inflammation.7 In this case, the patient was asked to change the plaster every day. At the next visit 3–6 months later, the steroid plaster was stopped if the stiffness and redness had disappeared. It was then replaced with heparinoid ointment (Hirudoid Soft Ointment; Maruho Co., Inc., Osaka, Japan) to keep the scar surface moist.
If, however, the stiffness with redness continued for 6 months despite the steroid plaster treatment, the lesion was deemed to have recurred. In this case, the steroid plaster was continued and steroid injection was added. This was true even if only a small part of the scar was refractory. The injection consisted of 5–10 mg of triamcinolone acetonide (Kenacort; Bristol-Myers Squibb K.K., Tokyo, Japan) that was diluted with 1% lidocaine to generate a 2–3 ml of solution. The injection was conducted with a 30G needle.
Primary Study Outcome and Other Variables
The primary outcome variable was recurrence in the 24-month follow-up period. Recurrence was defined as stiffness with redness in even a small part of the postoperative scar that did not respond to 6 months of steroid tape treatment. Other variables that were collected from the medical records of each patient were the size of the original keloid, whether the patient had postoperative complications such as wound dehiscence, pigmentation, depigmentation, and telangiectasia, whether steroid plaster was used in addition to silicone tape, how long the silicone tape and steroid plaster were used, how many steroid injections were given before scar maturation occurred, when the lesion recurred, and when the postoperative scars became mature scars (defined as scars without any redness).
Statistics
The variables were expressed as means or percentage. Groups were compared using Student’s t test or Chi-square test, as appropriate. All statistical analyses were performed using Microsoft Excel 97−2003 (Microsoft, Redmond, Wash.) and SPSS statistical software (SPSS, Chicago, Ill.). P values were considered to indicate statistically significant differences if they were <0.05.
RESULTS
The study included 141 lesions on 141 consecutive patients. The patients were on average 42.0 years old, and 62 were male and 79 were female. Of the 141 lesions, 82 (58.2%) and 59 (41.8%) were ≤10 and >10 cm in diameter, respectively.
Of the 141 postoperative scars, 46 (32.6%) exhibited stiffness for 3 months after surgery. In all cases, the silicone tape therapy was replaced with steroid plaster therapy. In 31 cases (67.4% of the 46 cases), the stiffness with redness disappeared completely within 3 months of starting steroid plaster therapy. The steroid plaster was then stopped. The remaining 15 cases (10.6% of the 141 lesions) were considered to be keloid recurrences, although the stiffness and redness only affected a small part of the postoperative scar. The average timepoint of steroid tape-resistant recurrence was 8.4 (range, 6–14) months after surgery. Of the 15 patients with recurrences, 8 and 7 were male and female, respectively. The recurrence and nonrecurrence patients did not differ significantly in terms of gender distribution (53.3% versus 38.3% male, P > 0.05). The 2 groups also did not differ significantly in terms of the size of the preoperative keloid (9.5 versus 8.9 cm, P > 0.05).
The 15 recurrent lesions were treated with steroid injection every 2–3 months. The daily steroid plaster application was also continued. On average, the 15 recurrence patients received a total of 3.8 steroid injections. All 15 lesions became mature scars (ie, scars without redness) but required longer to get to that point (33.8 months) than the scars of the nonrecurrence cases (26.8 months).
In terms of postoperative complications, 2 of the 141 postoperative scars (1.1%) exhibited temporary pigmentation on the irradiated area 3 months after surgery. The pigmentation improved spontaneously over the next 12 months. One case (0.5%) exhibited telangiectasia 24 months after surgery. It was treated by long-pulsed Nd:YAG laser. The radiotherapy did not cause depigmentation or wound dehiscence. All mature scars were soft and white (Figs. 2–4). The width of the postoperative scars ranged from 1 to 3 mm. The scars tended to be wider on the edge of the z-plasty.
Fig. 2.
