Abstract
Objective:
In 2013, Tanzania adopted the World Health Organization’s Option B+ guidelines for prevention of mother-to-child transmission of HIV (PMTCT), whereby all HIV-infected pregnant women initiate lifelong antiretroviral therapy. This study examined retention in PMTCT across critical junctures in the care continuum.
Methods:
This was a retrospective study of patient-level data for a cohort of women enrolled in PMTCT during the first year of Option B+ in Tanzania. Retention in care was described across three periods: (1) the first month of antenatal care (ANC), (2) pregnancy, and (3) the postpartum period. Logistic regression was used to identify factors associated with loss to follow up (LTFU) during the first month of ANC. Survival analyses were used to identify factors associated with LTFU during pregnancy and the postpartum periods.
Results:
650 participants were included in the cohort; 262 (40.3%) were newly diagnosed with HIV. Two years after delivery, 383/650 (58.7%) were LTFU. Of the 383 LTFU, 73 (19.1%) were lost during the first month of ANC, 44 (11.5%) during pregnancy, and 266 (69.5%) after delivery. Being newly diagnosed with HIV predicted higher LTFU during the first month of ANC (aOR 1.76; 95% CI: 1.06 – 2.94) and faster time to LTFU during the postpartum period (adjusted relative time, 0.68; 95% CI: 0.51 – 0.89).
Conclusions:
High LTFU occurred across the PMTCT continuum, including immediately after enrollment into ANC and the postpartum period. Ongoing research is needed to encourage treatment uptake and sustained engagement after delivery.
Keywords: PMTCT, HIV, Retention in care, LTFU, Option B+, Tanzania
Introduction
The prevention of mother-to-child transmission of HIV (PMTCT) is a key priority in efforts to reduce the global burden of HIV, including the UNAIDS target of achieving an “AIDS-free generation” (1). In 2012, the World Health Organization (WHO) released new treatment guidelines, termed Option B+, endorsing lifelong antiretroviral treatment (ART) for all pregnant women with HIV beginning at the first antenatal appointment (2). Option B+ was intended to simplify treatment regimens, prevent vertical transmission, decrease transmission in serodiscordant relationships, and improve the long-term health and wellbeing of women living with HIV (2).
The implementation of Option B+ in sub-Saharan Africa led to rapid increases in access to lifelong ART, particularly because pregnancy is a vital catch point for HIV testing and diagnosis (3). Despite widespread agreement that the new guidelines will increase ART coverage and improve individual and population health, concerns have been expressed that sub-optimal retention in PMTCT programs could compromise the success of Option B+ (4,5). Researchers have suggested that the “one size fits all” approach to treatment, namely that all HIV-infected pregnant women are encouraged to initiate lifelong treatment at the time of diagnosis, may leave little room for tailored approaches that are reflexive to women’s readiness to initiate daily medication for life (4,6). There has been concern that the early discrepancy between providers’ instructions and women’s readiness for treatment may make them more likely to disengage from care (7,8).
A recent systematic review identified 22 studies examining rates of retention under Option B+ in sub-Saharan Africa, along with 25 studies that explored predictors of retention under Option B+ in sub-Saharan Africa (5). In pooled analysis, retention in care was estimated to be 79.4%, 74.5%, and 69.3%, at 6, 12, and 24 months after initiation of ART, respectively. Qualitative studies highlighted the roll of stigma, fear of disclosure, and lack time to accept a new diagnosis of HIV as important barriers to long term care engagement (5,9–14). Many quantitative studies have reported that women who are younger and diagnosed with HIV during pregnancy are more likely to drop out of care (5,15,16). Data from outside sub-Saharan Africa, including the US and Europe, demonstrate similarly low levels of retention in care and viral suppression among pregnant and post-partum women (17).
While the existing studies provide important baseline data describing retention in Option B+ programs, nearly all the studies have measured retention from the time of initiation on ART, without accounting for the important life transitions women make from pregnancy into the postpartum period (5). Given that pregnancy, the birth of a child, and breastfeeding are central events in the PMTCT treatment cascade, these studies missed an opportunity to evaluate which transitions in PMTCT care are associated with an increased risk of loss to follow up (LTFU) (18). These data may be crucial to informing the content and timing of interventions aimed at improving ART uptake and retention throughout pregnancy and the postpartum period.
The purpose of this study is to examine retention in PMTCT care under Option B+ among a cohort of women in Tanzania, including an examination of LTFU at critical junctures in the PMTCT care continuum. We assess the risk of LTFU in women diagnosed with HIV for the first time during pregnancy and compare it to the risk of those who had previously established diagnoses, while examining other factors that may be associated with LTFU.
