Abstract
Purpose:
The use of traditional, complementary, and alternative medicine (TCAM) for cancer may influence the delivery or effectiveness of conventional cancer treatment. In this systematic review, we aimed to 1.) summarize the available prevalence data on traditional medicine use by cancer patients in less developed countries (LDCs), and 2.) stratify the prevalence data by world region and country income level.
Methods:
A literature search for cancer, TCAM, and low income (LI) and lower-middle income (LMI) countries was conducted across 5 databases. A total of 2,365 publications were reviewed for eligibility, of which 25 studies met inclusion criteria.
Results:
The combined sample size was 6,878 cancer patients, with a median of 54.5% reporting the use of TCAM for cancer care. Of the studies providing data on the concomitant use of TCAM and conventional cancer treatment (n = 4,872 cancer patients), a median of 26.7% of participants reported combining the two systems of medicine.
Conclusion:
From the data available, it is apparent that TCAM use among cancer patients in less developed countries is common; however, additional studies are needed to support the safe and effective management of cancer for patients in LI and LMI countries.
Keywords: Traditional Medicine, Global Health, Less Developed Countries, Cancer, Public Health
Introduction
The prevalence of traditional, complementary, and alternative medicine (TCAM) in less developed countries (LDCs) depends on numerous variables including cultural and historical influences, legal regulations, and access and affordability of TCAM in comparison to conventional medicine (World Health Organization, 2013). The World Health Organization (WHO) estimates the prevalence of TCAM use in LDCs reaches as high as 80% (Bodeker & Kronenberg, 2002; World Health Organization, 2002). For some patients, TCAM is the only option for care due to the lack of access to conventional medical facilities or expense associated with receiving conventional medical care (World Health Organization, 2013). A prime example is the continent of Africa, where the traditional healers/population ratio is estimated at 1:500, compared to the medical doctors/population ratio of 1:40,000 (Abdullahi, 2011; World Health Organization, 2013). Access to anti-neoplastic medication in LDCs partially depends on revenue for pharmaceutical companies, which in part depends on volume of medication sold and income of the local population (Ruff, Al-Sukhun, Blanchard, & Shulman, 2016; Villa, Compagni, & Reich, 2009). Due to low income levels in LDCs, there may be a lack of incentive for pharmaceutical companies to invest in cancer medications in LDCs, leading to a lack of access (Ruff et al., 2016). The expense associated with radiation therapy, required skillset and facilities required for surgical care, and legal regulations and cultural attitudes regarding palliative care medications also influence access to cancer care in LDCs (Ruff et al., 2016).
The high reliance on TCAM in LDCs may increase the concomitant use of TCAM with conventional medical practices when conventional practices are introduced into a country. An example is the expansion of conventional cancer care into less developed countries in order to address the increasing global cancer burden. On a global scale, cancer has become a significant public health concern. In 2012, there was 14 million new cancer cases and 8.2 million cancer deaths (Stewart & Wild, 2014). The rate of new cancer cases is expected to rise by 70% over the next two decades (Stewart & Wild, 2014). Less developed countries are experiencing the majority of this cancer burden, and greater than 60% of new cancer cases occur in Africa, Asia, Central America, and South America (Stewart & Wild, 2014). These same regions account for more than 70% of cancer deaths worldwide (Stewart & Wild, 2014). Cancer is now responsible for more deaths globally than AIDS, tuberculosis, and malaria combined (American Cancer Society, 2015), and is recognized as a significant global health concern by the National Cancer Institute, American Cancer Society, and the World Health Organization. The WHO collaborates with the International Agency for Research on Cancer (IARC) and additional United Nations organizations within the UN Non-communicable Diseases Interagency Taskforce to help address this global burden (WHO Cancer, 2015). These organizations, among others, are addressing the significant cancer crisis from a multitude of approaches. A main strategy is to expand conventional cancer health systems among less developed countries to provide treatment and care to as many patients as possible (INCTR Strategies, 2016).
The expansion of conventional medical practices in less developed countries creates an interface with previously established TCAM practices. Both benefits and concerns exist regarding the combination of TCAM and conventional cancer care. TCAM can play a role in supporting quality of life (QOL) for cancer patients, with a number of research studies reporting benefit of TCAM for QOL support (Blaes, Kreitzer, Torkelson, & Haddad, 2011). TCAM may also play a role in prevention, reduction of treatment side effects, and survivorship support. However, there are significant and justified concerns regarding TCAM use in cancer treatment including herb/drug and mechanism interactions with conventional treatment (Block & Gyllenhaal, 2002), lack of patient disclosure of TCAM use (Davis, Oh, Butow, Mullan, & Clarke, 2012), and delay in seeking conventional treatment or increased likelihood of incomplete treatments due to TCAM use (Obrist et al., 2014).
