Abstract
Historically, research on the cognitive processes that support human memory proceeded, to a large extent, independently of research on the neural basis of memory. Accumulating evidence from neuroimaging, however, has enabled the field to develop a broader and more integrative perspective. Here, we briefly outline how advances in cognitive neuroscience can potentially shed light on concepts and controversies in human memory research. We argue that research on the functional properties of cortico-hippocampal networks informs us about how memories might be organized in the brain, which, in turn, helps to reconcile seemingly disparate perspectives in cognitive psychology. Finally, we discuss several open questions and directions for future research.
Introduction
A key goal of any theory in cognitive neuroscience is to provide a coherent and compelling account of the relationship between neural phenomena, cognitive processes, and behavior. The field of memory can be seen as one of the major success stories in cognitive neuroscience, as there has been a long history of productive research on patients with amnesia, neuroimaging studies of healthy individuals, and corresponding research in animal models. It is often not clear, however, how to reconcile this rich body of evidence with concepts from cognitive psychology. For many years, the field had suffered from a kind of dualism, in which psychologists tended to explain phenomena in terms of theories about cognitive processes, and neuroscientists tended to favor descriptive taxonomies of different “memory systems”. In the past few decades, however, the two approaches have converged to a point in which we can arrive at a more unified view of memory processes at both the functional and neural levels of analysis.
The goal of this mini-review is to provide a synopsis of consistencies in the cognitive neuroscience literature, and we then describe how these findings might relate to theoretical constructs and phenomena discussed in cognitive psychology. We begin with a brief overview of the functional organization of cortico-hippocampal networks in the brain, and we then apply the organization of these networks as a framework for understanding cognition.
Representational structure of the medial temporal lobes
Virtually every account of how the brain forms memories for events focuses on the medial temporal lobes (Eichenbaum, Yonelinas, & Ranganath, 2007), and in particular the hippocampus. Many past reports have used the terms “hippocampus” and “medial temporal lobe(s)” interchangeably, such that the perirhinal cortex (PRC), parahippocampal cortex (PHC), and entorhinal cortex (EC) are treated as part of the “hippocampal system” or the hippocampus is treated as part of the “medial temporal lobe memory system” (Squire & Zola-Morgan, 1991). The grouping of regions in the medial temporal lobes is, to some extent, an accident of history (see Murray & Wise, 2012; Inhoff & Ranganath, 2017 for review), and there is near-consensus that the hippocampal formation supports memory in a manner that differs from the perirhinal and parahippocampal cortex (Brown & Aggleton, 2001; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; Eacott & Gaffan, 2005; Eichenbaum, Yonelinas, & Ranganath, 2007; Henson & Gagnepain, 2010; Huffman & Stark, 2014; Reagh & Yassa, 2014).
There are at least two ways to conceptualize the differences between the hippocampus and MTL neocortical areas. According to the “Complementary Learning Systems” (CLS; O’Reilly and McClelland, 1994) framework, and related views (Rolls & Kesner, 2006), the hippocampus and neocortex differ in terms of computational specializations. CLS proposes that the hippocampus is unique in that the various hippocampal subfields implement complementary computations – the dentate gyrus is proposed to encode sparse representations, such that similar events have minimal representational overlap (“pattern separation”) (Knierim & Neunuebel, 2016; O’Reilly & Norman, 2002; Yassa & Stark, 2011). The CA3 subfield, conversely, is proposed to support reinstatement of stored memories from partial or degraded cues in recurrent collateral networks (“pattern completion”) (Gold & Kesner, 2005; Nakazawa et al., 2002). In contrast to the hippocampus, the CLS framework proposes that neocortical areas encode information more slowly, with overlapping representations that support generalization across similar events.
Though most of the impetus for this model has come from computational simulations (McClelland, McNaughton, & O’Reilly, 1995; O’Reilly et al., 2014), electrophysiological recordings in rodents (Leutgeb et al., 2007; Neunuebel & Knierim, 2014) and human fMRI studies (Bakker et al., 2008; Berron et al., 2016) have provided indirect evidence for hippocampal pattern separation. Evidence for the pattern completion side of the story has been far more limited (but see Horner et al., 2015; Neunuebel et al., 2014). The CLS model is not without challenges, however. As is articulated in the representational-hierarchical theory (Kent et al., 2016), “pattern separation” may not be exclusive or even especially unique to the hippocampus. Indeed, there is evidence to suggest that fMRI activity thought to relate to hippocampal pattern separation in humans is also present in PRC, PHC, and EC (Reagh & Yassa, 2014).
Other models emphasize differences between the kinds of information that is represented by the hippocampus and neocortical areas. For instance, many theories propose that the hippocampus plays a unique role in representing associations amongst learned items (Brown & Aggleton, 2001; Eichenbaum, Yonelinas, & Ranganath, 2007; Konkel & Cohen, 2009) or a contextual space that specifies the relationships between the items in space and time (O’Keefe & Nadel, 1978; Ekstrom & Ranganath, 2017; Eichenbaum, 2017). The “Binding of Items and Contexts” (BIC) model (Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Ranganath, 2010) builds on these ideas by additionally proposing that the PHC encodes information about the spatial as well as the situational context in which items are encountered (see also: Aminoff, Gronau, & Bar, 2006; Davachi, 2006; Eacott & Gaffan, 2005; Knierim, 2006; Hayes, Nadel, & Ryan, 2007; Mayes, Montaldi, & Migo, 2007). The hippocampus, in turn, is proposed to represent how item or object-level representations in the PRC relate to one another within the dimensions of the contextual space specified by PHC. More recent formulations have additionally proposed that the hippocampus may intrinsically represent temporal information, or more specifically, the relative sequence of items that is encountered in a particular context (Cohn-Sheehy & Ranganath, 2017; Ranganath & Hsieh, 2016; Eichenbaum, 2017).
Consistent with the BIC model, differences in domain-selectivity between PRC and PHC are well documented in the neuroimaging literature, with the former showing sensitivity to items or objects and the latter showing sensitivity to scene content and spatial or nonspatial contextual information (Clarke & Tyler, 2014; Libby, Hannula, & Ranganath, 2014; Janzen & Van Turennout, 2004; Maguire et al., 1998 Miyashita, 1988). Hippocampal activity, in turn, is sensitive to successful encoding and retrieval of spatial, temporal, and situational information, and hippocampal activity patterns carry detailed information about particular objects-in-context (Berron et al., 2016; Horner et al., 2015; Hsieh et al., 2014; LaRocque et al., 2013; Liang, Wagner, & Preston, 2012; Ritchey et al., 2015; Tompary, Duncan, & Davachi, 2016).
At first glance, the BIC and CLS models may seem incompatible, but they are not mutually exclusive. The available evidence supports the idea that MTL cortical areas are sufficient to support distinct item-based and contextual representations, and there is good evidence to support the idea that the hippocampus facilitates resolution of interference among these representations. In other words, the unique role of the hippocampus in memory might reflect both its computational specializations for pattern separation/completion, and its ability to operate over both high-resolution information about the currently processed item(s) within the current spatial, temporal, and situational context. Moreover, depending on the cognitive demands at any given moment, the balance of item and context inputs to the hippocampus may vary.
Representation in broader cortico-hippocampal networks
Although a great deal of research in the cognitive neuroscience of memory has focused on MTL regions, there are a number of extra-MTL neocortical areas that are engaged during episodic retrieval. For instance, the angular gyrus, retrosplenial cortex, and precuneus reliably show activity enhancements during successful episodic memory retrieval, and the magnitude of activation in these regions during retrieval is linked to the subjective vividness of recollection (for a review, see Rugg & Vilberg, 2013). These findings have made it increasingly apparent that a focus on highly localized hubs in the brain is insufficient to explain the rich and dynamic nature of learning and remembering.