Methods
This study is based on a retrospective review of patient medical record data in the Tanzanian National AIDS Control Program (NACP) database. The data represent patients who were enrolled in PMTCT services under Option B+ treatment guidelines in the Kilimanjaro region during the first year Option B+ was implemented: February 1, 2014 to January 31, 2015. The study received ethical clearance from the Tanzanian National Institute of Medical Research and the IRBs at the Kilimanjaro Christian Medical Centre and Duke University. Permission to use national PMTCT data was received from the Ministry of Health, Community Development, Gender, Elderly, and Children, through the NACP and PMTCT coordination unit.
Database and Study Participants
The Tanzanian NACP coordinates HIV prevention, care and treatment, and also monitors the success of HIV programs through an aggregated national database. All individuals enrolled in HIV care are given a unique patient identification number that is used in medical record and NACP data. Tanzania’s national PMTCT guidelines stipulate that HIV-infected pregnant women should return to the clinic monthly for evaluation and ART refills (19,20). At each clinic visit, a health care provider updates the paper-based medical record with demographic, clinical, and health care utilization data. These data are later entered by clinic staff into a computerized database, which is synchronized and aggregated with regional and national data. Deaths and transfers between clinics are routinely recorded in the NACP database and individuals maintain the same unique identifier when they switch clinics; however, if a person transfers unofficially (i.e. without informing clinic staff and obtaining a formal letter of transfer), a new identifier is given at the new clinic, leading to a “silent” or uncaptured transfer.
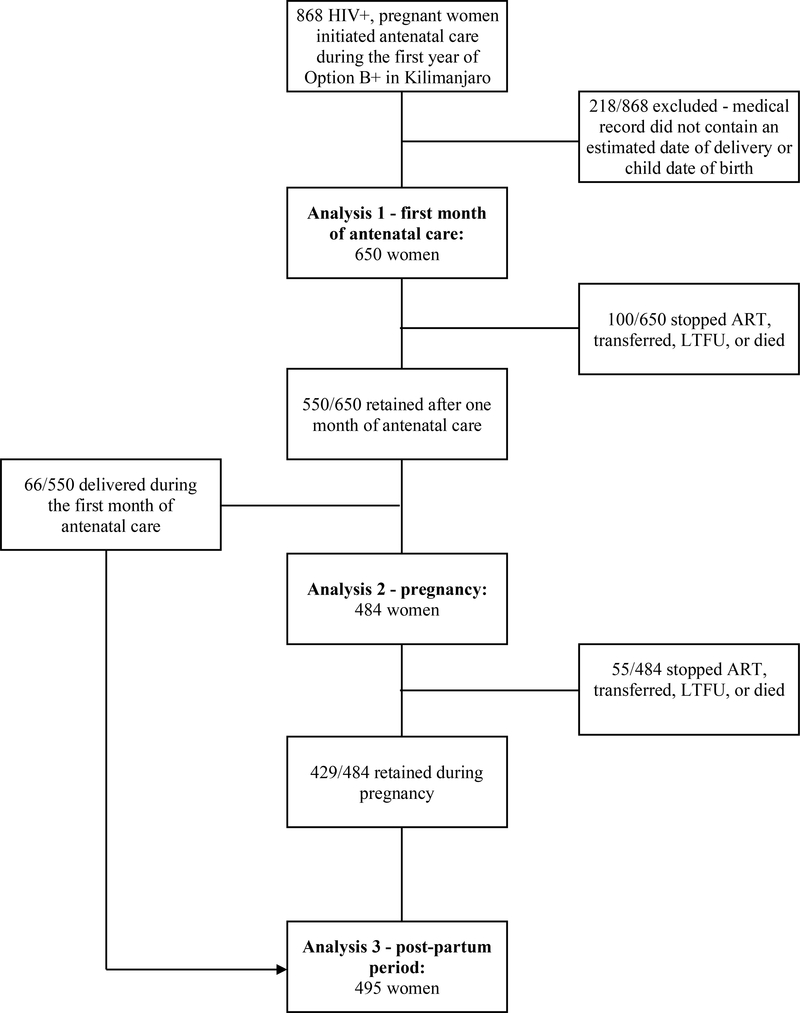
Under current guidelines, all women receiving antenatal care (ANC) in Tanzania are offered an HIV test at their first antenatal appointment. Women who test positive during pregnancy are captured in the NACP database, as are women known to be living with HIV who subsequently became pregnant. We used the NACP database to retrospectively identify a cohort of HIV-infected pregnant women who were registered in the database as pregnant during a one-year window between February 1, 2014 and January 31, 2015. Participants were included in the cohort if they attended their first ANC visit at a clinic in the Kilimanjaro region and were (a) known to be living with HIV prior to the first ANC visit, or (b) were diagnosed with HIV at their first ANC visit and successfully registered in the NACP database. A primary aim of this study was to map retention data onto the PMTCT care cascade, including the transition from pregnancy to the postpartum period. Therefore, women were excluded if their record in the NACP database did not contain either an expected date of delivery or an actual date of delivery for the index pregnancy (Figure 1).
