Abstract
Although scaled-up interventions and effective control efforts have drastically reduced malaria morbidity and mortality, malaria remains a serious threat to public health worldwide. Anopheles sinensis Wiedemann 1828 is a historically important vector of Plasmodium vivax (Haemosporida: Plasmodiidae) malaria in China. Insecticide resistance has become a major obstacle to vector-borne disease control. However, little is known about the insecticide resistance of An. sinensis in Wenzhou, an important coastal port city in Zhejiang province, China. The aim of this study was to examine insecticide resistance and mechanisms in An. sinensis field mosquito populations. Evidence of multiple insecticide resistance was found in An. sinensis adult female populations. Medium to high frequencies of target site kdr together with fixed ace-1 mutations was detected in both the Ruian and Yongjia populations. Both populations showed an association between kdr L1014 mutation and resistance phenotype when tested against deltamethrin and DDT. Significantly different metabolic enzyme activities were found between the susceptible laboratory strain and field-collected mosquitoes from both Ruian and Yongjia. Both field collected An. sinensis populations exhibited significantly higher P450 enzyme activity compared with the laboratory strain, while the field-collected resistant mosquitoes exhibited various GST and COE enzyme activities. These results indicate multiple resistance mechanisms in An. sinensis field populations. Effective implementation of insecticide resistance management strategies is urgently needed. The data collected in this study will be valuable for modeling insecticide resistance spread and vector-control interventions.
Keywords: Anopheles sinensis, insecticide resistance, kdr mutation, ace-1 mutation, metabolic detoxification enzyme
Although scaled-up interventions and control efforts have greatly reduced malaria morbidity and mortality worldwide, malaria remains a serious threat to public health (Lu et al. 2014). In 2017, there were approximately 219 million new cases of malaria infections and 435,000 malaria deaths worldwide, of which 92% of cases were in Africa, followed by 7% in Southeast Asia and 2% in the Eastern Mediterranean Region (WHO 2018). According to the latest report, 258 malaria cases and 2 deaths were documented from Wenzhou, Zhejiang province, China during 2007–2014, including 148 Plasmodium vivax (Haemosporida: Plasmodiidae) cases, 106 P. falciparum (Haemosporida: Plasmodiidae) cases, and 4 P. ovale (Haemosporida: Plasmodiidae) cases (Ni et al. 2015).
In the past decades, prevention and control of vector-borne diseases have relied largely on vector control using insecticides (Committee for the Study on Malaria Prevention and Control 1991). There are four major classes of insecticides available for malaria vector control: pyrethroids, organochlorines, carbamates, and organophosphates. Pyrethroids are the only insecticide class currently recommended by the WHO for use on long-lasting insecticidal nets due to their relatively low mammalian toxicity and fast biodegradation. However, extensive use of chemical insecticides has resulted in resistance problems in disease vectors, including malaria vector Anopheles mosquitoes (Rivero et al. 2010). Numerous investigations have reported insecticide resistance in most malaria vectors, including the major African malaria vectors Anopheles gambiae Giles (Diptera: Culicidae) (Wanjala et al. 2015, Antonio-Nkondjio et al. 2016, Chabi et al. 2016, Churcher et al. 2016, Dery et al. 2016, Hakizimana et al. 2016, Ondeto et al. 2017, Philbert et al. 2017), An. arabiensis Patton (Diptera: Culicidae) (Ochomo et al. 2015, Mzilahowa et al. 2016, Randriamaherijaona et al. 2016, Alemayehu et al. 2017, Mbepera et al. 2017), and An. funestus Giles (Diptera: Culicidae) (Djouaka et al. 2016a,b; Menze et al. 2016; Mzilahowa et al. 2016; Riveron et al. 2016; Samb et al. 2016; Barnes et al. 2017; Irving and Wondji 2017); and the major Southeast Asian malaria vectors An. sinensis Wiedemann 1828 (Diptera: Culicidae) (Wang 1999, 2000; Cui et al. 2006; Zhong et al. 2013; Chang et al. 2014; Qin et al. 2014; Dai et al. 2015; Zhang et al. 2015; Sun et al. 2017) and An. minimus Theobald (Diptera: Culicidae) (Verhaeghen et al. 2009, Dash et al. 2012, Pemo et al. 2012, Dev and Manguin 2016, Chaumeau et al. 2017, Marcombe et al. 2017). These studies have shown different resistance levels to multiple insecticides with complex resistance mechanisms. Monitoring and surveillance for insecticide resistance would provide baseline information essential for resistance management.
Two main mechanisms have been documented for insecticide resistance in malaria vectors: 1) target-site resistance mutations in knockdown resistance (kdr) at codon 1014 of the para-type sodium channel gene and at codon 119 of the acetylcholinesterase (ace-1) gene; and 2) metabolic detoxification resistance mechanisms conferred by cytochrome P450 monooxygenases (P450s), glutathione S-transferases (GSTs), and carboxylesterases (COEs) (Martinez-Torres et al. 1998, Zhong et al. 2013, Ibrahim et al. 2016, Menze et al. 2016, Safi et al. 2017). Other resistance mechanisms have also been reported, such as physiological resistance and behavioral resistance (Chareonviriyaphap et al. 2013, Yahouedo et al. 2017, Zalucki and Furlong 2017). Anopheles sinensis is a historically important vector of Plasmodium vivax malaria in China (Pan et al. 2012, Zhu et al. 2013, Zhang et al. 2016, Feng et al. 2017). Extensive and multiple insecticide resistance has been reported in An. sinensis mosquitoes from central China (Zhong et al. 2013, Chang et al. 2014, Zhang et al. 2015, Chang et al. 2016) and from other regions in China (Zhong et al. 2013, Qin et al. 2014, Dai et al. 2015, Feng et al. 2015, Sun et al. 2017). In Zhejiang province, however, there are only a few older reports of insecticide resistance in An. sinensis larvae (Wang 1999, 2000; Cui et al. 2006). Resistance to pyrethroids was first reported in Cangnan population, in which larval mortality of 61.2% for deltamethrin and 64.9% for permethrin were observed when comparing to mortality in susceptible laboratory strains of larvae, and adult mortality of 58.1% for 0.01% deltamethrin (Wang 1999). A significantly higher median lethal concentration (LC50) of deltamethrin was also found in other five natural larval populations, including the Wenzhou population, which had a resistance ratio (RR50) of 11 relative to its susceptible strain (Wang 2000). The current status of insecticide resistance and mechanisms in An. sinensis adult mosquitoes in Zhejiang province is largely unclear.