We (Inhoff & Ranganath, 2017; Ranganath & Ritchey, 2012; Ritchey, Libby, & Ranganath, 2015) recently proposed that the PHC is a key component of a posterior medial (PM) network that also includes retrosplenial, posterior cingulate, medial parietal, and ventrolateral parietal cortex; PRC, in turn, is situated in a broader anterior-temporal (AT) network that includes ventral temporopolar cortex, anterior fusiform cortex, the amygdala, and the lateral orbitofrontal cortex. In addition to anatomical connections among regions in these respective networks, it is important to note that regions within the AT and PM networks show highly correlated activity patterns over time across a variety of states (i.e., “intrinsic functional connectivity;” Kahn et al., 2008; Wang et al., 2016; Zhuo et al., 2016). Studies of anatomical connectivity in animal models and intrinsic functional connectivity in humans converge in suggesting that connectivity is higher amongst regions within each network than it is across networks. Given that functional specialization in the neocortex is thought to depend on the “connectional fingerprint,” or unique pattern of connectivity for a given region (Passingham et al., 2002; Young et al., 1994), the findings suggest an anatomical basis for functional differentiation between regions in the PM and AT networks.
Evidence regarding the functional characteristics of the PM and AT networks has been reviewed in depth in recent papers (Inhoff & Ranganath, 2017; Ranganath & Ritchey, 2012; Ritchey, Libby, & Ranganath, 2015). Briefly, this work suggests that the PM and AT networks exhibit characteristics that are directly related to the representational differences between the PHC and PRC, respectively (Inhoff & Ranganath, 2017). More specifically, the evidence (Inhoff & Ranganath, 2017; Ranganath & Ritchey, 2012; Ritchey, Libby, & Ranganath, 2015) indicates that the PM network may encode contextual associations that are used to generate a representation of the spatial, temporal, and broader causal relationships between different elements of an event (i.e., a “situation model”). The AT network, in turn, encodes semantic and perceptual information about the attributes and motivational significance of people and objects (i.e. “entities”).
It is important to note how a focus on distributed cortico-hippocampal networks can provide a broader perspective that differs from simply focusing on regions within the MTL. The world does not come with labels for “objects” and “scenes” or for “items” and “contexts”, so incoming sensory information must be processed in different ways to culminate in the distinct representational properties observed in MTL cortex. Moreover, the process of reconstructing and re-experiencing a past event depends critically on an interaction between recovery of information about items encountered in a specific event context and general knowledge about the structure of real-world events (Bartlett & Burt, 1933; Radvansky & Zacks, 2014). Moreover, information about past events is often required in order to generate and update semantic knowledge (Winocur & Moscovitch, 2011) that is used to plan for the future (Schacter, Addis, & Buckner, 2007) or to make inferences about the hidden characteristics of people and things.
To help clarify the relative roles of PRC, PHC, and the PM and AT networks, consider an overly-simplified cartoon example. Walking through a building, visual context information represented by PHC might be used to orient oneself relative to one’s knowledge of the topology of the building, accessed via a spatial situation model (Rinck et al.,1996) supported by activated representations in extra-MTL PM cortical areas, such as retrosplenial cortex, posterior cingulate, and precuneus. Upon encountering a familiar person in the room, activation of the corresponding PRC representation of the person’s face can be linked with knowledge about the person’s traits, via activation of representations in AT network areas, such as the amygdala and temporopolar, orbitofrontal, and insular cortex (Olson et al., 2013). Although this account undoubtedly underestimates the complexity and dynamics of brain activity during even a simple event, it helps to illustrate how representations in the MTL can be understood as parts of a whole that instantiated in distributed cortical networks with distinct but complementary cognitive functions (see Kravitz et al., 2011; Livne & Bar, 2016; Nadel & Peterson, 2013, for related views). In sum, the broader network perspective can give us “why” and “how” in addition to “what” and “where.”
How does an understanding of cortico-hippocampal networks illuminate key aspects of memory?
Having discussed the organization of cortico-hippocampal systems and the sorts of representations they seem to support, we can now turn to how these ideas might relate to major themes and phenomena in cognitive psychology. Below, we consider some major distinctions and topics from memory research, noting how these different perspectives of memory phenomenology are informed by cortico-hippocampal network dynamics.
Recollection- and Familiarity-based recognition:
Many theories have proposed that people can recognize a person or thing based on two processes: by sensing the overall strength of its match with the contents of memory (“familiarity”), or by recalling specific details associated with the context in which that person or thing was last encountered (“recollection”) (Cary & Reder, 2003; Chan & McDermott, 2007; Hintzman, Caulton, & Levitin, 1998; Jacoby, 1991; Yonelinas, 1994, 2002). In a typical laboratory study, recollection- and familiarity-based recognition is assessed by having the subject discriminate between studied and unstudied items using confidence ratings (Yonelinas, 2002) or “Remember/Know” judgments (Tulving, 1985). One way to think about these decisions is that a participant is shown an item, and s/he must indicate whether the item cues retrieval of contextual information (“recollection”), or if it instead recognized due to fluency of processing or the overall strength of the memory elicited by the item (“familiarity”).
If familiarity is based, at least in part, on item strength or fluency of item processing, we would expect that it should reflect activation of representations in PRC (Eichenbaum, Yonelinas, & Ranganath, 2007; Diana, Yonelinas, & Ranganath, 2007; Kafkas & Montaldi, 2014). A key property of PRC is representation of the perceptual and/or conceptual features of objects (Clarke & Tyler, 2014). A common finding in the literature is that, during learning, PRC activation scales positively with memory confidence when the item is subsequently tested (e.g., Haskins et al., 2008; Ranganath et al., 2004). Interestingly, during retrieval, we see the opposite finding, such that activity is reduced with increasing familiarity (e.g., Montaldi et al. 2006; Wang, Ranganath, & Yonelinas, 2014). A popular interpretation of this phenomenon is that more activation during encoding leads to a stronger item representation, and stronger representations lead to faster processing at retrieval, such that activation is reduced in duration or magnitude (Grill-Spector, Henson, & Martin, 2006). Faster, and more efficient processing at the neural level, should give rise to more fluent processing of the corresponding item at the behavioral level. Fluency of item processing, in turn, can be attributed to familiarity for that item (Kelley & Jacoby, 1998). It should be noted that, although familiarity is usually discussed in relation to items, there is evidence to support the idea of context-based familiarity as well (Addante, Ranganath, & Yonelinas, 2012). Context-based familiarity might be related to the phenomenon of memory distortion, which we discuss later in this review.
Whereas familiarity might simply reflect the strength of representations in PRC and PHC and affiliated brain regions, recollection, in turn, might reflect more complex dynamics. In general, it is thought that recollection involves retrieving contextual associations among items or concepts, and retrieving a particular episode based on these associations. Accordingly, during learning, we would predict that the hippocampus should encode memory traces that link inputs about specific items (conveyed via PRC) with temporally contiguous inputs about the context in which the item was encountered (conveyed via PHC and other regions in the PM network). We would expect that the hippocampus would assign distinct representations to similar items that were encoded at different times due to pattern separation and the difference in contextual information associated with each item. During retrieval, input about the item cue from PRC could drive hippocampal pattern completion, thereby leading to reactivation of associated context representations in PHC and of representations of other items encountered during the event, via PRC (Diana, Yonelinas, & Ranganath, 2007; Staresina, Duncan, & Davachi, 2011; Staresina et al., 2013). Activation of these regions, in turn, would be expected to instantiate reactivation of higher-level semantic relationships with that item in the AT network and recovery of the corresponding situational context via reinstatement in the PM network.