Figure 1:
Women enrolling in PMTCT care during the first year of Option B+ in Kilimanjaro
Follow up data were available for the cohort through March 1, 2017. A minimum of 25 months of data were available from the time of the first ANC visit, with a maximum of 37 months of data. The first delivery in the cohort occurred in February 2014 and the last delivery in September 2015; a minimum of 18 months of postpartum follow up data was available, with a maximum of 36 months of data.
Outcomes
The primary outcomes of interest in this study was LTFU across the PMTCT care continuum. Within each individual’s NACP record, the date of every clinic visit is listed. We defined LTFU as a gap in clinical care >90 days, implying a woman who was LTFU would be without medicine for over two months; this definition is frequently used in studies measuring retention in care under Option B+ (21–23).
Retention in care was described across three discrete time periods: (1) the first month of ANC, (2) the remainder of pregnancy, excluding the first month of ANC, and (3) the postpartum period. Deaths, official transfers from one clinic to another, and known changes in ART status (e.g., a patient stopped taking ART or initiated ART) were captured in the NACP database. For this study, we considered LTFU and death as a composite outcome; the occurrence of death cannot be assumed to be independent from LTFU since those who stop taking ART are more likely to have poor clinical outcomes. Death was an infrequent occurrence in this study population (10 deaths were recorded out of 650 participants). No information about the cause of death was captured in the database. Women who officially transferred care or who stopped taking ART but continued to attend clinic appointments were considered retained in care.
Covariates
Within the NACP database, basic demographic information including age, address, and relationship status are typically recorded upon entry into HIV care. Clinical information is captured at baseline and each subsequent visit; recorded data include date of HIV diagnosis, date of ART initiation and indication, WHO staging, nutritional status, and any laboratory testing.
We used the recorded date of HIV diagnosis to dichotomize participants as either having a new diagnosis (diagnosed with HIV at the first ANC appointment of the index pregnancy) or an established diagnosis (known to have HIV at the time of first ANC appointment). As a secondary analysis, we used the date of ART initiation to compare women who were initiating ART at the first ANC visit to those were continuing ART.
Other co-variates of interest included age, marital/relationship status, gestational age of pregnancy at the first ANC visit, type of health facility for first ANC visit, location of the health facility, and date of the first ANC visit. Age was dichotomized as either being younger than 25 or 25 and older, consistent with other studies (13,24,25). Relationship status was dichotomized as either being in a relationship (married or cohabiting) or not (single, divorced or widowed). Of note, the measure of relationship status was limited in that it was not time stamped in the NACP database and may not have been regularly updated to accurately represent current relationship status. Gestational age at first ANC visit was calculated based upon the estimated due date and dichotomized as < 28 weeks (1st and 2nd trimester) or >28 weeks (3rd trimester). The type of facility at first ANC visit was categorized as a local clinic (dispensary or health center) or hospital. The location of the health care facility at the first ANC visit was classified as either urban (for facilities located within the Moshi Urban District of Kilimanjaro) or rural (for all other facilities in Kilimanjaro). To control for implementation issues associated with the rollout of Option B+, women were either classified as having attended their first ANC visit during the first 6 months of Option B+ or 6–12 months after the policy was implemented.
We were unable to consider other variables, including WHO clinical stage and laboratory data due the quality and consistency of these data in the NACP database.
Data analysis
Differences in baseline characteristics among participants with established HIV compared to those newly diagnosed with HIV during the index pregnancy were described with proportions and tested using the χ2 test. Multiple imputation was used to estimate relationship status for the 97/650 (14.9%) women in the cohort who had missing data.