The aim of this study was to examine the current status of insecticide resistance and mechanisms in field-collected adult An. sinensis mosquitoes from Wenzhou, the most populous city in Zhejiang province, China. Anopheles sinensis mosquitoes were collected from Yongjia and Ruian counties of Wenzhou city. Insecticide susceptibility tests were performed on adult female mosquitoes to determine resistance status. Target site mutations (kdr and ace-1) and metabolic detoxification enzyme (P450, GST, and COE) activities were examined to determine resistance mechanisms.
Materials and Methods
Mosquito Collection
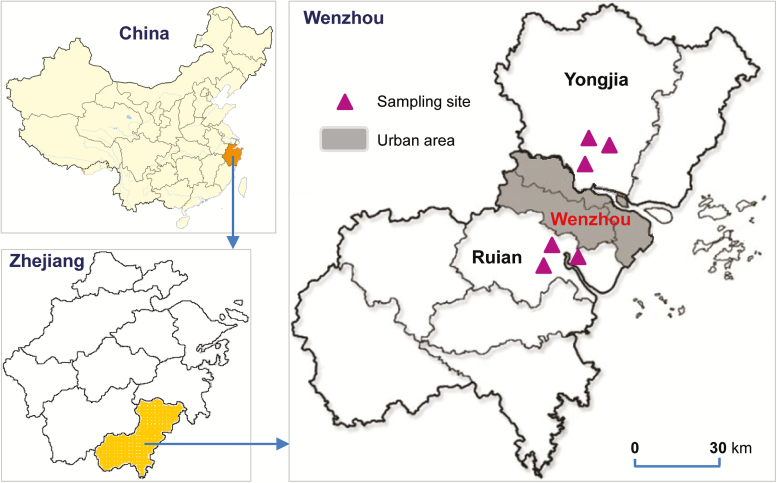
Anopheles sinensis mosquito larvae and pupae were collected from rice fields in Yongjia (28°6′S; 120°40′E) and Ruian (27°49′S; 120°35′E) counties of Wenzhou city, Zhejiang province, from July to August 2015 (Fig. 1). Wenzhou is a city on China’s east coast in Zhejiang province, which is adjacent to Anhui Province, a malaria endemic region. Wenzhou is an important port and industrial city surrounded by mountains, the East China Sea, and 436 islands. As the most populous city in Zhejiang province, Wenzhou has a population of 9,122,100, of which 31.16% are migrant workers (Wikipedia contributors 2017). Organochlorine pesticides (DDT) were used intensively in agriculture at these sites from 1950 to 1980 (Voldner and Li 1995). Organophosphates have been used for malaria vector control since the mid-1960s. Since the 1980s, pyrethroid insecticides have been used for agricultural pest control and malaria vector control, resulting in a sharp decline in malaria cases in these areas (Wang 2000). In each locality, at least 100 aquatic habitats in three villages were randomly selected for larvae and pupae collection in order to minimize the sampling of siblings. The number of larvae and pupae sampled in each habitat was no more than 10 individuals using a standard 350-ml capacity mosquito dipper. Field-collected larvae and pupae were transported to the insectary at Wenzhou Medical University in a large plastic container. Larvae and pupae were reared to adults under standard insectary conditions at 27 ± 1°C, approximately 80% relative humidity, and a photoperiod of 12:12 (L:D) h. The emerged female adults were provided with cotton balls soaked in 8% glucose solution. Adult Anopheles mosquitoes were morphologically identified using previously published keys (Dong 2010).
Fig. 1.
Map of sampling sites in Ruian and Yongjia counties of Wenzhou city in Zhejiang province, China. The map was created using the R package ‘maptools,’ version: 0.9–2, URL: http://r-forge.r-project.org/projects/maptools/
Insecticide Susceptibility Test and Biochemical Assay
Following the standard WHO insecticide susceptibility tube-test procedures (WHO 2016), 3–5 d postemergence, nonblood fed, female mosquitoes were used to conduct bioassays to determine susceptibility to three classes of insecticides: pyrethroids-deltamethrin (0.05%), organochlorine-DDT (4.0%), and organophosphate-malathion (5.0%). The control test papers were treated with silicone oil, risella oil, and olive oil, respectively. For each insecticide, a total of 100–150 female mosquitoes were tested, with 20–25 mosquitoes per tube and two control tubes without insecticide. All the live mosquitoes (including the control survivors exposed to no insecticide control papers) after bioassays were stored at −80C for biochemical assays and DNA extraction following our previous study method (Qin et al. 2014). The dead mosquitoes exposed to different insecticides were kept individually at −20°C for further molecular analysis. Enzyme activities of cytochrome P450 monooxygenases (P450s), glutathione S-transferases (GSTs), and carboxylesterases (COEs) were measured in live mosquitoes after bioassays using the mosquito body with one leg removed according to the published methods (Zhong et al. 2013, Chang et al. 2014, Qin et al. 2014). For each insecticide, a subset of 20 individuals from the survivors after exposure to insecticide and no-insecticide control mosquitoes were examined in duplicate for the activity of each metabolic detoxification enzyme. An An. sinensis susceptible laboratory strain (Chang et al. 2014), maintained for more than 10 yr with no insecticide exposure, was used as a control for comparison.