Putting this all together, one can make a strong case that recollection and familiarity are related to different neural dynamics, but these differences do not boil down to a clean distinction between the hippocampus and neocortex. Moreover, our understanding of episodic memory retrieval becomes clearer when we consider not only the roles of PRC and PHC, but also the broader cortico-hippocampal networks of which they are components, that allow recollected information to be reconstructed into a coherent episodic memory. For example, familiarity-based memory has been found to modulate activity in AT regions including the temporal poles (Leveroni et al., 2000; Pourtois et al., 2005) and orbitofrontal cortex (Elliott, Dolan, & Frith, 2000), whereas recollection-based recognition drives activity in PM regions such as the angular gyrus and precuneus (Rugg & Vilberg, 2013). Activity in the extended PM and AT networks may be especially relevant to the subjective experiences of familiarity and recollection, which are likely to reflect access to information about semantics and situational context.
Episodic and Semantic Memory:
Tulving (1972) proposed that there is a fundamental distinction between episodic memory, which supports representations of past events that are specific to a place and time, and semantic memory, which supports generalized knowledge (Tulving, 1972). Though this distinction remains controversial, our current understanding of cortico-hippocampal networks provides some insight into how to conceptualize these forms of memory.
An important prediction of the PMAT framework is that there are at least two qualitatively and neurally dissociable forms of semantic knowledge. As noted above, there is considerable evidence to suggest that regions in the AT network support perceptual and semantic knowledge about objects (Clarke & Tyler, 2014), whereas PM regions may support the kind of relational semantic knowledge that is used to build situation models. The distinction between entity- and situation-based semantic knowledge can be contrasted with the general use of the term “schema,” to refer to virtually any form of organized semantic knowledge (Ghosh & Gilboa, 2014).
A strong dissociation between semantic knowledge putatively represented by the PM and AT networks was recently observed in an elegant study by Boylan, Trueswell, and Thompson-Schill (2017). In this experiment, participants were presented with word pairs and instructed to link nouns, which varied in whether they were linked with one another by attributes (e.g., “zebra clam” which denotes a type of striped clam) or relations (e.g., “mountain lake” which denotes two things that are situationally linked). The authors reported greater anterior temporal lobe engagement during attributive linking and greater angular gyrus engagement during relational linking, consistent with different aspects of semantics arising from different cortical networks. Importantly, we propose that PM and AT networks are not simply locked into maintaining specific or general representations. Generality in either network can arise from overlap among features, which is not mutually exclusive with representation of specificity in these networks.
A second key prediction of the PMAT model is that real-life episodic memory builds on a scaffold that is provided by semantic knowledge in the PM and AT networks. Whereas recollection-based recognition relies on brief reactivation of a past context, recall of an entire episode requires one to reconstruct the events that unfolded over a sustained period of time. Many theories suggest that this entails generating a situation model in which one assigns particular entities to roles that specify how they would relate to one another in a particular context (Zwaan, Langston, & Graesser 1995). Rather than a simple representation of space and time, a situation model is a higher-order cognitive representation used to encode or retrieve a particular state of affairs (Bailey & Zacks, 2015).
We hypothesize that the PM network essentially provides the event scaffold at different levels of abstraction (context, situation model, and schema), whereas the AT network contributes specific local representations as well as semantic information that is laid onto that scaffold. To illustrate how this process might work, consider a simple example of remembering a birthday dinner. To begin the process, you first construct a “birthday” situation, which constrains the subsequent local and contextual features that can occupy that situation, and which is itself constrained by the types of things that happen in your schema of a birthday dinner (perhaps disproportionately supported by ventromedial prefrontal cortex). Concurrently, you generate a semantic framework for local item features, such as general birthday decor, candles, and cake provided by the AT network. In the absence of a functioning hippocampus, one would expect the retrieved memory to be vague and schematic. The story changes, however, if processing of the birthday schema and relevant retrieval cues drives hippocampal pattern completion. In this case, we would expect hippocampal feedback to drive reinstatement of activity patterns in the PM and AT network that approximates the trajectory of activity states in these networks during processing of a specific birthday event (Figure 1). Thus, hippocampal feedback can enable reactivation of specific contextual information (e.g., which room you were in when you blew out your candles, and how the party attendees were spatially arranged) and specific information about local entities (e.g., the flavor of your cake, and your relation to the particular person was sitting to your left) that constrain the general situation model to a particular event. Importantly, these specific details need not be true. We might expect that the likelihood of inserting erroneous details into the reconstructed event depends on the degree to which hippocampal feedback can effectively constrain patterns of activity within cortical regions (van Kesteren et al., 2013), or the extent to which the target details interfere with other stored representations.
Figure 1: Reinstatement of a specific event representation in cortico-hippocampal networks.
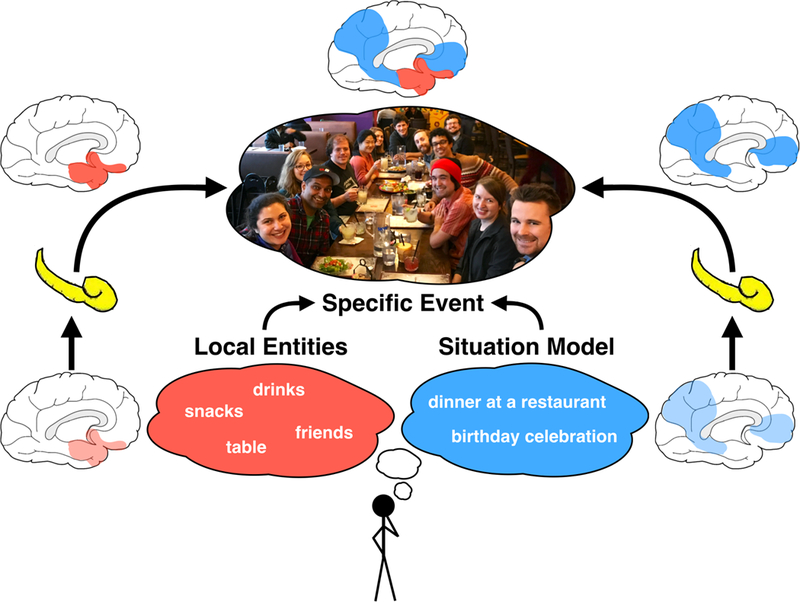
The process of recalling a particular event or episode often begins with the construction of a situation model, here a birthday dinner at a restaurant. We propose that such a situation model, informed by your schemas of a birthday celebration and dining at a restaurant, are constructed by posterior-medial cortical areas (blue). The situation model is populated with local entities, such as drinks, snacks, friends, and a table. We propose that these local features are represented in anterior-temporal cortical areas (red). Through interactions with the hippocampus, these two cortical networks are able to sharpen their activity patterns into a representation of a specific event such that specific people, foods, and drinks are recalled in a particular arrangement at a particular table and a particular restaurant. Moreover, the hippocampus facilitates the integration of information across the two networks.