We used a multivariable logistic regression to assess factors associated with LTFU during the first month after the first ANC visit. LTFU during the pregnancy and postpartum periods were modeled using multivariable parametric survival analyses, assuming an underlying generalized gamma distribution. Only those participants who were retained during the first month of ANC and had not delivered were included in the pregnancy survival analysis (484/650; Figure 1). Similarly, only those who were retained until delivery were included in the postpartum survival analysis (495/650; Figure 1). A composite event of LTFU and death was used. Women were considered retained in care and administratively censored from the analyses if a transfer was documented or if they stopped taking ART and continue with clinic visits. We used a generalized gamma distribution to derive relative times to event, rather than typical relative hazards produced using a Cox regression. This approach allowed for the modeling of non-proportional hazards (26). The modeling parameters produced from the primary analysis presented in Table 3 are given in Supplemental Table 2. In order to assess the stability of the results produced through multiple imputation, a sensitivity analysis was conducted using complete data only. All analyses were completed in STATA (27).
Table 3:
Factors associated with LTFU during first month of ANC care, pregnancy and the post-partum period
| LTFU – first month of ANC (n = 650) | LTFU – pregnancy (n = 484) | LTFU- post-partum (n = 495) | |
|---|---|---|---|
| aOR [95% CI] | adjusted relative times* [95% CI] | ||
| HIV status | |||
| New diagnosis (REF: established diagnosis) | 1.76 [1.06 – 2.94]** | 0.61 [0.27 – 1.36] | 0.68 [0.51 – 0.89]** |
| Age at index pregnancy | |||
| < 25 (REF: ≥ 25) | 1.34 [0.76 – 2.38] | 1.33 [0.49 – 3.59] | 0.66 [0.47 – 0.91]** |
| Relationship status | |||
| Single, widowed or divorced (REF: married or cohabiting) | 1.02 [0.58 – 1.80] | 0.71 [0.32 – 1.59] | 1.18 [0.88 – 1.59] |
| Gestational age (1stvisit) | |||
| 3rd trimester (REF: 1st/2nd trimester) | 1.20 [0.68 – 2.12] | 1.21 [0.33 – 4.43] | 1.21 [0.90 – 1.63] |
| Type of health facility (1stvisit) | |||
| Local clinic (REF: Hospital) | 0.72 [0.43 – 1.20] | 1.28 [0.57 – 2.85] | 0.67 [0.51 – 0.88]** |
| Location of health facility (1stvisit) | |||
| Urban (REF: Rural) | 2.03 [1.22 – 3.38]** | 0.84 [0.39 – 1.81] | 0.92 [0.70 – 1.21] |
| Timing of first ANC visit | |||
| ≤ 6 months after Option B+ (REF: > 6 months) | 1.39 [0.85 – 2.30] | 1.37 [0.63 – 2.97] | 0.97 [0.74 – 1.26] |
All three models presented above were adjusted for all of the variables listed in the table above.
LTFU - initial month of PMTCT: logistic regression.
LTFU - pregnancy: parametric survival analysis (supplemental Table 2).
LTFU - post-partum: parametric survival analysis (supplemental Table 2).
A relative time describes the time to event compared to the reference group. If the value is less than 1, that variable is associated with a faster time to LTFU than the reference group. For example, participants newly diagnosed with HIV were LTFU 32% faster than those with established HIV during the post-partum period.
Represents a significant association with LTFU based upon the 95% confidence interval and p <.05.
Results
A total of 868 women enrolled in PMTCT care in the Kilimanjaro region of Tanzania during the first year of Option B+, and 650 women were included in the study cohort (Figure 1). Participant characteristics are described in Table 1 (also see Supplemental Table 1). Of the 650 participants, 262 (40.3%) were newly diagnosed with HIV. Compared to women with established HIV diagnoses at the time of pregnancy, those who were newly diagnosed were more likely to be in a relationship (67.4% vs. 59.3%; p-value =.05), to initiate care at a local clinic (61.8% vs. 39.4%; p-value <.001), and to initiate care in an urban setting (50.0% vs. 40.2%; p-value =.01). Of the 388 with established HIV diagnoses, 147 (37.9%) were initiating ART for the first time.