Molecular Assays for kdr and ace-1 Mutations
DNA was extracted from one leg of each mosquito using the EZNA MicroElute Genomic DNA Kit (Promega, Madison, WI). Molecular species identification for An. sinensis confirmation was performed by rDNA PCR following the method of Joshi et al. (2010). To determine point mutations of the kdr gene at codon 1014, a 325-bp fragment was amplified in An. sinensis using forward primer TGCCACTCCGTGTGTTTAGA and reverse primer GAGCGATGATGATCCGAAAT, following the methods previously described by Zhong et al. (2013). The PCR product was directly sequenced from both ends using the same PCR primers by the Life Genetic Service Facility (Invitrogen, Shanghai, China). Genotyping of point mutations of the ace-1 gene at codon 119 was performed by PCR-RFLP as described previously (Qin et al. 2014). A subset of 20 genotyped samples were confirmed by PCR and DNA sequencing using forward primer TAGGTCACGGTGAGTCCGTA and reverse primer ACCACGATCACGTTCTCCTC, following the methods previously described by Chang et al. (2014). In total, 240 An. sinensis mosquitoes were sequenced for kdr mutations and genotyped for ace-1 mutations.
Statistical Analysis
Mortality rates of adult female mosquitoes were calculated after bioassay for each insecticide and population. Abbott’s formula was used to correct mortality rates if mortality in the control tube was between 5 and 20% (Abbott 1925). If mortality in the control tube was more than 20%, the test data were discarded. Mosquito resistance status was determined in accordance with current WHO guidelines (WHO 2016): resistant if mortality is <90%, probably resistant if mortality is between 90 and 98% (requires additional bioassay or molecular assay for known resistance), and susceptible if mortality is between 98 and 100%. Analysis of variance (ANOVA) was performed to determine among-population differences in mosquito mortality rates and metabolic enzyme activity. Comparisons of enzyme activity between the susceptible laboratory strain and field-collected resistant and field-collected unexposed mosquitoes in each population were conducted by the nonparametric Wilcoxon–Mann–Whitney U test using JMP version 12.2.0. Chi-square (χ2) tests and odds ratios were used to analyze the association between target site mutations and the resistance phenotype.
Results
Insecticide Susceptibility Bioassays and Molecular Identification of Species
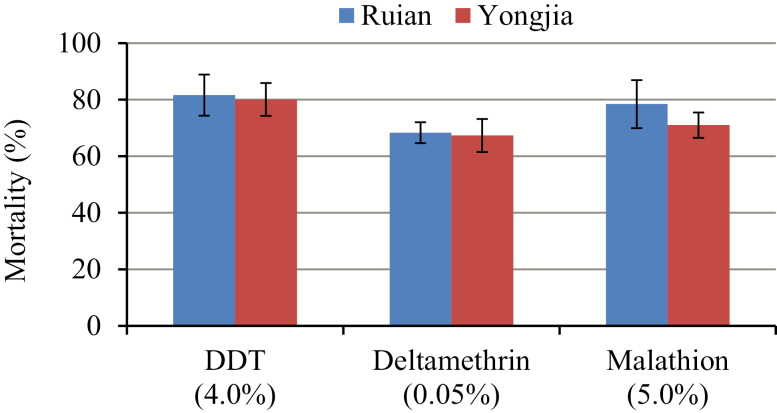
Approximately 3,000 Anopheles mosquito larvae and pupae were collected from the two study sites (Fig. 1). Higher larvae density was found in Ruian county compared with Yongjia county. In total, 334 female adult mosquitoes from Ruian and 342 from Yongjia were bioassayed against three insecticides. All of the Anopheles mosquitoes were identified as An. sinensis by species-specific PCR. Mortality rates of An. sinensis mosquitoes from Ruian and Yongjia were 81.6 and 80.1%, respectively, when tested against DDT, 68.3 and 67.4% against deltamethrin, and 78.4 and 71.0% against malathion (Fig. 2). These results suggest that for both study sites, mosquito populations were resistant to all the tested insecticides. No significant difference in mortality was found between the two study sites when tested against any of the three insecticides.
Fig. 2.
Mortality rates of multiple insecticides in An. sinensis adult mosquitoes from Wenzhou city in Zhejiang province, China.