Autobiographical Memory:
The hippocampus and PM network are often found to be engaged during autobiographical retrieval (e.g., Andrews-Hanna et al., 2014; St. Jacques & De Brigard, 2015; Svoboda, McKinnon, & Levine, 2006), whereas the contributions of the AT system are less clear. Theoretical accounts, however, emphasize both semantic and episodic components of autobiographical memory (Levine et al., 2002), thereby raising an interesting question: If retrieval of personal events involves semantic knowledge, including knowledge about specific people and things, why does the PM network seemingly dominate this process? Consider that, when recollecting an autobiographical experience, we often first focus on recall of situational or contextual details, and sustain this representation throughout the experience of remembering local entities. The recollective process usually prioritizes placing these people or things in a particular event context, rather than abstractly contemplating their characteristics. Though the PM network seems to be heavily engaged in autobiographical retrieval, we predict that the relative balance of PM and AT involvement depends on the content being retrieved. For instance, one can easily conceive of cases in which goal-directed autobiographical memory retrieval processes can shift focus to knowledge about specific people or things (e.g., recalling the occupation of the person that you met at the party; see Renoult et al., 2012; Viskontas et al., 2000).
Sheldon and Levine (2018) recently reported results that align well with the above account. Briefly, in this study, participants were tasked with creating mental representations under conditions of autobiographical retrieval, generating a spatial framework, or relating conceptual/perceptual features of objects. The authors seeded anterior and posterior hippocampal ROIs, and examined interregional correlations during these three task conditions. Autobiographical retrieval largely drove anterior hippocampal correlations with PM areas, including precuneus, angular gyrus, medial prefrontal cortex, and PHC. Spatial retrieval drove both anterior and posterior hippocampal correlations, which similarly included PM regions. Given our perspective outlined above, it makes sense that spatial cognition would recruit a similar network of PM brain areas involved in reconstructing past experiences. Interestingly, conceptual retrieval drove hippocampal correlations (mostly anterior) with AT regions such as the temporal pole and insula. Thus, in this case, the hippocampus demonstrated a distinct correlational structure as individuals were tasked with elaborating about a local entity rather than its place in a situation or a spatial framework. Overall, this is highly consistent with a role for the hippocampus in interfacing between distinct cortical networks in orchestrating cognitive processes, flexibly adapting its functional relationships to suit the task at hand.
Coming back to the concept of semantic and episodic autobiographical memory, the key idea is that there are different forms of knowledge supported by the PM and AT networks. During the experience of an event, we envision unique patterns of activity in these networks associated with the processing of specific situations or people. Going back to the CLS framework, however, we can expect that the PM and AT networks exhibit overlapping representations of similar situations and entites, respectively (i.e., overlapping PM representations of birthday parties and overlapping AT representations of birthday cakes). Thus, if hippocampal pattern completion were to fail, one would primarily generate semantic information during retrieval. If successful, however, hippocampal feedback could lead these networks to approximate the specific pattern of activity associated with the event in question (see Figure 1).
There is one interesting area of autobiographical memory research that we have not yet addressed—many researchers have argued that self-knowledge (or personal semantics), can be differentiated from knowledge of others (Conway & Pleydell-Pierce, 2000). Adding further complexity to the issue, there may be an important distinction between “experience near” personal semantics that are tied to particular events and “experience far” semantic knowledge that is not tied to any event Grilli & Verfaellie, 2016). Although a deep exploration of this issue is beyond the scope of this paper, it is notable that a review by Grilli & Verfaellie (2018) pointed out that patients with amnesia often show specific deficits in personal semantic memory that is tied to particular events, whereas deficits in memory for autobiographical facts was generally observed only in a subset of patients with damage extending to regions of the AT network. Other evidence has suggested that ventromedial and medial frontopolar PFC might play a role in reflection on self-related characteristics (Simons et al., 2015), and that this process might depend on “simulation” (i.e., activation of a self-relevant situation model) that additionally engage the PM network (Benoit et al., 2010).
Memory Specificity and Distortion.
Across many paradigms, researchers have found that people can confidently endorse new information as having been studied (Roediger & McDermott, 1995), and people can even voluntarily generate memories for events that did not happen (Loftus, 1997). In some cases, this involves actively presenting participants with conflicting information (e.g., framing recall cues such that a viewed stop sign in a video was later remembered as a yield sign; Loftus & Pickerell, 1995), whereas in other cases mere contextual associates among studied items (e.g., presenting subjects with novel words that are semantically related to those studied; Roediger & McDermott, 1995) leads to this phenomenon. Traditional theories of memory would describe these phenomena as simple errors that reflect a relatively weak response criterion (Miller & Wolford, 1999), or a close match between an unstudied foil and the average of several studied items (e.g., Raaijmakers & Shiffrin, 1992), but it has become clear that these accounts cannot explain the results from Roediger & McDermott’s (1995) paradigm, let alone the range of memory distortions seen in a broader range of paradigms (Gallo, 2010).
Drawing from our account of cortico-hippocampal networks, we can consider a few sources of memory errors. The simplest account, in cases of false item recognition, can be that processing of multiple items that share similar features can activate a shared conceptual representation supported by regions in the AT network, thereby supporting fluent processing, and attributions of familiarity to a similar, but nonstudied item (Wang & Yonelinas, 2012; Norman & O’Reilly, 2003; Wang et al., 2010; Wang, Ranganath, & Yonelinas, 2014). Another source of memory distortion, however, can arise from overreliance on PM-mediated representations. For instance, during processing of a thematically-related word list (e.g., smoke, beef, backyard), we would expect activation of contextual representations that could lead one to generate a situation model (“barbecue”) during encoding. In this case, processing of a novel, but contextually-related item (“grill”), reactivation of this situation model might lead one to confidently infer that the item was previously encountered, a phenomenon known as gist-based memory distortion (Johnson & Raye, 1998; Koutstaal and Schacter, 1997; Loftus & Pickrell, 1995).
We can consider the line between memory retrieval and memory accuracy as reflecting the balance between different types of information. Overreliance on a PM-mediated situation model could lead one to erroneously ignore local details that are inconsistent with past experiences, whereas overemphasis on local features may lead one to misattribute fluent processing to having previously encountered a particular item (Jacoby, Bjork, & Kelley, 1994). The hippocampus, can play a key role in mediating the balance by constraining the activation of AT and PM network representations, thereby enabling accurate recollection of details processed in a specific spatiotemporal context. Even in the context of relatively accurate recollection, however, presentation of novel, but situationally-consistent information (Loftus & Pickerell, 1995) could lead to modification of the existing memory trace or the formation of a new, competing cortico-hippocampal memory trace. Whether the memory is updated, or a new trace is formed, we would expect that processing of new, situationally-consistent information to lead to memory distortions, via the same cortico-hippocampal circuitry that supports accurate recollection.
Alternative Accounts and Currently Unresolved Issues
The foundation for our review has been that there is a broad base of reliable findings from cognitive neuroscience that can be linked with cognitive processes that support memory. Our interpretation of these findings has been guided by multiple theoretical accounts, including the CLS and BIC models, and by the “PMAT” framework (Inhoff & Ranganath, 2017; Ranganath & Ritchey, 2012; Ritchey, Libby, & Ranganath, 2015), which suggests complementary contributions of the PM and AT networks cognition. We note that there are related, plausible accounts, however, that speak to similar issues. For instance, Robin and Moscovitch (2017) recently proposed a model arguing for a gradient of memory specificity along the hippocampal longitudinal axis, extending into anterior and posterior cortical areas. Specifically, the posterior hippocampus is thought to support memory for details, whereas the anterior hippocampus is thought to support gist-like representations. Following this hippocampal gradient, posterior cortical regions are proposed to represent specific perceptual details, whereas anterior regions are proposed to represent general, gist-based information. This model also explicitly posits a specific role for ventromedial prefrontal cortex in supporting schemas, in line with proposals by Schlichting and Preston (2015). Robin and Moscovitch (2017) argue that hippocampal specificity gradients are of central importance, and that this drives distinctions in connected cortical areas rather than information content per se. Conversely, PMAT proposes that anterior and posterior cortical regions involved in memory are largely distinguished by the types of information supported (i.e., entities versus situational representations). Although the PM and AT networks would be expected to differentially interact with the anterior and the posterior hippocampus (Kahn et al., 2008; Libby et al., 2012; Poppenk, Evensmoen, & Moscovitch, 2013), the PMAT model proposes that the basis for differences between the two networks goes beyond the direct influence of hippocampal specificity gradients. Though a full exploration of this issue is beyond the scope of the present review, we believe these models to be largely complimentary. For instance, representation of situation models in PM regions could be viewed as more granular than representations of local features in AT regions. However, predictions about the primary direction of influence between the hippocampus and neocortical areas differ. Future studies can be designed to elaborate on the extent to which cortico-hippocampal interactions are driven by generality/specificity versus the manner of information being represented. It is certainly possible that, to an extent, both phenomena are at play.