The aim of this systematic review is to evaluate the existing prevalence data on traditional, complementary, and alternative medicine in cancer patients in low income and lower-middle income countries. The review will stratify TCAM prevalence data by country income level and by world region. This data are intended to provide a global perspective on TCAM use among cancer patients in low and lower-middle income countries as well as highlight areas for additional research needs. The protocol and manuscript for this systematic review was created using PRISMA guidelines. The protocol is listed on the PROSPERO registry, with registration number CRD42016053447.
Methods
Literature Search
The search terms for this systematic review were created with the assistance of a health sciences librarian familiar with formatting search terms across multiple databases. Medical subject headings (MeSH) terminology was applied when possible. A literature search was completed using 5 databases, which included PubMed, EMBASE, Web of Science, Global Health Database, and PsycINFO. The searches were completed on the following dates; 1.) studies with any date of publication up to January 11, 2017, and 2.) an updated search using identical search terms including studies from January 1, 2017 to June 30, 2017 (PubMed and Embase) and January 1, 2017 to July 7, 2017 (Web of Science, Global Health Database, PsycINFO). The following search terms were used across all databases with formatting changes applied per specific database requirement:
Cancer AND Complementary Medicine OR Alternative Medicine OR Complementary Therapies OR Traditional Medicine AND (86 iterations of ways to say less developed country + the name and name variations of low income or lower-middle income countries (81 country names/name variations))
Systematic reviews and reference sections of included studies were screened for additional relevant studies. A second independent abstract screening was conducted on 10% of imported studies to verify the correct application of inclusion and exclusion criteria. Each study was either excluded for irrelevancy/failure to meet eligibility criteria, or moved to full text screening.
Included Countries
The World Bank Atlas Method version September, 2016, was used to determine the list of countries with a low income or lower-middle income country stratification (“World Bank list of economies (September 2016),” 2016). See appendix 1 for countries included in the search terms (n = 81).
Eligibility Criteria
The following eligibility criteria were applied to all studies: Study Designs: 1.) studies must be published in a peer-reviewed medical journal, 2.) there is no restriction on the year of publication or language of publication for included studies, 3.) studies may be of any design style as long as all other inclusion criteria are met, 4.) in-vitro and animal studies are excluded. Population: 1.) studies can include children and adults of any age with a current or previous diagnosis of cancer, 2.) the study sample must include only participants with a diagnosis of cancer, 3.) studies including caregiver or next-of-kin representation for adult cancer patients are excluded, 4.) parental or caregiver representation for a child with cancer is eligible, 5.) the study sample must reside in a low income or lower-middle income country at the time of the study, 6.) there is no restriction on sample size for included studies. TCAM: 1.) studies must document patient reported use of at least 1 TCAM modality for symptom or treatment support of their cancer illness, and/or patients who report seeking a TCAM practitioner specifically for their cancer illness. TCAM is defined as medical recommendations or practitioners using modalities not considered standard of care in conventional medical approaches. An additional definition used for this study can be found at the National Center for Complementary and Integrative Health (NCCIH) website. Outcome: 1.) Studies must include prevalence of TCAM use, and/or prevalence of seeking a TCAM practitioner for care related to cancer among cancer patients in low income or lower-middle income countries. Studies of questionable eligibility were presented to a senior reviewer to decide on the inclusion or exclusion of the study.
Quality and Risk of Bias Review
Each study meeting inclusion criteria after the full-text review was then further reviewed independently by two researchers for quality and risk of bias. The researchers used the Cincinnati Children’s LEGEND: Evidence Appraisal of a Single Study, Descriptive Study, Epidemiology Study, Case Series, template for review. The studies were either graded as, G: Good Quality, or, L: Low Quality, based on the cumulative available information per study. A quality rating was determined for each study through discussion and consensus by the quality review researchers.