Metabolic Enzyme Activity Assays and Association with Resistance
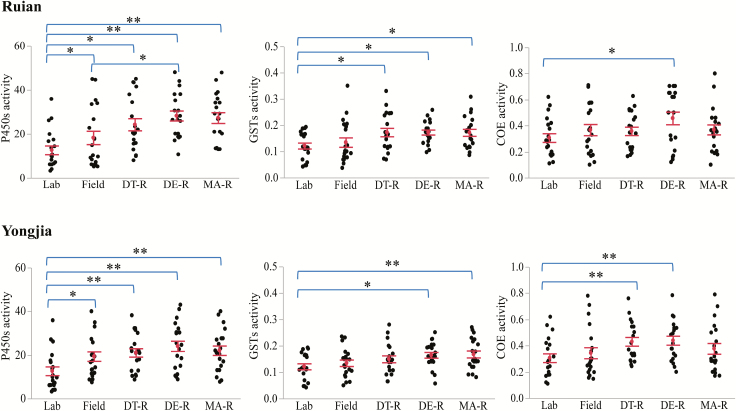
To examine the role of metabolic enzyme activity in insecticide resistance, field-collected resistant mosquitoes were compared with the susceptible laboratory strain without blood feeding, without exposure to insecticide, and at the similar age (WHO 2012, 2016). To determine the differences in constitutive enzyme activity between resistant and susceptible mosquitoes, the enzyme activity in field-collected insecticide-unexposed mosquitoes was also included for comparison (WHO 2016). Significantly different expression of metabolic enzyme activities was found between the susceptible laboratory strain and field-collected mosquitoes from both Ruian (P450s: F4, 95 = 6.81, P < 0.001; GSTs: F4, 95 = 3.15, P < 0.05; COEs: F4, 95 = 1.89, P = 0.11) and Yongjia (P450s: F4, 95 = 4.21, P < 0.05; GSTs: F4, 95 = 2.67, P < 0.05; COEs: F4, 95 = 2.35, P = 0.06) (Fig. 3). Both the field-collected unexposed and exposed mosquitoes from the two An. sinensis populations exhibited significantly higher P450 enzyme activity compared with the laboratory strain (P < 0.05 or <0.001), while the field-collected resistant mosquitoes exhibited various GST and COE enzyme activities. The field-collected deltamethrin-resistant mosquitoes showed significantly higher P450 activity than the unexposed field mosquitoes in the Ruian population, but not those in the Yongjia population. The GST activity in deltamethrin-resistant and malathion-resistant mosquitoes from both Ruian and Yongjia was significantly higher than that of the laboratory strain (P < 0.05). In DDT-resistant mosquitoes, GST activity was higher than the laboratory strain in Ruian but not in Yongjia. The COE activity in deltamethrin-resistant mosquitoes from both Ruian and Yongjia was higher than that of the laboratory strain (P < 0.05). In DDT-resistant mosquitoes, COE activity was higher than the laboratory strain in Yongjia but not in Ruian (Fig. 3). These results suggest that P450 metabolic detoxification enzymes may play a role in all three insecticide resistances, while GSTs and COEs may play different roles in different insecticide resistances.
Fig. 3.
Metabolic detoxification enzyme activity in Anopheles sinensis from Ruian county (upper panel) and Yongjia county (lower panel). Comparisons of field-collected resistant mosquitoes (DT-R, DDT-live; DE-R, deltamethrin-live, and MA-R, malathion-live) to susceptible laboratory strain (Lab) and field-collected insecticide unexposed (Field) mosquitoes. P450 activity was measured by pmoles of 7-HC produced/min/mg protein; GST activity was measured by umoles of cDNB produced/min/mg protein; COE activity was measured by umoles of p-nitrophenol formed/min/mg protein. Mean activity and standard error are shown by a red dot with error bar. *P < 0.05 and **P < 0.001 represent significant differences by pairwise comparison.
Association Between Target Site Mutations and Insecticide Resistance
Three types of alleles (TTG, TTT, and TGT) were detected at codon 1014 of the para-type sodium channel gene. The wild-type TTG codes for leucine; TTT (L1014F) codes for phenylalanine, and TGT (L1014C) codes for cysteine (GenBank MG953790–MG953799). Both homozygous and heterozygous kdr genotypes were detected in live mosquitoes and dead mosquitoes in both the Ruian and Yongjia populations, with the exception of homozygous genotype CC, which was not detected in the Ruian population (Table 1). Frequencies of the L1014F kdr allele in both deltamethrin-live mosquitoes (54.3–64.6%) and DDT-live mosquitoes (50.0–56.8%) were significantly higher than in dead mosquitoes (26.0–31.3% in deltamethrin-dead and 30.0–32.6% in DDT-dead) in both the Ruian and Yongjia populations (odds ratios ranging from 2.67 to 5.79, P < 0.05), while the frequencies of the L1014C kdr allele in resistant mosquitoes were low in both Ruian (6.3–7.9%) and Yongjia (9.1–13.0%). No significant association was detected between the L101C kdr allele and deltamethrin- or DDT-resistant phenotypes (P > 0.05) (Table 1). These results indicate that the L1014F kdr allele is linked to deltamethrin- and DDT-resistant phenotypes in both the Ruian and Yongjia populations. The role of the L101C allele in resistant phenotypes is not clear. In total, 80 malathion-live and -dead mosquitoes in the two An. sinensis populations were genotyped for ace-1 mutation at codon position 119 (G119S) by PCR-RFLP. Twenty individuals were used to confirm the mutation by DNA sequencing analysis, from which four haplotypes were identified (GenBank MG953800–MG953803). The ace-1 homozygous mutation was detected in the two populations (Ruian and Yongjia) with 100% frequency both in live mosquitoes and in dead mosquitoes (Table 2), suggesting that the G119S mutation may not play a role in malathion resistance in these two populations.
Table 1.
Relationship between kdr mutant allele and phenotypic resistance in An. sinensis populations from Wenzhou, Zhejiang province, China
| Insecticide | Population | Phenotype | N | Genotype | Odds ratio (95% CI) | Fisher Exact Test: P | |||||||
| LL | LF | FF | FC | CC | LC | L1014F | L1014C | L1014F | L1014C | ||||
| Deltamethrin | Ruian | Live | 24 | 4 | 5 | 12 | 2 | 0 | 1 | 5.79 (2.36,14.22) | 2.43 (0.44,13.52) | 0.0001 | 0.3002 |
| Dead | 25 | 11 | 10 | 1 | 1 | 0 | 2 | ||||||
| Yongjia | Live | 23 | 3 | 7 | 9 | 0 | 2 | 2 | 3.22 (1.32,7.87) | 2.90 (0.71,11.88) | 0.0092 | 0.1292 | |
| Dead | 24 | 9 | 9 | 2 | 2 | 0 | 2 | ||||||
| DDT | Ruian | Live | 19 | 4 | 7 | 5 | 2 | 0 | 1 | 2.67 (1.03,6.92) | 5.06 (0.48,52.88) | 0.0406 | 0.1407 |
| Dead | 20 | 10 | 6 | 3 | 0 | 0 | 1 | ||||||
| Yongjia | Live | 22 | 3 | 7 | 9 | 0 | 1 | 2 | 2.89 (1.17,7.12) | 1.39 (0.32,5.97) | 0.0197 | 0.6600 | |
| Dead | 23 | 9 | 7 | 3 | 2 | 1 | 1 |
N, number of females tested; LL, homozygous leucine/leucine; LF, heterozygotes leucine/phenylalanine; FF, homozygous phenylalanine/phenylalanine; FC, heterozygotes phenylalanine/cysteine; CC, homozygous cysteine/cysteine; LC, heterozygotes leucine/cysteine.