Table 1:
Participant characteristics (n = 650)
| Variable | All Participants (n = 650) | Established HIV (n = 388) | New HIV (n = 262) | P-value* |
|---|---|---|---|---|
| Age at index pregnancy | ||||
| < 25 | 138 (21.2%) | 74 (19.1%) | 64 (24.4%) | .10 |
| ≥ 25 | 512 (78.8%) | 314 (80.9%) | 198 (75.6%) | |
| Relationship status** | ||||
| Married or cohabiting | 346 (62.6%) | 197 (59.3%) | 149 (67.4%) | .05 |
| Single, widowed or divorced | 207 (37.4%) | 135 (40.7%) | 72 (32.6%) | |
| Gestational age at 1stANC visit | ||||
| 1st or 2nd trimester | 475 (73.1%) | 273 (70.4%) | 202 (77.1%) | .06 |
| 3rd trimester | 175 (26.9%) | 115 (29.6%) | 60 (22.9%) | |
| Type of health facility (1stvisit) | ||||
| Local clinic | 315 (48.5%) | 153 (39.4%) | 162 (61.8%) | < .001 |
| Hospital | 335 (51.5%) | 235 (60.6%) | 100 (38.2%) | |
| Location of health facility (1stvisit) | ||||
| Rural | 363 (55.8%) | 232 (59.8%) | 131 (50.0%) | .01 |
| Urban | 287 (44.2%) | 156 (40.2%) | 131 (50.0%) | |
| Timing of first ANC visit | ||||
| ≤ 6 months after Option B+ | 318 (48.9%) | 182 (46.9%) | 136 (51.9%) | .21 |
| > 6 months after Option B+ | 332 (51.1%) | 206 (53.1%) | 126 (48.1%) | |
| ART status | ||||
| Initiating ART | 409 (62.9%) | 147 (37.9%) | 262 (100.0%) | |
| Continuing ART | 241 (37.1%) | 241 (62.1%) | 0 (0.0%) |
P-value derived from χ2 test.
Missing 97/650 values for “relationship status” variable; 56/388 with established HIV; 41/259 with new HIV.
After two years of postpartum follow up, 383/650 (58.9%) women were LTFU, 121/650 (18.6%) remained in care at the same clinic, 126/650 (19.4%) transferred to another clinic, 10/650 (1.5%) died, and 10/650 (1.5%) were noted as having stopped ART (Table 2). Of the 383 participants LTFU, 73 (19.1%) were lost during the first month of ANC, 44 (11.5%) during the remainder of the pregnancy, and 266 (69.5%) during the postpartum period. A total of 484 women contributed 104.0 years of person-time of follow up data to the pregnancy period; a total of 495 women contributed 557.4 years of person-time of follow up data to the postpartum period (Figure 1).
Table 2:
Care engagement across the PMTCT cascade
| Outcomes | Number (%) |
|---|---|
| 1stmonth of ANC (n = 650) | |
| Retained | 550 (84.6%) |
| Stopped ART - continued care | 3 (0.5%) |
| Transferred | 22 (3.4%) |
| Died | 2 (0.3%) |
| LTFU* | 73 (11.2%) |
| Pregnancy (excluding 1stmonth of antenatal care) (n = 484) | |
| Retained to delivery | 429 (88.6%) |
| Stopped ART - continued care | 2 (0.4%) |
| Transferred | 9 (1.9%) |
| Died | 0 (0.0%) |
| LTFU* | 44 (9.1%) |
| Post-partum (n = 495) | |
| Retained up to 2 years | 121 (24.4%) |
| Stopped ART - continued care | 5 (1.0%) |
| Transferred | 95 (19.2%) |
| Died | 8 (1.6%) |
| LTFU* | 266 (53.7%) |
The number of participants included in each step of the care cascade is described in Figure 1. Cumulatively, 121/650 were retained up to 2 years post-partum, 10/650 stopped ART, 126/650 transferred, 10/650 died, and 383/650 were LTFU.
LTFU is defined as going >90 days without clinical care.
LTFU in the month after the first ANC visit
In the month following the first ANC visit, 550/650 (84.5%) women were retained in care, 73/650 (11.2%) women were LTFU, 22/650 (3.4%) transferred, 3/650 (0.5%) stopped ART but continued attending clinic appointments, and 2/650 (0.3%) died (Table 2). In both crude and adjusted analyses (Table 3; see also Supplemental Table 3a), women who had a new HIV diagnosis were more likely to be LTFU than women with an established HIV diagnosis (aOR 1.76; 95% CI: 1.06 – 2.94). Additionally, women who initiated care at a facility in an urban area were more likely to be LTFU (aOR 2.03; 95% CI: 1.22 – 3.38). Age, relationship status, gestational age, and facility type were not associated with LTFU in crude or adjusted analysis.