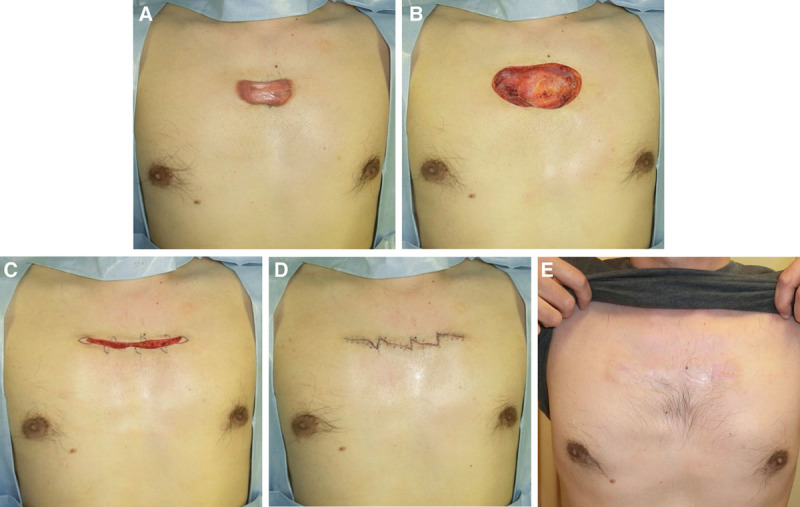
The case of a 39-year-old man with an anterior chest wall keloid. A, Preoperative view. B, Immediately after excision. C, Immediately after deep and superficial fascial suturing and the design of the z-plasty. D, Immediately after the operation. E, Two years after the operation.
Fig. 4.
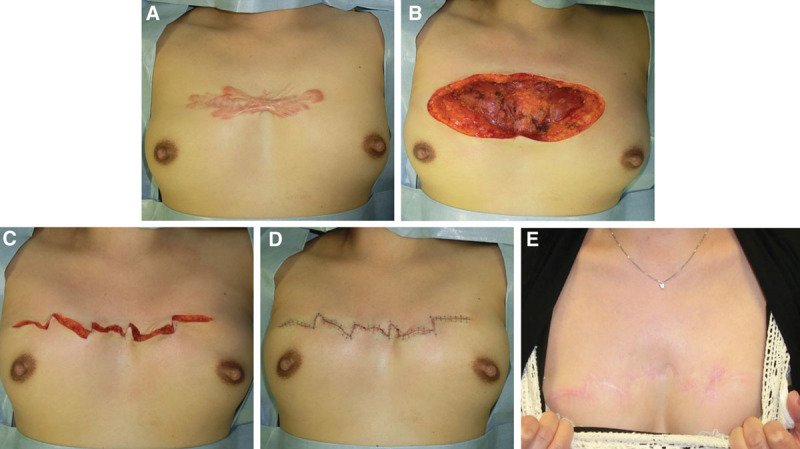
The case of a 42-year-old woman with an anterior chest wall keloid. A, Preoperative view. B, Immediately after excision. C, Immediately after deep and superficial fascial suturing and the design of the z-plasties. D, Immediately after the operation. E, Two years after the operation.
Fig. 3.
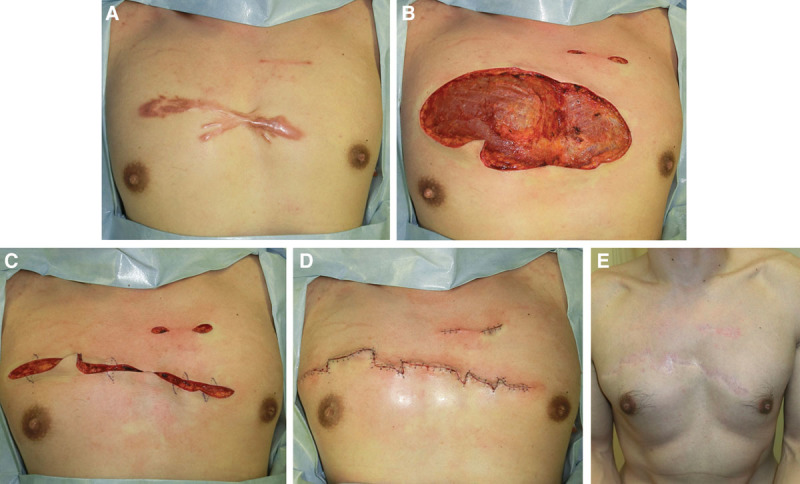
The case of a 41-year-old man with 1 large and several small anterior chest wall keloids. A, Preoperative view. B, Immediately after excision. C, Immediately after deep and superficial fascial suturing and the design of the z-plasties. D, Immediately after the operation. E, Two years after the operation.