Table 2.
ace-1 genotype and mutation frequency of An. sinensis from malathion bioassay in Wenzhou, Zhejiang province, China
| Population | Phenotype | N | ace-1 genotype | G119S frequency (%) | ||
| GGC/GGC | GGC/AGC | AGC/AGC | ||||
| Ruian | Live | 21 | 0 | 0 | 21 | 100 |
| Dead | 20 | 0 | 0 | 20 | 100 | |
| Yongjia | Live | 19 | 0 | 0 | 19 | 100 |
| Dead | 20 | 0 | 0 | 20 | 100 |
N, number of females tested; GGC, wild-type codon; AGC, mutant codon.
Discussion
Anopheles sinensis Wiedemann 1828 (Diptera: Culicidae) is widely distributed in rural agricultural areas and is the only malaria vector that has ever been a major health problem in Zhejiang province, China (Guo et al. 2014, Feng et al. 2017). In 2010, the government of China launched a malaria elimination plan to achieve the goal of malaria-free status by 2020 (Zhou et al. 2016). Wenzhou city, located in the extreme southeast of Zhejiang province, has prevented local vivax malaria transmission since 2010. However, with increasing international trade in Wenzhou, the proportion of imported falciparum malaria cases increased each year from 2010 to 2014 (Ni et al. 2015). Because synthetic insecticide-based vector control is the primary method for prevention and elimination of malaria, understanding the current status of insecticide resistance is important for the deployment of appropriate insecticides. Larval bioassays demonstrated more than a decade ago that An. sinensis populations in Wenzhou had developed resistance to deltamethrin (Wang 1999, 2000), but the current resistance status to various insecticides is unknown. In this study, the resistance to multiple insecticides was investigated in An. sinensis adult female populations from rural areas in Wenzhou, Zhejiang province, China. The results indicated that An. sinensis populations had developed resistance to three classes of insecticides: pyrethroids (deltamethrin), organochlorines (DDT), and organophosphates (malathion). An increased deltamethrin resistance was also evidenced in the study compared with a previous study reported by Wang (2000). Multiple insecticide resistance in An. sinensis suggests that the insecticides currently used for vector control may only be partially effective or even ineffective. This calls for urgent implementation of insecticide resistance management strategies. Multiple insecticide resistance in An. sinensis field populations has been reported in various geographic regions in China, such as central and southwest China (Chang et al. 2014), northern China (Dai et al. 2015), and Hainan island (Zhong et al. 2013). Our study fills the gap in An. sinensis resistance data in eastern China. The data collected in this study will be valuable for modeling insecticide resistance spread and vector-control interventions.
Understanding resistance mechanisms and monitoring resistance patterns is crucial to managing insecticide resistance in field populations of disease vectors, including An. sinensis mosquitoes. In the two An. sinensis populations examined in the present study, two types of kdr mutant alleles were found, including L1014F and L1014C. Similar mutation patterns have also been identified in An. sinensis mosquito populations from central China (Zhong et al. 2013). No other mutant allele was detected, such as L1014S and L1014W, which have been reported in An. sinensis from other locations in China (Tan et al. 2012) or Southeast Asia (Verhaeghen et al. 2010). Our results indicated that L1014F allele frequencies were significantly associated with deltamethrin-resistant and DDT-resistant phenotypes, but this was not the case for the L1014C allele. This may be explained by the medium to high L1014F allele frequencies (>50%) and low L1014C allele frequencies (<10%) observed in the two field populations. Some authors have argued that the kdr allele cannot be used as a definitive biomarker for pyrethroid resistance (Brooke 2008, Donnelly et al. 2009). Whether the kdr allele can predict resistance at the population level may be contingent on a number of factors, such as kdr frequency and the relative importance of metabolic resistance (Qin et al. 2014). In further examination of target site mutation at the ace-1 gene, the G119S allele frequency of a homozygous mutation was showing in 100% of the mosquitoes from the two An. sinensis populations, indicating that it is not involved in malathion resistance.
Previous studies have indicated the involvement of metabolic detoxification in resistance in An. sinensis mosquitoes from other populations in China (Zhong et al. 2013). The two An. sinensis populations examined here exhibited significantly higher P450 enzyme activity compared with the susceptible laboratory strain, while the field-collected resistant mosquitoes exhibited various enzyme activities. Overall, the field-collected deltamethrin-resistant mosquitoes exhibited higher P450, GST, and COE enzyme activity compared with the susceptible laboratory strain, but various enzyme activities were detected in field-collected DDT-live and malathion-live mosquitoes. Similar patterns of metabolic resistance have also been found in other An. sinensis populations (Zhong et al. 2013, Qin et al. 2014). These results, coupled with the results for the target site mutations, suggest that multiple mechanisms are involved in insecticide resistance in the study population. This is further evidence that the extensive use of insecticides for vector control and agricultural purposes has resulted in multiple insecticide resistance in An. sinensis. Further study is needed to confirm the role of metabolic detoxification enzyme activity.
Although malaria epidemic due to pyrethroid resistance has been reported since 2000 (Maharaj et al. 2005), several studies indicated that insecticide resistance may have little effect on malaria control. For example, in Bioko Island, the frequency of kdr in African anopheline mosquitoes increases but transmission level and malaria cases decrease (Ranson et al. 2011). In Burundi and Cote d’Ivoire, distribution of insecticide-treated nets resulted in a reduction in malaria incidence despite a high kdr frequency in Anopheles gambiae (Henry et al. 2005, Protopopoff et al. 2008). This might explain why the selection of high level of insecticide resistance in An. sinenesis by vector control in this area was not associated with an increase in malaria prevalence.