A recurrent theme in the frameworks described above is that both make reference to the role of schemas. Schemas have been the focus of many recent studies of perceptual and mnemonic processes (van Kesteren et al., 2013). The most frequently discussed candidate region supporting schemata is ventromedial prefrontal cortex (Robin & Miscovitch, 2017; Schlichting & Preston, 2015; van Kesteren et al., 2013), though others have suggested - as we do here - that regions of a broader PM network may be involved (Aly et al., 2017). Proposals for the function of schemas in guiding decisions (Kumaran et al., 2009; Rumelhart, 1980), facilitating inference (Preston & Eichenbaum, 2013; van Kesteren et al., 2013), and expediting retrieval (Anderson & Pichert, 1978) have been put forward.
Making sense of these different ideas is complicated by the fact that the meaning of a “schema” is ambiguous. Some have used the term to refer to a collection of associated features or concepts, whereas others reserve the term to refer to structured knowledge about events and situations. In future studies, it will be important for researchers to clarify their operational definition of a schema, and furthermore to flesh out situations in which it is (and is not) useful to think of cognitive or neural processes as involving schema. The types of cognition that are influenced by a schema, and the underlying neural substrates might critically depend on the way in which a schema is operationalized in a given study.
We believe that it may be useful to think of a “schema” in reference to a class of situations that specifies the roles of, and relationships amongst, particular individuals, thereby enabling predictions about what is likely to occur in the near future. One can define “context” in terms of a particular place or situation, and the schema, along with context-specific cues, can be used to generate a situation model which guides processing of the event. For example, while ordering espresso at a café, the available cues at the café can trigger activation of a schema for the general experience of being in a café. The schema, along with features of the specific café and its inhabitants can be used to generate a situation model which enables predictions about roles and likely sequences of events. In this example, the situation model would allow us to infer that the customer will give money to the cashier, the barista will make the espresso, and customer will drink it.
Another issue warranting further investigation is the relative importance of spatial information in memory (Lisman et al., 2017). The hippocampus and a majority of regions comprising the PM network are reliably engaged during active navigation (Aguirre et al., 1996; Sherrill et al., 2013), and to a lesser extent, during scene perception (Epstein & Kanwisher, 1998; Park & Chun, 2009). Echoing the ideas of O’Keefe and Nadel (1978), Robin, Buchsbaum, and Moscovitch (2018) argued that spatial context plays a privileged role in episodic memory, and in representation of memories by the hippocampus and PM network. At the same time, considerable evidence suggests that these areas might encode dimensional information that is used to understand and represent particular events and situations, rather than simply locations within a 3-dimensional environment (Eichenbaum & Cohen, 2014). For example, a recent study by Constantinescu, O’Reilly, and Behrens (2017) reported that hexagonally-distributed fMRI adaptation signals, previously taken as evidence for “grid-cell-like” responses in human virtual reality navigation studies (Doeller, Barry, & Burgess, 2010), may also carry information about abstract feature space in PM regions. Specifically, they report “grid-like” signals encoding conceptual knowledge about the relative length of legs and necks of birds. A related study by Mack, Love, and Preston (2016) found similar evidence for abstract feature space representations in the hippocampus using complex objects (in this case, beetles separated into classes based on antenna and leg configurations). In a similar vein, Tavares et al. (2015) reported that activity in the hippocampus and PM network tracked distances from oneself and others in a two-dimensional space defined by social characteristics of Power and Affiliation.
Though further studies are necessary to come to a fuller understanding of these findings, they may suggest that space is not especially privileged among types of contextual information. However, it is possible that temporal and spatial context together play a key role in discovering the behaviorally-relevant features of the current situation (Ekstrom & Ranganath, 2017). For instance, people tend to use statistics derived across experiences within a recent time window in order to ascertain the relevant feature space in complex decision tasks (Clapper & Bower, 2002; Tervo et al., 2016).
Although many arguments about spatial representation focus on the hippocampus (and to a lesser extent, the PM network), it is important to note that some findings highlight a role for the PRC in spatial cognition. For instance, Connor & Knierim (2017) argued that PRC, in concert with the lateral entorhinal cortex and hippocampus collectively form an “external navigation” system that represents information about local landmarks and their spatial relationships. The Connor and Knierim model was designed to account for a relatively narrow range of findings about PRC in the context of perception and free foraging tasks, and it does not address a broad range of tasks that show preferential recruitment of PRC, along with other regions in the AT network. Nonetheless, their model highlights the fact that, rather than being totally independent, in many situations, the PM and AT networks may heavily interact with one another (via reciprocal connections between lateral and medial entorhinal cortex), and with modality-specific cortical regions. Thus, it may be insufficient to consider whether “spatial” information plays a privileged role in memory, and instead, it may be necessary to consider how kinds of representations may be brought together to accomplish particular tasks.
General conclusions
Many researchers have argued about whether neuroimaging, and more broadly, cognitive neuroscience, can explain anything about human cognition (Coltheart, 2006; Henson, 2006; Jonides et al., 2006). This debate is, in our opinion, based on the flawed premise that there should be a one-way flow of information from neuroscience to cognitive psychology. The present review shows how ideas derived from cognitive neuroscience can be accommodated with, and potentially provide insights into, findings, phenomena, and controversies in the psychology of human memory. The significance of the data goes beyond simple models that only make predictions about behavior. We believe that the field has reached a point where researchers can promote unified models of cognition and brain function that make predictions at both the behavioral and neural levels (Love, 2016). Although our knowledge is still quite limited, we are optimistic that major advances in our understanding of memory will come from a understanding the functional organization of the cortico-hippocampal networks that collectively support the process of remembering.
Acknowledgments
This work was supported by T32 AG 50061–2 to Z.M.R., and Vannevar Bush Faculty Fellowship (Office of Naval Research Grant N00014–15-1–0033) to C.R. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research or the U.S. Department of Defense.