DISCUSSION
Our keloid therapy protocol presently consists of excision with tension-reducing suturing, z-plasty, postoperative radiation therapy, and postoperative wound self-care. The present study of 141 cases of small to moderately sized anterior chest wall keloids showed that this protocol had excellent outcomes: only 10.6% of the cases recurred. Moreover, our salvage therapy with steroid tape and injections resulted in the complete resolution of even these recurrences. These good outcomes are encouraging given the well-known intractability of keloids to surgery: simple surgical excision of keloid associates with high recurrence rates that range from 45% to 100%.4
Keloid Etiology and Pathomechanisms
Keloids are driven by genetic, environmental, systemic, and local factors. The genetic susceptibility factors include single nucleotide polymorphisms.8,9 Moreover, patients with multiple keloids tend to have a family history10 and several genetic factors related to familial keloid have been reported.11 In addition, keloids rarely occur in people with Down’s syndrome.12 Keloids also exhibit geographic variation that may relate to population genetics and/or environmental factors: their incidence ranges from 0.09% in the United Kingdom to 16% in Congo,13 and darker-skinned populations have 2- to 19-fold higher incidences of keloid than lighter-skinned populations.14
Although many keloids arise from large skin injuries such as those induced by trauma, burn, and surgical incision, they can also be triggered by minor inflammatory skin injuries such as acne and folliculitis. In the present study, we focused exclusively on keloids that arose on the anterior chest from these small injuries. We reported previously that half of these simple keloids occur on the anterior chest wall area.1
Keloids (and hypertrophic scars) are the result of chronic inflammation in the reticular dermis. This chronic inflammation is largely driven by mechanical tension on the wound/scar edges, although it can also be worsened by systemic factors such as hypertension,16 pregnancy (sex hormones),17 and conditions that associate with high circulating levels of inflammatory cytokines such as interleukin-6.18 In particular, the cyclical skin stretching that occurs during daily bodily movements is a strong keloidogenic factor.2,3 The anterior chest skin is especially prone to such mechanical stresses given its constant motion due to arm movements. Moreover, since breathing cyclically stretches the chest wall, it may also promote keloidogenesis, albeit not as strongly as the arm movements. These stresses cause keloids to grow along the lines of predominant skin tension. This explains why keloids on specific body areas adopt characteristic shapes, including the crab claw shape on the anterior chest wall.15
Intraoperative and Postoperative Methods That Reduce Mechanical Tension and Keloid Recurrence
The observations above suggest that keloid therapeutic protocols should comprise multiple methods that together reduce the local mechanics and systemic factors that drive the development and progression of keloids. Specifically, when keloids are removed surgically, it is important to minimize the tension on the reticular dermis because that is where the chronic inflammation evolves. This can be achieved by using tension-reducing sutures and z-plasties intraoperatively and by stabilizing the scar with taping fixation. Moreover, any reinflammation can be quelled by applying postoperative radiation and steroid therapy.
The tension-reducing sutures involve multiple layers of sutures, starting with the strong suturing together of first the deep fascia and then the superficial fascia. The latter sutures cause the wound edges to attach naturally to each other. Our previous study showed that deep fascia suturing reduced about 90% of the tension on the wound edge, whereas superficial fascia suturing reduced the remaining 10% (Fig. 5).19 It is very important to realize that dermal sutures on their own cannot release the tension on the dermis: only deep sutures can achieve this. Moreover, it is essential that we confirm the wound edges are naturally attached to each other before starting the dermal sutures. We believe that this concept is the key to prevent the formation of pathological scars after surgery.
Fig. 5.
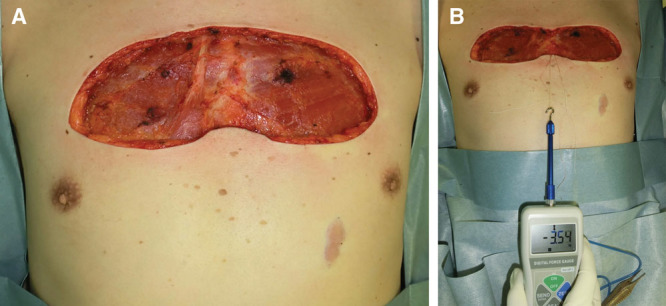
Measurement of the ability of deep and superficial fascial sutures of the pectoralis major muscle to reduce the tension on the dermis after excision of an anterior chest wall keloid. The tension needed to close the wound after keloids were excised from the anterior chest wall was measured before the deep fascial sutures were placed (A), and before the superficial fascial sutures were placed (B).