This study found that multiple insecticide resistance exists in An. sinensis from Wenzhou, an important coastal port city in China. Medium to high frequencies of target site kdr mutations and pure ace-1 mutation were detected in the two field mosquito populations. Examination of the resistance mechanism indicated that both target site kdr mutations and metabolic detoxification enzyme (P450, GST, and COE) activity played important roles in resistance in An. sinensis field populations. Effective implementation of insecticide resistance management strategies is urgently needed. The data collected in this study will be valuable for modeling insecticide resistance spread and vector-control interventions.
Acknowledgments
This work is supported by grants from Wenzhou Science and Technology Bureau (Y20140666); doctoral research funding from Wenzhou Medical University (89215003); student research funding from Wenzhou Medical University (wyx2015101054); and National Institutes of Health grants D43 TW001505 and U19AI089672.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Alemayehu E., Asale A., Eba K., Getahun K., Tushune K., Bryon A., Morou E., Vontas J., Van Leeuwen T., Duchateau L., et al. 2017. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasit. Vectors. 10: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Nkondjio C., Poupardin R., Tene B. F., Kopya E., Costantini C., Awono-Ambene P., and Wondji C. S.. 2016. Investigation of mechanisms of bendiocarb resistance in Anopheles gambiae populations from the city of Yaoundé, Cameroon. Malar. J. 15: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K. G., Irving H., Chiumia M., Mzilahowa T., Coleman M., Hemingway J., and Wondji C. S.. 2017. Restriction to gene flow is associated with changes in the molecular basis of pyrethroid resistance in the malaria vector Anopheles funestus. Proc. Natl Acad. Sci. USA. 114: 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke B. D. 2008. kdr: can a single mutation produce an entire insecticide resistance phenotype? Trans. R. Soc. Trop. Med. Hyg. 102: 524–525. [DOI] [PubMed] [Google Scholar]
- Chabi J., Baidoo P. K., Datsomor A. K., Okyere D., Ablorde A., Iddrisu A., Wilson M. D., Dadzie S. K., Jamet H. P., and Diclaro J. W., II. 2016. Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae (sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasit. Vectors. 9: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Zhong D., Fang Q., Hartsel J., Zhou G., Shi L., Fang F., Zhu C., and Yan G.. 2014. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. Plos Negl. Trop. Dis. 8: e2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Zhong D., Lo E., Fang Q., Bonizzoni M., Wang X., Lee M. C., Zhou G., Zhu G., Qin Q., et al. 2016. Landscape genetic structure and evolutionary genetics of insecticide resistance gene mutations in Anopheles sinensis. Parasit. Vectors. 9: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareonviriyaphap T., Bangs M. J., Suwonkerd W., Kongmee M., Corbel V., and Ngoen-Klan R.. 2013. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors. 6: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeau V., Cerqueira D., Zadrozny J., Kittiphanakun P., Andolina C., Chareonviriyaphap T., Nosten F., and Corbel V.. 2017. Insecticide resistance in malaria vectors along the Thailand–Myanmar border. Parasit. Vectors. 10: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher T. S., Lissenden N., Griffin J. T., Worrall E., and Ranson H.. 2016. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife 5: e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Study on Malaria Prevention and Control 1991. Status review and alternative strategies, pp. 45 In S. C. Oaks V. S. Jr. Mitchell G. W. Pearson and C. C. J. Carpenter (eds.), Malaria: obstacles and opportunities. Division of International Health, Institute of Medicine, National Academy Press, Washington, DC. [Google Scholar]
- Cui F., Raymond M., and Qiao C. L.. 2006. Insecticide resistance in vector mosquitoes in China. Pest Manag. Sci. 62: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Dai Y., Huang X., Cheng P., Liu L., Wang H., Wang H., and Kou J.. 2015. Development of insecticide resistance in malaria vector Anopheles sinensis populations from Shandong province in China. Malar. J. 14: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S., Panda B. K., Sahu S. S., and Mohanty P. K.. 2012. Preliminary study on susceptibility status of Anopheles minimus Theobald and An. fluviatilis James (Diptera: Culicidae) to DDT and deltamethrin in Keonjhar district of Odisha, India. J. Commun. Dis. 44: 25–28. [PubMed] [Google Scholar]
- Dery D. B., Segbaya S., Asoalla V., Amoyaw F., Amoako N., Agyeman-Budu A., Oduro A., Owusu-Agyei S., and Asante K. P.. 2016. Anopheles gambiae (Diptera: Culicidae) susceptibility to insecticides and knockdown resistance genes prior to introduction of indoor residual spraying in 11 districts in Ghana. J. Med. Entomol. 53: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Dev V., and Manguin S.. 2016. Biology, distribution and control of Anopheles (Cellia) minimus in the context of malaria transmission in northeastern India. Parasit. Vectors. 9: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka R., Riveron J. M., Yessoufou A., Tchigossou G., Akoton R., Irving H., Djegbe I., Moutairou K., Adeoti R., Tamò M., et al. 2016a. Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasit. Vectors. 9: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka R. J., Atoyebi S. M., Tchigossou G. M., Riveron J. M., Irving H., Akoton R., Kusimo M. O., Bakare A. A., and Wondji C. S.. 2016b. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in South West Nigeria. Malar. J. 15: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. D. 2010. The mosquito fauna of Yunnan, vol. 1. Yunnan Publishing Group Corporation, Yunnan Science & Technology Press, Kunming, China. [Google Scholar]