References
- 1.Addante RJ, Ranganath C, Yonelinas AP (2012). Examining ERP correlates of recognition memory: Evidence of accurate source recognition without recollection. Neuroimage 62(1):439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre GK, Detre JA, Alsop DC, D’Esposito M (1996). The parahippocampus subserves topographical learning in man. Cerebral cortex 6(6):823–829. [DOI] [PubMed] [Google Scholar]
- 3.Aly M, Chen J, Turk-Browne NB, Hasson U (2017). Learning naturalistic temporal structure in the posterior medial network. bioRxiv 196287. [DOI] [PMC free article] [PubMed]
- 4.Aminoff E, Gronau N, Bar M (2006). The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex 17(7):1493–1503. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RC, Pichert JW (1978). Recall of previously unrecallable information following a shift in perspective. Journal of verbal learning and verbal behavior 17(1):1–12. [Google Scholar]
- 6.Andrews-Hanna JR, Saxe R, Yarkoni T (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage 91:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey HR, Zacks JM (2015). Situation model updating in young and older adults: Global versus incremental mechanisms. Psychology and aging, 30(2), 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett FC, Burt C (1933). Remembering: A study in experimental and social psychology. British Journal of Educational Psychology, 3(2), 187–192. [Google Scholar]
- 9.Benoit RG, Gilbert SJ, Volle E, Burgess PW (2010). When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage, 50(3), 1340–1349. [DOI] [PubMed] [Google Scholar]
- 10.Berron D, Schütze H, Maass A, Cardenas-Blanco A, Kuijf HJ, Kumaran D, Düzel E (2016). Strong evidence for pattern separation in human dentate gyrus. Journal of Neuroscience, 36(29), 7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boylan C, Trueswell JC, Thompson-Schill SL (2017). Relational vs. attributive interpretation of nominal compounds differentially engages angular gyrus and anterior temporal lobe. Brain and language 169:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MW, Aggleton JP (2001). Recognition memory: what are the roles of the perirhinal cortex and hippocampus?. Nature Reviews Neuroscience 2(1):51–61. [DOI] [PubMed] [Google Scholar]
- 13.Burgess N (2002). The hippocampus, space, and viewpoints in episodic memory. The Quarterly Journal of Experimental Psychology: Section A 55(4):1057–1080. [DOI] [PubMed] [Google Scholar]
- 14.Cary M, Reder LM (2003). A dual-process account of the list-length and strength-based mirror effects in recognition. Journal of Memory and Language, 49(2):231–248. [Google Scholar]
- 15.Chan JC, McDermott KB (2007). The testing effect in recognition memory: a dual process account. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33(2):431–437. [DOI] [PubMed] [Google Scholar]
- 16.Clarke A, Tyler LK (2014). Object-specific semantic coding in human perirhinal cortex. Journal of Neuroscience 34(14):4766–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapper JP, Bower GH (2002). Adaptive categorization in unsupervised learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 28(5), 908. [DOI] [PubMed] [Google Scholar]
- 18.Cohn-Sheehy BI, Ranganath C (2017). Time regained: How the human brain constructs memory for time. bioRxiv 186601. [DOI] [PMC free article] [PubMed]
- 19.Connor CE, Knierim JJ (2017). Integration of objects and space in perception and memory. Nature neuroscience, 20(11), 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coltheart M (2006). Perhaps functional neuroimaging has not told us anything about the mind (so far). Cortex, 42(3), 422–427. [DOI] [PubMed] [Google Scholar]
- 21.Constantinescu AO, O’Reilly JX, Behrens TE (2016). Organizing conceptual knowledge in humans with a gridlike code. Science 352(6292):1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conway MA, Pleydell-Pearce CW (2000). The construction of autobiographical memories in the self-memory system. Psychological review, 107(2), 261. [DOI] [PubMed] [Google Scholar]
- 23.Davachi L (2006). Item, context and relational episodic encoding in humans. Current opinion in neurobiology 16(6):693–700. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh SS, Knierim JJ (2013). Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus 23(4):253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diana RA, Yonelinas AP, Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in cognitive sciences 11(9):379–386. [DOI] [PubMed] [Google Scholar]
- 26.Diana RA, Yonelinas AP, & Ranganath C (2008). High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus 18(6):536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doeller CF, Barry C, Burgess N (2010). Evidence for grid cells in a human memory network. Nature 463(7281):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eacott MJ, Gaffan EA (2005). The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. The Quarterly Journal of Experimental Psychology Section B 58(3–4):202–217. [DOI] [PubMed] [Google Scholar]
- 29.Eichenbaum H (2017). On the Integration of Space, Time, and Memory. Neuron 95(5):1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichenbaum H, Yonelinas AP, Ranganath C (2007). The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekstrom AD, Ranganath C (2017). Space, Time and Episodic Memory: the Hippocampus is all over the Cognitive Map. Hippocampus [DOI] [PubMed]
- 32.Elliott R, Dolan RJ, Frith CD (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral cortex, 10(3), 308–317. [DOI] [PubMed] [Google Scholar]
- 33.Epstein R, Kanwisher N (1998). A cortical representation of the local visual environment. Nature 392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- 34.Fortin NJ, Agster KL, Eichenbaum HB (2002). Critical role of the hippocampus in memory for sequences of events. Nature neuroscience 5(5):458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo DA (2010). False memories and fantastic beliefs: 15 years of the DRM illusion. Memory & Cognition, 38(7), 833–848. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh VE, Gilboa A (2014). What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia, 53, 104–114. [DOI] [PubMed] [Google Scholar]
- 37.Gold AE, Kesner RP (2005). The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus, 15(6), 808–814. [DOI] [PubMed] [Google Scholar]
- 38.Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD (2005). Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron, 47(5):751–761. [DOI] [PubMed] [Google Scholar]
- 39.Grill-Spector K, Henson R, Martin A (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends in cognitive sciences, 10(1), 14–23. [DOI] [PubMed] [Google Scholar]
- 40.Hannula DE, Tranel D, & Cohen NJ (2006). The long and the short of it: relational memory impairments in amnesia, even at short lags. Journal of Neuroscience 26(32):8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haskins AL, Yonelinas AP, Quamme JR, Ranganath C (2008). Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron 59(4):554–560. [DOI] [PubMed] [Google Scholar]
- 42.Hasson U, Chen J, Honey CJ (2015). Hierarchical process memory: memory as an integral component of information processing. Trends in cognitive sciences 19(6):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes SM, Nadel L, Ryan L (2007). The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus 17(9):873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henson R (2006). What has (neuro) psychology told us about the mind (so far)? A reply to Coltheart (2006). Cortex, 42(3), 387–392. [DOI] [PubMed] [Google Scholar]
- 45.Henson RN, Gagnepain P (2010). Predictive, interactive multiple memory systems. Hippocampus 20(11):1315–1326. [DOI] [PubMed] [Google Scholar]
- 46.Hintzman DL, Caulton DA, Levitin DJ (1998). Retrieval dynamics in recognition and list discrimination: Further evidence of separate processes of familiarity and recall. Memory & Cognition, 26(3):449–462. [DOI] [PubMed] [Google Scholar]
- 47.Horner AJ, Bisby JA, Bush D, Lin WJ, Burgess N. (2015). Evidence for holistic episodic recollection via hippocampal pattern completion. Nature communications, 6, 7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron 81(5):1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huffman DJ, Stark CEL (2014). Multivariate pattern analysis of the human medial temporal lobe revealed representationally categorical cortex and representationally agnostic hippocampus. Hippocampus 24(11):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inhoff MC, Ranganath C (2017). Dynamic cortico-hippocampal networks underlying memory and cognition: the PMAT framework. In The Hippocampus from Cells to Systems (pp. 559–589). Springer, Cham. [Google Scholar]
- 51.Jacoby LL (1991). A process dissociation framework: Separating automatic from intentional uses of memory. Journal of memory and language, 30(5):513–541. [Google Scholar]
- 52.Jacoby LL, Bjork RA, Kelley CM (1994). Illusions of comprehension, competence, and remembering. Learning, remembering, believing: Enhancing human performance, 57–80.