An intraoperative approach that also reduces dermal tension on the horizontal plane is to use z-plasties. The first z-plasty was performed by Horner in 1837.20 One of the primary goals of z-plasty is to interrupt the tension lines on a scar.21–23 Notably, the present study showed that the size of the keloid did not influence the recurrence rate. Thus, our protocol is suitable for small to moderately sized keloids. To ensure tensile reduction after excision of larger keloids, it may be necessary to combine fascial suturing with multiple z-plasties. The usefulness of z-plasties combined with 3-layer tension-reducing sutures and postoperative radiation is supported by our previous study; we reported that when only 3-layer sutures and postoperative radiation therapy were used for simple chest keloids, the recurrence rate was 14.3%.24,25 Thus, adding z-plasty to this protocol improved the therapeutic outcome. It should be noted that z-plasties can only be used with keloids whose size and shape allow primary closure with z-plasty. Other keloids will require flap surgery.
A key element of our keloid treatment protocol was the use of postoperative radiation therapy. Although these therapies are currently routinely used to treat malignant disease, postoperative external radiotherapy of scars also has a century-long history.26 Many studies have shown that ionizing irradiation can inhibit keloid fibroblast proliferation and induce their senescence.27 Indeed, keloids have been treated with x-rays (superficial or orthovoltage), β-rays (electron beams, 32P, or 90Sr/90Y), and γ-rays (60Co or 192Ir) with teletherapy or brachytherapy modalities (contact, high dose rate superficial, or low dose rate superficial).26 Radiotherapy combined with surgical removal is more effective for keloids than either monotherapy.28 However, moderately high doses are needed for keloids, regardless of the keloid site.29 A large variety of radiation techniques have been devised to treat keloids. They vary in terms of the radiation source, total dose, fractionation, and schedule. Each keloid treatment facility has chosen a particular technique that in their judgment best reduces keloid recurrence while avoiding radiation-induced complications such as malignancies or pigmentation.
In our department, we started performing radiation therapy in anterior chest wall keloid cases in 2002. The regimen consisted of 15 Gy/3 fractions/3 days.24 In 2007, we changed this regimen to 20 Gy/4 fractions/4 days.25 This change improved the recurrence rate from 43.1% to 14.3%.24,25 We then found that fine-tuning the radiation therapy regimen according to body region could further limit the radiation dose without affecting the recurrence rate. Therefore, we presently employ the following postoperative radiotherapy modality for keloids on the anterior chest: a 4-MeV electron beam at a total dose of 18 Gy in 3 fractions for 3 consecutive days, starting on postoperative day 1. Full shielding to protect the normal tissues is routinely employed.30
At present, there is no widely accepted radiation regimen for keloid treatment. Guidelines, like those that have been developed for cancer, are needed. The German Cooperative Group on Radiotherapy for Benign Disease recently proposed a possible national consensus guideline on the basis of the available evidence, namely, low-energy X-rays (150–200 kV) or electrons (4–10 MeV) or brachytherapy applied immediately after excision in single 2–5 Gy doses or a total dose of 16–20 Gy given in 4–5 fractions/wk.31 Our anterior chest keloid wall radiation therapy protocol adheres to this proposed guideline. However, more studies that identify the optimal timing and dose of adjuvant radiation therapy after keloid excision surgery are warranted.
Despite the careful use of tension-reducing operative strategies and radiation, the resulting wounds are highly likely to be subject to the same local cyclical mechanical stimulation that drove the development of the original keloid. Since this stimulation can trigger keloid recurrence, it is essential to both limit it and extinguish any inflammation that is provoked. Consequently, surgery and radiation therapy must be combined with scar self-management, namely, the routine application of silicone tape and, if necessary, steroid plaster. Steroid plaster is an effective way to treat keloids.7 In the United States, a preparation containing 4 μg/cm2 flurandrenolide (a medium strength steroid) is available. In the United Kingdom, the commercially available formulation comprises a tape impregnated with 4 μg/cm2 fludroxycortide (a medium strength steroid). In Japan, 2 steroid tape formulations are available: a 4-μg/cm2 fludroxycortide type and a 20-μg/cm2 deprodone propionate plaster (a higher potency steroid). In our experience, the deprodone propionate plaster most effectively treats and prevents keloids.7 Another postoperative treatment option could be to inject other drugs such as 5-fluorouracil and immunosuppressants, which have been reported to be effective for keloids.5 However, the fact that deprodone propionate plaster is both highly effective and can be easily applied by the patients themselves for long periods after surgery makes it a particularly valuable adjunct in our protocol.