LTFU during pregnancy
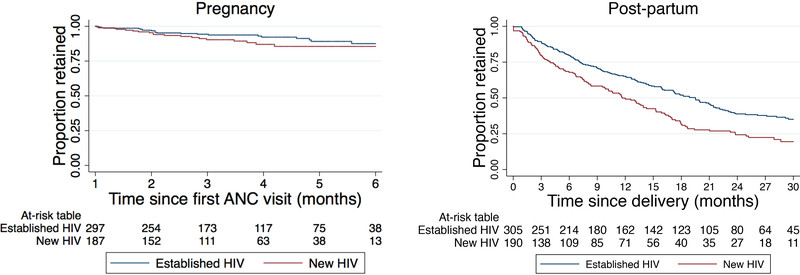
Of the 484 women who were in care after the first month of ANC care and had not delivered, 429 (88.6%) were retained in care until the time of delivery, 44 (9.1%) were LTFU, 9 (1.9%) transferred, and 2 (0.4%) stopped ART but continued attending clinic appointments (Table 2). Using Kaplan-Meier estimates (Figure 2), retention in care for the 484 pregnant women who completed the first month of PMTCT care was 96.2%, 92.8%, 90.2%, 87.6%, and 86.5%, at the end of the 2nd, 3rd, 4th, 5th, and 6th month of ANC, respectively. No variables were associated with faster times to LTFU in crude or adjusted models (Table 3; see also Supplemental Table 3b).
Figure 2:
Retention in care during pregnancy and the post-partum periods (Kaplan-Meier)
LTFU during the postpartum period
Of the 495 women who were in care up until the point of delivery, 121 (24.4%) participants were retained in care 2 years after delivery, 266 (53.7%) were LTFU, 95 (19.2%) transferred, 8 (1.6%) died, and 5 (1.0%) stopped ART but continued attending clinic appointments (Table 2). Using Kaplan-Meier estimates (Figure 2), postpartum retention was 85.7%, 75.1%, 59.1%, 44.6%, and 33.5%, at 3, 6, 12, 18, and 24 months after delivery, respectively. Women who were diagnosed with HIV during the index pregnancy had higher LTFU than those with established diagnoses (Figure 2); those who were newly diagnosed were LTFU 32% faster than those with established HIV (aRT.68, 95% CI:.51 –.89) (Table 3; see also Supplemental Table 3c). Women who were younger (<25) were LTFU faster than those who were older (≥ 25) (aRT.66; 95% CI:.47 -.91). Additionally, women who initiated PMTCT care at a local clinic were LTFU faster than those who initiated care at a hospital (aRT.67; 95% CI:.51 –.88).
Initiating vs. continuing ART
As a secondary analysis, we compared women who were initiating ART to those who were continuing ART. The results and inferences were largely similar (Supplemental Table 4), although initiating ART during the index delivery did not increase the odds of being LTFU during the first month of ANC. New ART initiators were LTFU at significantly faster times during the postpartum period (aRT.70, 95% CI:.53 –.94). Additionally, younger women and those receiving care at local clinics experienced faster times to LTFU during the postpartum period, consistent with the findings from the primary analyses.
Sensitivity analysis
Analyzing the complete data only (Supplemental Table 5) yielded similar results and inferences produced in the primary analysis.
Discussion
In this cohort of pregnant women entering PMTCT services during the first year of Option B+ implementation in the Kilimanjaro region of Tanzania, high rates of LTFU were observed in the first month of ANC, pregnancy, and postpartum periods. The risk of being LTFU was highest during the first month of ANC and the postpartum period. Women who were newly diagnosed with HIV had higher LTFU than women with established diagnoses, both during the initial month of ANC and in the postpartum period.
This study found rates of retention that are similar to other studies from East and Southern Africa (5). Cumulative LTFU was 11.2% at the end of the first month of ANC care, 18.0% at the end of pregnancy, and 58.9% two years after delivery. A recent systematic review found that retention in care under Option B+ ranged from 42% - 97% twelve months after initiation of ART (5). The heterogeneity of retention likely stems from the different methodologies used to estimate retention (e.g. retrospective medical record reviews compared to prospective cohort studies). The estimate of retention two years after delivery is within the range of published studies, and, like other retrospective studies may be biased by silent or undocumented transfers (28). In aggregate, this study together with previously published data suggests that significant attrition from PMTCT programs occurs throughout the care continuum.
Being diagnosed with HIV during the index pregnancy emerged as an important risk factor for subsequent LTFU, consistent with other quantitative studies of retention in care under Option B+ (5). Women who initiate ART on the same day of diagnosis may be more likely to drop out of care due to stigma, fear of disclosure, lack of social support, lack of time to accept HIV status, side effects of ART, or poor counseling and communication at the clinic (5). Additionally, being younger was associated with increased LTFU during the post-partum period, consistent with previous findings (5). Other factors associated with increased LTFU in this study, including attending a PMTCT clinic in an urban area and attending a local clinic, likely depend on local context and may vary across regions and countries. Identifying these types of epidemiologic associations may lead to the development of evidence-based models of differentiated care that seek to improve support for those at particularly high risk of poor outcomes (3,29,30); for example, a recent study evaluated the role of adherence clubs for women initiating ART during pregnancy (31).