- 53.Janzen G, Van Turennout M (2004). Selective neural representation of objects relevant for navigation. Nature neuroscience 7(6):673–677. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JD, Rugg MD (2007). Recollection and the reinstatement of encoding-related cortical activity. Cerebral Cortex 17(11):2507–2515. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MK, Raye CL (1998). False memories and confabulation. Trends in cognitive sciences 2(4):137–145. [DOI] [PubMed] [Google Scholar]
- 56.Jonides J, Nee DE, Berman MG (2006). What has functional neuroimaging told us about the mind? So many examples, so little space. Cortex, 42(3), 414–417. [DOI] [PubMed] [Google Scholar]
- 57.Kafkas A, Montaldi D (2014). Two separate, but interacting, neural systems for familiarity and novelty detection: A dual-route mechanism. Hippocampus, 24(5), 516–527. [DOI] [PubMed] [Google Scholar]
- 58.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008) Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley CM, Jacoby LL (1998). Subjective reports and process dissociation: Fluency, knowing, and feeling. Acta Psychologica 98(2):127–140. [Google Scholar]
- 60.Kent BA, Hvoslef-Eide M, Saksida LM, & Bussey TJ (2016). The representational–hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiology of learning and memory, 129, 99–106. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Delcasso S, Lee I (2011). Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. Journal of Neuroscience 31(47):16991–17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knierim JJ (2006). Neural representations of location outside the hippocampus. Learning & Memory, 13(4), 405–415 [DOI] [PubMed] [Google Scholar]
- 63.Knierim JJ, Neunuebel JP (2016). Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiology of learning and memory 129:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konkel A, Cohen NJ (2009). Relational memory and the hippocampus: representations and methods. Frontiers in neuroscience 3(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koutstaal W, Schacter DL (1997). Gist-based false recognition of pictures in older and younger adults. Journal of Memory and Language 37(4):555–583. [Google Scholar]
- 66.Kravitz DJ, Saleem KS, Baker CI, Mishkin M (2011) A new neural framework for visuospatial processing. Nat Rev Neurosci 12(4):217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA (2009). Tracking the emergence of conceptual knowledge during human decision making. Neuron, 63(6):889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaRocque KF, Smith ME, Carr VA, Witthoft N, Grill-Spector K, Wagner AD (2013). Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. Journal of Neuroscience, 33(13), 5466–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM (2000). Neural systems underlying the recognition of familiar and newly learned faces. Journal of Neuroscience, 20(2), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M (2002). Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and aging, 17(4), 677. [PubMed] [Google Scholar]
- 71.Liang JC, Wagner AD, Preston AR (2012). Content representation in the human medial temporal lobe. Cerebral Cortex 23(1):80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libby LA, Ekstrom AD, Ragland JD, Ranganath C (2012). Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. Journal of Neuroscience, 32(19), 6550–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libby LA, Hannula DE, Ranganath C (2014). Medial temporal lobe coding of item and spatial information during relational binding in working memory. Journal of Neuroscience 34(43):14233–14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Litman L, Awipi T, Davachi L (2009). Category-specificity in the human medial temporal lobe cortex. Hippocampus 19(3):308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu P, Bilkey DK (1998a). Excitotoxic lesions centered on perirhinal cortex produce delay-dependent deficits in a test of spatial memory. Behavioral neuroscience 112(3), 512–524. [DOI] [PubMed] [Google Scholar]
- 76.Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Rangananth C, Redish AD (2017). Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nature, 20, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livne T, Bar M (2016). Cortical integration of contextual information across objects. Journal of cognitive neuroscience 28(7):948–958. [DOI] [PubMed] [Google Scholar]
- 78.Loftus EF (1997). Creating false memories. Scientific American 277(3):70–75. [DOI] [PubMed] [Google Scholar]
- 79.Loftus EF, Pickrell JE (1995). The formation of false memories. Psychiatric annals, 25(12):720–725. [Google Scholar]
- 80.Love BC (2016). Cognitive models as bridge between brain and behavior. Trends in cognitive sciences, 20(4), 247–248. [DOI] [PubMed] [Google Scholar]
- 81.Mack ML, Love BC, Preston AR (2016). Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proceedings of the National Academy of Sciences, 113(46), 13203–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maguire EA, Frith CD, Burgess N, Donnett JG, O’Keefe J (1998). Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. Journal of Cognitive Neuroscience 10(1):61–76. [DOI] [PubMed] [Google Scholar]
- 83.Mandler G (2008). Familiarity breeds attempts: A critical review of dual-process theories of recognition. Perspectives on Psychological Science 3(5):390–399. [DOI] [PubMed] [Google Scholar]
- 84.Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N (2002). Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus 12(3):325–340. [DOI] [PubMed] [Google Scholar]
- 85.Mayes A, Montaldi D, Migo E (2007). Associative memory and the medial temporal lobes. Trends in cognitive sciences, 11(3), 126–135. [DOI] [PubMed] [Google Scholar]
- 86.McClelland JL, McNaughton BL, & O’reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological review, 102(3), 419. [DOI] [PubMed] [Google Scholar]
- 87.McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H (2014). Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron 83(1):202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishkin M, Suzuki WA, Gadian DG, Vargha–Khadem F (1997). Hierarchical organization of cognitive memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences 352(1360):1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyashita Y (1988). Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature 335(6193):817–820. [DOI] [PubMed] [Google Scholar]
- 90.Montaldi D, Spencer TJ, Roberts N, & Mayes AR (2006). The neural system that mediates familiarity memory. Hippocampus 16(5):504–520. [DOI] [PubMed] [Google Scholar]
- 91.Mumby DG (2001). Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behavioural brain research 127(1):159–181. [DOI] [PubMed] [Google Scholar]
- 92.Mundy ME, Downing PE, Dwyer DM, Honey RC, Graham KS (2013). A critical role for the hippocampus and perirhinal cortex in perceptual learning of scenes and faces: complementary findings from amnesia and fMRI. Journal of Neuroscience 33(25):10490–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray EA, Wise SP (2012). Why is there a special issue on perirhinal cortex in a journal called hippocampus? The perirhinal cortex in historical perspective. Hippocampus, 22(10), 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nadel L, Peterson MA (2013) The hippocampus: part of an interactive posterior representational system spanning perceptual and memory systems. J Exp Psychol Gen 142(4):1242–1254. [DOI] [PubMed] [Google Scholar]
- 95.Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002). Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science, 297(5579), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neunuebel JP, & Knierim JJ (2014). CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron, 81(2), 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norman KA, O’Reilly RC (2003). Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological review 110(4), 611–646. [DOI] [PubMed] [Google Scholar]
- 98.O’Keefe J, Nadel L (1978). The hippocampus as a cognitive map Oxford: Clarendon Press. [Google Scholar]
- 99.Olson IR, McCoy D, Klobusicky E, Ross LA (2013). Social cognition and the anterior temporal lobes: a review and theoretical framework. Social cognitive and affective neuroscience, 8(2), 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Reilly RC, McClelland JL (1994). Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4(6):661–682. [DOI] [PubMed] [Google Scholar]
- 101.O’Reilly RC, Norman KA (2002). Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in cognitive sciences 6(12):505–510. [DOI] [PubMed] [Google Scholar]
- 102.Park S, Chun MM (2009). Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. Neuroimage 47(4):1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Passingham RE, Stephan KE, Kötter R (2002). The anatomical basis of functional localization in the cortex. Nature Reviews Neuroscience, 3(8), 606. [DOI] [PubMed] [Google Scholar]
- 104.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science 321(5894):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P (2005). View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage, 24(4), 1214–1224. [DOI] [PubMed] [Google Scholar]