CONCLUSIONS
There is currently no universally accepted combination treatment algorithm for anterior chest wall keloids. However, the present study shows that our combination treatment successfully treated simple keloids on the anterior chest wall. Consequently, we recommend surgical removal followed by postsurgical radiation therapy consisting of 18 Gy administered in 3 fractions over 3 days and postsurgical self-management of scars with silicone tape and steroid plaster. The safety of our radiation therapy regimen will be carefully assessed by close monitoring of our large and growing case series.
Footnotes
Published online 13 March 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157. [DOI] [PubMed] [Google Scholar]
- 2.Harn HI, Ogawa R, Hsu CK, et al. The tension biology of wound healing. Exp Dermatol. In press. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;Mar. 18(3):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustoe TA, Cooter RD, Gold MH, et al. ; International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. [DOI] [PubMed] [Google Scholar]
- 6.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67:1017–1025. [DOI] [PubMed] [Google Scholar]
- 7.Goutos I, Ogawa R. Steroid tape: a promising adjunct to scar management. Scars Burn Heal. 2017;3:2059513117690937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima M, Chung S, Takahashi A, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42:768–771. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa R, Watanabe A, Than Naing B, et al. Associations between keloid severity and single-nucleotide polymorphisms: importance of rs8032158 as a biomarker of keloid severity. J Invest Dermatol. 2014;134:2041–2043. [DOI] [PubMed] [Google Scholar]
- 10.John C, Murray MD. Keloids and hypertrophic scars. Clin Dermatol. 1994;12:27–37. [DOI] [PubMed] [Google Scholar]
- 11.Marneros AG, Norris JE, Olsen BR, et al. Clinical genetics of familial keloids. Arch Dermatol. 2001;137:1429–1434. [DOI] [PubMed] [Google Scholar]
- 12.Baek KH, Zaslavsky A, Lynch RC, et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark JA, Turner ML, Howard L, et al. Description of familial keloids in five pedigrees: evidence for autosomal dominant inheritance and phenotypic heterogeneity. BMC Dermatol. 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly AP. Keloids. Dermatol Clin. 1988;6:413–424. [PubMed] [Google Scholar]
- 15.Akaishi S, Akimoto M, Ogawa R, et al. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445–451. [DOI] [PubMed] [Google Scholar]
- 16.Arima J, Huang C, Rosner B, et al. Hypertension: a systemic key to understanding local keloid severity. Wound Repair Regen. 2015;23:213–221. [DOI] [PubMed] [Google Scholar]
- 17.Park TH, Chang CH. Keloid recurrence in pregnancy. Aesthetic Plast Surg. 2012;36:1271–1272. [DOI] [PubMed] [Google Scholar]
- 18.Quong WL, Kozai Y, Ogawa R. A case of keloids complicated by Castleman’s disease: interleukin-6 as a keloid risk factor. Plast Reconstr Surg Glob Open. 2017;5:e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa R, Capek L, Akaishi S, et al. Measurement of tension-reduction effect on dermis after deep fascia suture of pectoralis major muscle in the surgery of anterior chest wall keloid. Scar Management. 2015;9:65–68. [Google Scholar]
- 20.Borges AF, Gibson T. The original Z-plasty. Br J Plast Surg. 1973;26:237–246. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto EA, Liang H, Mahadevan L. Topology, geometry, and mechanics of Z-plasty. Phys Rev Lett. 2018;120:068101. [DOI] [PubMed] [Google Scholar]
- 22.Hudson DA. Some thoughts on choosing a Z-plasty: the Z made simple. Plast Reconstr Surg. 2000;106:665–671. [DOI] [PubMed] [Google Scholar]
- 23.Ellur S. A mathematical model to predict the requirement for multiple Z plasties. Indian J Plast Surg. 2008;41:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–53; discussion 554. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59:688–691. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201. [DOI] [PubMed] [Google Scholar]
- 27.Ji J, Tian Y, Zhu YQ, et al. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol. 2015;42:56–63. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsson F. The treatment of keloids at Radium-hemmet, 1921-1941. Acta Radiol. 1948;29:251–267. [DOI] [PubMed] [Google Scholar]
- 29.Renz P, Hasan S, Gresswell S, et al. Dose effect in adjuvant radiation therapy for the treatment of resected keloids. Int J Radiat Oncol Biol Phys. 2018;102:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch. 2016;83:46–53. [DOI] [PubMed] [Google Scholar]
- 31.Seegenschmiedt MH, Micke O, Muecke R; German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD). Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br J Radiol. 2015;88:20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]