One way to strengthen the health systems and PMTCT programs is by examining retention across each step of PMTCT care cascade. Typically, studies evaluating treatment outcomes in PMTCT analyze retention from the time of ART initiation, without reference to the time of delivery (5). This approach neglects the important life transitions and events that occur for women as they move through pregnancy and delivery and begin caring for their newborn. By accounting for the timing of childbirth and analyzing retention across three different periods, we were able to identify times of particularly high risk (initiation of ANC and early in the postpartum period) and factors associated with LTFU during each period. Further research is needed to identify the more nuanced barriers and facilitators to care engagement as women transition through each juncture of the PMTCT cascade. This information can lead to the development and evaluation of interventions that target the most vulnerable women and do so at the most critical time points in their care.
There were several important limitations to this study. First and most importantly, this was a retrospective analysis using routinely collected data captured in medical records. The estimates of retention in care are limited by the quality of the available data and do not have the ability to capture silent transfers between clinics. Second, there was a significant number of women in the NCAP database who were marked as pregnant during the time period of interest but were not included in the final analysis because their record was incomplete and missing the date of delivery or an estimated due date. These women were not more or less likely to be LTFU than those included in the analysis, but it is possible that the exclusion of these women resulted in residual confounding.
Under the global push for universal test and treat policies, PMTCT programs remain an important entry point for HIV testing and initiation of ART. Sustained engagement in care is critical for the long-term success of the Option B+ guidelines for PMTCT programs. Retention in this cohort of women in Tanzania was similar to previously published studies and showed particularly high rates of loss to follow up in the first month of ANC and in the postpartum period, with women who were newly diagnosed, younger, in urban locales, and initiating at a local clinic at higher risk for LTFU. Future studies should evaluate retention across each step of the care-continuum, and evidence-based interventions are needed that target both early retention during the initiation of ANC and the early postpartum period, as well as women who may be at higher risk for LTFU, including those diagnosed with HIV during pregnancy.
Supplementary Material
Acknowledgements:
This study was supported by a grant from the NIH National Institute of Allergies and Infectious Diseases (NIAID): Postpartum HIV care engagement in the context of Option B+ in Tanzania (R21 AI124344, MPI Watt/Mmbaga). Additionally, this work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at Duke University; Cody Cichowitz is a Doris Duke International Clinical Research Fellow. Finally, we acknowledge support received from the Duke Center for AIDS Research (P30 AI064518). None of the funders had any involvement in the study or the preparation of this manuscript.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Compliance with Ethical Standards
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.UNAIDS. On the fast-track to an AIDS-free generation 2016. http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf (accessed November 14, 2017).
- 2.World Health Organization. Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants 2012. http://www.who.int/hiv/PMTCT_update.pdf (accessed November 14, 2017).
- 3.Geldsetzer P, Yapa HMN, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc 2016; 19(1): 20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black S, Zulliger R, Marcus R, Mark D, Myer L, Bekker L-G. Acceptability and challenges of rapid ART initiation among pregnant women in a pilot programme, Cape Town, South Africa. AIDS Care 2014; 26(6): 736–41. [DOI] [PubMed] [Google Scholar]
- 5.Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, et al. Retention in HIV care during pregnancy and the postpartum period in the Option B+ era: a systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr 2018; 77(5): 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalua T, Tippett Barr BA, van Oosterhout JJ, Mbori-Ngacha D, Schouten EJ, Gupta S, et al. Lessons learned from Option B+ in the evolution toward “test and start” from Malawi, Cameroon, and the United Republic of Tanzania. J Acquir Immune Defic Syndr 2017; 75(Suppl 1): S43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwadz M, Cleland CM, Applegate E, Belkin M, Gandhi M, Salomon N, et al. Behavioral intervention improves treatment outcomes among HIV-infected individuals who have delayed, declined, or discontinued antiretroviral therapy: a randomized controlled trial of a novel intervention. AIDS Behav 2015; 19(10): 1801–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberer JE, Sabin L, Amico KR, Orrell C, Galarraga O, Tsai AC, et al. Improving antiretroviral therapy adherence in resource-limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc 2017; 20(1): 21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill MM, Umutoni A, Hoffman HJ, Ndatimana D, Ndayisaba GF, Kibitenga S, et al. Understanding Antiretroviral Treatment Adherence Among HIV-Positive Women at Four Postpartum Time Intervals: Qualitative Results from the Kabeho Study in Rwanda. AIDS Patient Care STDS 2017; 31(4): 153–166. [DOI] [PubMed] [Google Scholar]