- Donnelly M. J., Corbel V., Weetman D., Wilding C. S., Williamson M. S., and Black W. C. IV. 2009. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 25: 213–219. [DOI] [PubMed] [Google Scholar]
- Feng X., Yang C., Yang Y., Li J., Lin K., Li M., and Qiu X.. 2015. Distribution and frequency of G119S mutation in ace-1 gene within Anopheles sinensis populations from Guangxi, China. Malar. J. 14: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Zhang S., Huang F., Zhang L., Feng J., Xia Z., Zhou H., Hu W., and Zhou S.. 2017. Biology, bionomics and molecular biology of Anopheles sinensis Wiedemann 1828 (Diptera: Culicidae), main malaria vector in China. Front Microbiol. 8: 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Ling F., Hou J., Wang J., Fu G., and Gong Z.. 2014. Mosquito surveillance revealed lagged effects of mosquito abundance on mosquito-borne disease transmission: a retrospective study in Zhejiang, China. Plos One. 9: e112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakizimana E., Karema C., Munyakanage D., Iranzi G., Githure J., Tongren J. E., Takken W., Binagwaho A., and Koenraadt C. J.. 2016. Susceptibility of Anopheles gambiae to insecticides used for malaria vector control in Rwanda. Malar. J. 15: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. C., Assi S. B., Rogier C., Dossou-Yovo J., Chandre F., Guillet P., and Carnevale P.. 2005. Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Côte d’Ivoire. Am. J. Trop. Med. Hyg. 73: 859–864. [PubMed] [Google Scholar]
- Ibrahim S. S., Riveron J. M., Stott R., Irving H., and Wondji C. S.. 2016. The cytochrome P450 CYP6P4 is responsible for the high pyrethroid resistance in knockdown resistance-free Anopheles arabiensis. Insect Biochem. Mol. Biol. 68: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving H., and Wondji C. S.. 2017. Investigating knockdown resistance (kdr) mechanism against pyrethroids/DDT in the malaria vector Anopheles funestus across Africa. BMC Genet. 18: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D., Park M. H., Saeung A., Choochote W., and Min G. S.. 2010. Multiplex assay to identify Korean vectors of malaria. Mol. Ecol. Resour. 10: 748–750. [DOI] [PubMed] [Google Scholar]
- Lu G., Zhou S., Horstick O., Wang X., Liu Y., and Müller O.. 2014. Malaria outbreaks in China (1990–2013): a systematic review. Malar. J. 13: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj R., Mthembu D. J., and Sharp B. L.. 2005. Impact of DDT re-introduction on malaria transmission in KwaZulu-Natal. S. Afr. Med. J. 95: 871–874. [PubMed] [Google Scholar]
- Marcombe S., Bobichon J., Somphong B., Phommavan N., Maithaviphet S., Nambanya S., Corbel V., and Brey P. T.. 2017. Insecticide resistance status of malaria vectors in Lao PDR. Plos One. 12: e0175984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D., Chandre F., Williamson M. S., Darriet F., Bergé J. B., Devonshire A. L., Guillet P., Pasteur N., and Pauron D.. 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 7: 179–184. [DOI] [PubMed] [Google Scholar]
- Mbepera S., Nkwengulila G., Peter R., Mausa E. A., Mahande A. M., Coetzee M., and Kweka E. J.. 2017. The influence of age on insecticide susceptibility of Anopheles arabiensis during dry and rainy seasons in rice irrigation schemes of Northern Tanzania. Malar. J. 16: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze B. D., Riveron J. M., Ibrahim S. S., Irving H., Antonio-Nkondjio C., Awono-Ambene P. H., and Wondji C. S.. 2016. Multiple insecticide resistance in the malaria vector Anopheles funestus from Northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. Plos One. 11: e0163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzilahowa T., Chiumia M., Mbewe R. B., Uzalili V. T., Luka-Banda M., Kutengule A., Mathanga D. P., Ali D., Chiphwanya J., Zoya J., et al. 2016. Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar. J. 15: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Q. X., Zhang X. H., Chen Y., Wei J. J., Yu X. H., Shao Y. Q., and Pan Q. J.. 2015. Epidemiological analysis of malaria in Wenzhou City during 2007–2014. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 33: 205–207. [PubMed] [Google Scholar]
- Ochomo E., Subramaniam K., Kemei B., Rippon E., Bayoh N. M., Kamau L., Atieli F., Vulule J. M., Ouma C., Gimnig J., et al. 2015. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasit. Vectors. 8: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondeto B. M., Nyundo C., Kamau L., Muriu S. M., Mwangangi J. M., Njagi K., Mathenge E. M., Ochanda H., and Mbogo C. M.. 2017. Current status of insecticide resistance among malaria vectors in Kenya. Parasit. Vectors. 10: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. Y., Zhou S. S., Zheng X., Huang F., Wang D. Q., Shen Y. Z., Su Y. P., Zhou G. C., Liu F., and Jiang J. J.. 2012. Vector capacity of Anopheles sinensis in malaria outbreak areas of central China. Parasit. Vectors. 5: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemo D., Komalamisra N., Sungvornyothin S., and Attrapadung S.. 2012. Efficacy of three insecticides against Anopheles dirus and Anopheles minimus, the major malaria vectors, in Kanchanaburi Province, Thailand. Southeast Asian J. Trop. Med. Public Health. 43: 1339–1345. [PubMed] [Google Scholar]
- Philbert A., Lyantagaye S. L., Pradel G., Ngwa C. J., and Nkwengulila G.. 2017. Pyrethroids and DDT tolerance of Anopheles gambiae s.l. from Sengerema District, an area of intensive pesticide usage in north-western Tanzania. Trop. Med. Int. Health. 22: 388–398. [DOI] [PubMed] [Google Scholar]
- Protopopoff N., Van Bortel W., Marcotty T., Van Herp M., Maes P., Baza D., D’Alessandro U., and Coosemans M.. 2008. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am. J. Trop. Med. Hyg. 79: 12–18. [PubMed] [Google Scholar]