- 106.Preston AR, Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology 23(17):R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raaijmakers JG, Shiffrin RM (1992). Models for recall and recognition. Annual review of psychology, 43(1), 205–234. [DOI] [PubMed] [Google Scholar]
- 108.Radvansky GA, Zacks JM (2014). Event cognition Oxford University Press. [Google Scholar]
- 109.Ramos JM (2017). Perirhinal cortex involvement in allocentric spatial learning in the rat: Evidence from doubly marked tasks. Hippocampus 27(5):507–517. [DOI] [PubMed] [Google Scholar]
- 110.Ranganath C (2010). A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20(11):1263–1290. [DOI] [PubMed] [Google Scholar]
- 111.Ranganath C, Hsieh LT (2016). The hippocampus: a special place for time. Annals of the New York Academy of Sciences 1369(1):93–110. [DOI] [PubMed] [Google Scholar]
- 112.Ranganath C, Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience 13(10). [DOI] [PubMed] [Google Scholar]
- 113.Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, & D’Esposito M (2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42(1):2–13. [DOI] [PubMed] [Google Scholar]
- 114.Reagh ZM, Yassa MA (2014). Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proceedings of the National Academy of Sciences 111(40):E4264–E4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Renoult L, Davidson PS, Palombo DJ, Moscovitch M, Levine B (2012). Personal semantics: at the crossroads of semantic and episodic memory. Trends in cognitive sciences, 16(11), 550–558. [DOI] [PubMed] [Google Scholar]
- 116.Rinck M, Williams P, Bowe GH, Becker ES (1996). Spatial situation models and narrative understanding: Some generalizations and extensions. Discourse Processes, 21(1), 23–55. [Google Scholar]
- 117.Ritchey M, Libby LA, Ranganath C (2015). Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Progress in brain research 219:45–64. [DOI] [PubMed] [Google Scholar]
- 118.Ritchey M, Montchal ME, Yonelinas AP, Ranganath C (2015). Delay-dependent contributions of medial temporal lobe regions to episodic memory retrieval. Elife 4:e05025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ritchey M, Yonelinas AP, Ranganath C (2014) Functional connectivity relationships predict similarities in task activation and pattern information during associative memory encoding. J Cogn Neurosci 26(5):1085–1099. [DOI] [PubMed] [Google Scholar]
- 120.Robin J, Moscovitch M (2017). Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Current Opinion in Behavioral Sciences 17:114–123. [Google Scholar]
- 121.Robin J, Buchsbaum BR, Moscovitch MF (2018). The primacy of spatial context in the neural representation of events. Journal of Neuroscience, 38(11), 2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roediger HL, McDermott KB (1995). Creating false memories: Remembering words not presented in lists. Journal of experimental psychology: Learning, Memory, and Cognition, 21(4):803–814. [Google Scholar]
- 123.Rolls ET, Kesner RP (2006). A computational theory of hippocampal function, and empirical tests of the theory. Progress in neurobiology 79(1):1–48. [DOI] [PubMed] [Google Scholar]
- 124.Rugg MD, Vilberg KL (2013). Brain networks underlying episodic memory retrieval. Current opinion in neurobiology 23(2):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rumelhart DE (1980). On evaluating story grammars. Cognitive Science 4(3):313–316. [Google Scholar]
- 126.Scaplen KM, Gulati AA, Heimer-McGinn VL, & Burwell RD (2014). Objects and landmarks: hippocampal place cells respond differently to manipulations of visual cues depending on size, perspective, and experience. Hippocampus 24(11):1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schacter DL, Addis DR, Buckner RL (2007). Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience, 8(9), 657. [DOI] [PubMed] [Google Scholar]
- 128.Schapiro AC, Turk-Browne NB, Botvinick MM, Norman KA (2017). Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Phil. Trans. R. Soc. B 372(1711):20160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schapiro AC, Turk-Browne NB, Norman KA, Botvinick MM (2016). Statistical learning of temporal community structure in the hippocampus. Hippocampus 26(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schlichting ML, Preston AR (2015). Memory integration: neural mechanisms and implications for behavior. Current opinion in behavioral sciences 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sheldon S, Levine B (2018). The medial temporal lobe functional connectivity patterns associated with forming different mental representations. Hippocampus [DOI] [PubMed]
- 132.Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE (2013). Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. Journal of Neuroscience 33(49):19304–19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW (2005). Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. Journal of Neurophysiology, 94(1), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Squire LR, Zola-Morgan S (1991). The medial temporal lobe memory system. Science 253(5026):1380–1386. [DOI] [PubMed] [Google Scholar]
- 135.Staresina BP, Cooper E, Henson RN (2013). Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. Journal of Neuroscience, 33(35), 14184–14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.St. Jacques PL, De Brigard F (2015). Neural correlates of autobiographical memory. The Wiley Handbook on the Cognitive Neuroscience of Memory 265–286.
- 137.Svoboda E, McKinnon MC, Levine B (2006). The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44(12):2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D (2015). A map for social navigation in the human brain. Neuron, 87(1), 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tervo DGR, Tenenbaum JB, Gershman SJ (2016). Toward the neural implementation of structure learning. Current opinion in neurobiology, 37, 99–105. [DOI] [PubMed] [Google Scholar]
- 140.Tompary A, Duncan K, Davachi L (2016). High-resolution investigation of memory-specific reinstatement in the hippocampus and perirhinal cortex. Hippocampus 26(8):995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tulving E (1972). Episodic and semantic memory. Organization of memory 1:381–403. [Google Scholar]
- 142.Tulving E, Markowitsch HJ (1998). Episodic and declarative memory: role of the hippocampus. Hippocampus 8(3):198–204. [DOI] [PubMed] [Google Scholar]
- 143.van Kesteren MT, Beul SF, Takashima A, Henson RN, Ruiter DJ, Fernández G (2013). Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: from congruent to incongruent. Neuropsychologia 51(12):2352–2359 [DOI] [PubMed] [Google Scholar]
- 144.Viskontas IV, McAndrews MP, Moscovitch M (2000). Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. Journal of Neuroscience, 20(15), 5853–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang WC, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP (2010). The medial temporal lobe supports conceptual implicit memory. Neuron, 68(5), 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang S-F, Ritchey M, Libby LA, Ranganath C (2016) Functional connectivity based parcellation of the human medial temporal lobe. Neurobiol Learn Mem 134:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang WC, Ranganath C, Yonelinas AP (2014). Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia 52:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang WC, Yonelinas AP (2012). Familiarity and conceptual implicit memory: Individual differences and neural correlates. Cognitive neuroscience, 3(3–4), 213–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ward AM, Schultz AP, Huijbers W, Dijk KR, Hedden T, Sperling RA(2014). The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Human brain mapping 35(3):1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winocur G, Moscovitch M (2011). Memory transformation and systems consolidation. Journal of the International Neuropsychological Society, 17(5), 766–780. [DOI] [PubMed] [Google Scholar]
- 151.Yassa MA, Stark CEL (2011). Pattern separation in the hippocampus. Trends in neurosciences 34(10):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yonelinas AP (1994). Receiver-operating characteristics in recognition memory: evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(6):1341–1354. [DOI] [PubMed] [Google Scholar]
- 153.Yonelinas AP (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of memory and language, 46(3):441–517. [Google Scholar]
- 154.Young MP, Scanneil JW, Burns GA, Blakemore C (1994). Analysis of connectivity: neural systems in the cerebral cortex. Reviews in the Neurosciences, 5(3), 227–250. [DOI] [PubMed] [Google Scholar]
- 155.Zwaan RA, Langston MC, Graesser AC (1995). The construction of situation models in narrative comprehension: An event-indexing model. Psychological science, 6(5), 292–297 [Google Scholar]
- 156.Zhuo J, Fan L, Liu Y, Zhang Y, Yu C, Jiang T (2016) Connectivity profiles reveal a transition subarea in the parahippocampal region that integrates the anterior temporal-posterior medial systems. J Neurosci 36(9):2782–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]