- 10.Elwell K Facilitators and barriers to treatment adherence within PMTCT programs in Malawi. AIDS Care 2016; 28(8): 971–975. [DOI] [PubMed] [Google Scholar]
- 11.Cataldo F, Chiwaula L, Nkhata M, van Lettow M, Kasende F, Rosenberg NE, et al. Exploring the experiences of women and health care workers in the context of PMTCT Option B Plus in Malawi. J Acquir Immune Defic Syndr 2017; 74(5): 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth 2017; 17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tweya H, Gugsa S, Hosseinipour M, Speight C, Ng’ambi W, Bokosi M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health 2014; 19(11): 1360–66. [DOI] [PubMed] [Google Scholar]
- 14.Napua M, Pfeiffer JT, Chale F, Hoek R, Manuel J, Michel C, et al. Option B+ in Mozambique: Formative Research Findings for the Design of a Facility-Level Clustered Randomized Controlled Trial to Improve ART Retention in Antenatal Care. J Acquir Immune Defic Syndr. 2016; 72(Suppl 2): S181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, O’Donnell K. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care 2016; 28(7): 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzangare J, Takarinda KC, Harries AD, Tayler-Smith K, Mhangara M, Apollo TM, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on “Option B+” in rural Zimbabwe. Trop Med Int Health 2016; 21(2): 202–09. [DOI] [PubMed] [Google Scholar]
- 17.Myer L, Phillips TK. Beyond “Option B+”: Understanding Antiretroviral Therapy (ART) Adherence, Retention in Care and Engagement in ART Services Among Pregnant and Postpartum Women Initiating Therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr 2017. June 1;75 Suppl 2:S115–22. [DOI] [PubMed] [Google Scholar]
- 18.Psaros C, Remmert JE, Bangsberg DR, Safren SA, Smit JA. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Curr HIVAIDS Rep 2015; 12(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The United Republic of Tanzania Ministry of Health and Social Welfare. National guidelines for comprehensive care services for prevention of mother-to-child transmission of HIV and keeping mothers alive. 2013. http://ihi.eprints.org/3335/1/tz_guidelines_ccs_optionb_all.pdf (accessed May 22, 2017).
- 20.The United Republic of Tanzania Ministry of Health and Social Welfare. Tanzania elimination of mother to child transmission of HIV plan 2012–2015. 2012. http://www.healthpromotiontanzania.org/index.php/en/library122/doc_view/152-costed-emtct-plan-final-16-may-2.html (accessed October 13, 2017).
- 21.Atanga PN, Ndetan HT, Achidi EA, Meriki HD, Hoelscher M, Kroidl A. Retention in care and reasons for discontinuation of lifelong antiretroviral therapy in a cohort of Cameroonian pregnant and breastfeeding HIV-positive women initiating “Option B+” in the South West Region. Trop Med Int Health. 2017; 22(2): 161–70. [DOI] [PubMed] [Google Scholar]
- 22.Auld AF, Shiraishi RW, Couto A, Mbofana F, Colborn K, Alfredo C, et al. A decade of antiretroviral therapy scale-up in Mozambique: evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004–2013. J Acquir Immune Defic Syndr 2016; 73(2): e11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s Option B+ programme: an observational cohort study. Lancet HIV. 2016; 3(4): e175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman RM, Phiri K, Parent J, Grotts J, Elashoff D, Kawale P, et al. Factors associated with retention in Option B+ in Malawi: a case control study. J Int AIDS Soc 2017; 20(1): 21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitiku I, Arefayne M, Mesfin Y, Gizaw M. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc 2016; 19(1): 20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox C, Chu H, Schneider MF, Muñoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med 2007; 26(23): 4352–74. [DOI] [PubMed] [Google Scholar]
- 27.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp, LLC; 2017. [Google Scholar]
- 28.Ford D, Muzambi M, Nkhata MJ, Abongomera G, Joseph S, Ndlovu M, et al. Implementation of antiretroviral therapy for life in pregnant/breastfeeding HIV+ women (Option B+) alongside rollout and changing guidelines for ART initiation in rural Zimbabwe: the Lablite Project experience. J Acquir Immune Defic Syndr 2017; 74(5): 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016; 19(1):21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimsrud A, Lesosky M, Kalombo C, Bekker L-G, Myer L. Implementation and operational research: community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr 2016; 71(1): e16–23. [DOI] [PubMed] [Google Scholar]
- 31.Myer L, Iyun V, Zerbe A, Phillips TK, Brittain K, Mukonda E, et al. Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. J Int AIDS Soc 2017; 20(Suppl 4): 21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.