- Qin Q., Li Y., Zhong D., Zhou N., Chang X., Li C., Cui L., Yan G., and Chen X. G.. 2014. Insecticide resistance of Anopheles sinensis and An. vagus in Hainan Island, a malaria-endemic area of China. Parasit. Vectors. 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamaherijaona S., Velonirina H. J., and Boyer S.. 2016. Susceptibility status of Anopheles arabiensis (Diptera: Culicidae) commonly used as biological materials for evaluations of malaria vector control tools in Madagascar. Malar. J. 15: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H., N’guessan R., Lines J., Moiroux N., Nkuni Z., and Corbel V.. 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27: 91–98. [DOI] [PubMed] [Google Scholar]
- Rivero A., Vézilier J., Weill M., Read A. F., and Gandon S.. 2010. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? Plos Pathog. 6: e1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveron J. M., Osae M., Egyir-Yawson A., Irving H., Ibrahim S. S., and Wondji C. S.. 2016. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: implications for malaria control. Parasit. Vectors. 9: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi N. H., Ahmadi A. A., Nahzat S., Ziapour S. P., Nikookar S. H., Fazeli-Dinan M., Enayati A., and Hemingway J.. 2017. Evidence of metabolic mechanisms playing a role in multiple insecticides resistance in Anopheles stephensi populations from Afghanistan. Malar. J. 16: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samb B., Konate L., Irving H., Riveron J. M., Dia I., Faye O., and Wondji C. S.. 2016. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasit. Vectors. 9: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. W., Wang G. Z., Zeng L. H., Li S. G., He C. H., Hu X. M., and Wang S. Q.. 2017. Extensive resistance of Anopheles sinensis to insecticides in malaria-endemic areas of Hainan Province, China. Am. J. Trop. Med. Hyg. 97: 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W. L., Li C. X., Wang Z. M., Liu M. D., Dong Y. D., Feng X. Y., Wu Z. M., Guo X. X., Xing D., Zhang Y. M., et al. 2012. First detection of multiple knockdown resistance (kdr)-like mutations in voltage-gated sodium channel using three new genotyping methods in Anopheles sinensis from Guangxi Province, China. J. Med. Entomol. 49: 1012–1020. [DOI] [PubMed] [Google Scholar]
- Verhaeghen K., Van Bortel W., Trung H. D., Sochantha T., and Coosemans M.. 2009. Absence of knockdown resistance suggests metabolic resistance in the main malaria vectors of the Mekong region. Malar. J. 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen K., Van Bortel W., Trung H. D., Sochantha T., Keokenchanh K., and Coosemans M.. 2010. Knockdown resistance in Anopheles vagus, An. sinensis, An. paraliae and An. peditaeniatus populations of the Mekong region. Parasit. Vectors. 3: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldner E. C., and Li Y.-F.. 1995. Global usage of selected persistent organochlorines. Sci. Total Environ. 160–161: 201–210. [Google Scholar]
- Wang J. 1999. Resistance to two pyrethroids in Anopheles sinensis from Zhejiang, China. J. Am. Mosq. Control Assoc. 15: 308–311. [PubMed] [Google Scholar]
- Wang J. 2000. Resistance and response to selection to deltamethrin in Anopheles sinensis from Zhejiang, China. J. Am. Mosq. Control Assoc. 16: 9–12. [PubMed] [Google Scholar]
- Wanjala C. L., Mbugi J. P., Ototo E., Gesuge M., Afrane Y. A., Atieli H. E., Zhou G., Githeko A. K., and Yan G.. 2015. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, Western Kenya. Emerg. Infect. Dis. 21: 2178–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2016. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, 2nd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- WHO 2018. World malaria report 2018. World Health Organization; Geneva, Switzerland: https://www.who.int/malaria/media/world-malaria-report-2018/en/ (last accessed 15 December 2018). [Google Scholar]
- Wikipedia contributors 2017. Wenzhou people, Wikipedia, The Free Encyclopedia https://en.wikipedia.org/w/index.php?title=Wenzhou_people&oldid=805937556 (last accessed 2 November 2017).
- Yahouédo G. A., Chandre F., Rossignol M., Ginibre C., Balabanidou V., Mendez N. G. A., Pigeon O., Vontas J., and Cornelie S.. 2017. Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci. Rep. 7: 11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalucki M. P., and Furlong M. J.. 2017. Behavior as a mechanism of insecticide resistance: evaluation of the evidence. Curr. Opin. Insect Sci. 21: 19–25. [DOI] [PubMed] [Google Scholar]
- Zhang H. W., Liu Y., Hu T., Zhou R. M., Chen J. S., Qian D., Yang C. Y., Zhao Y. L., Li S. H., Cui J., et al. 2015. Knockdown resistance of Anopheles sinensis in Henan province, China. Malar. J. 14: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Sun J., Zhang Z., Geng Q., Lai S., Hu W., Clements A. C., and Li Z.. 2016. Risk assessment of malaria in land border regions of China in the context of malaria elimination. Malar. J. 15: 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D., Chang X., Zhou G., He Z., Fu F., Yan Z., Zhu G., Xu T., Bonizzoni M., Wang M. H., et al. 2013. Relationship between knockdown resistance, metabolic detoxification and organismal resistance to pyrethroids in Anopheles sinensis. Plos One. 8: e55475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Li Z., Cotter C., Zheng C., Zhang Q., Li H., Zhou S., Zhou X., Yu H., and Yang W.. 2016. Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar. J. 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Xia H., Zhou H., Li J., Lu F., Liu Y., Cao J., Gao Q., and Sattabongkot J.. 2013. Susceptibility of Anopheles sinensis to Plasmodium vivax in malarial outbreak areas of central China. Parasit. Vectors. 6: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]