Statistical Analysis
All included data was extracted independently by two researchers. Differences in extraction data were resolved through discussion and consensus. The data were collected in a Microsoft Excel spreadsheet. Extracted data for each study is included in Appendix 2. The primary outcome of this review is the prevalence of overall TCAM use for cancer. The secondary outcome is the prevalence of concomitant TCAM and conventional medicine use for cancer. Descriptive statistics were used to summarize the data. Median percentage values were used to summarize the prevalence data for the total amount of studies, and per world region and income level. The median value was selected due to the robustness of this statistic. In addition, we performed hypothesis tests on the difference of the prevalence of overall TCAM and concomitant TCAM and conventional medicine use between different country income levels and world regions, using analysis of variance (ANOVA). The analyses were done using R version 3.3.0. P-values < 0.05 are considered to be significant.
Results
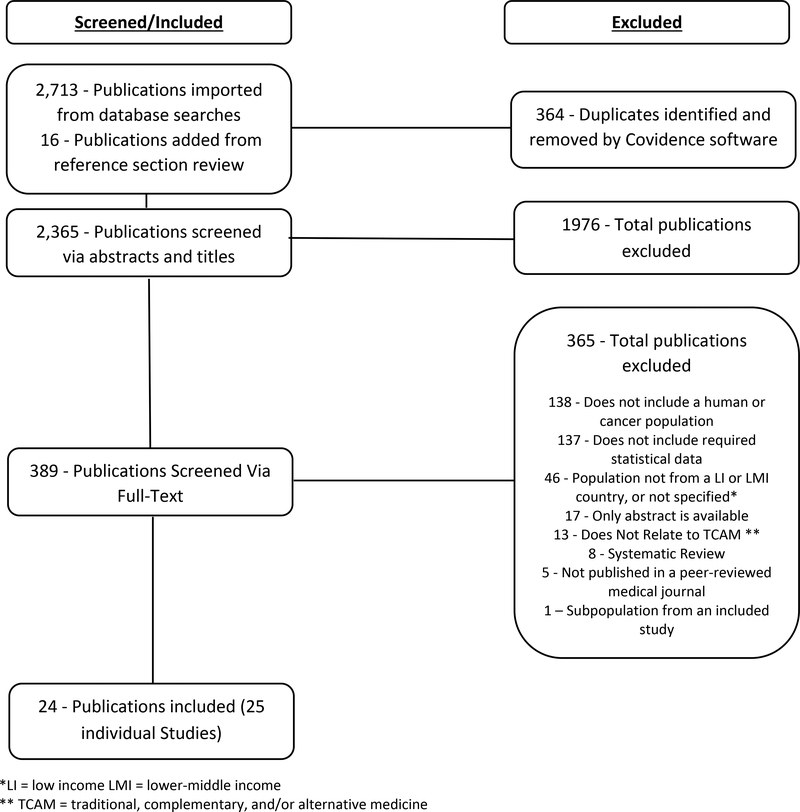
A total of 2,729 publications were imported for screening, of which 16 were added to screening from reference section review. A de-duplication program was run and initially excluded 364 duplicate publications. Title and abstract screening was completed on 2,365 publications. Full text screening was completed on 389 publications, of which 365 were excluded (Figure 1). A total of 24 publications providing prevalence data for 25 individual studies met inclusion criteria. Eligibility criteria for the 25 studies was verified by a second reviewer. The language of publication for the studies includes English (n = 21) and French (n = 3).
Figure 1:
Inclusion/Exclusion Flow Chart
The sample size of all included studies was 6,878 cancer patients (see appendix 2 for included studies). A total of 14 different countries were represented, including 3 (21.4%) low-income countries and 11 (78.6%) lower-middle income countries (Appendix 2). Country sample sizes and percent representation of total sample, were: India (2,200, 32.0%), Pakistan (811, 11.8%), Cameroon (587, 8.5%), Nigeria (560, 8.1%), Sri Lanka (500, 7.3%), Morocco (484, 7.0%), Mongolia (482, 7.0%), Tanzania (449, 6.5%), Ethiopia (264, 3.8%), Ghana (187, 2.7%), Myanmar (107, 1.6%), West Bank/Palestine (103, 1.5%), Guatemala (100, 1.5%), and Mali (44, 0.6%). The highest represented countries by study count were Pakistan (n = 4 studies), followed by India (n = 3), and Ethiopia, Ghana, Cameroon, Morocco, Nigeria, and Tanzania (n = 2 for each).
Included survey participants were 57.7% female and 42.3% male. Of the 25 total studies, 14 provided data on the percent of male and female TCAM users. Participants reporting TCAM use for their cancer care were 58.1% female, and 41.9% male. The most common cancer types surveyed were hematological (30.6%), breast (24.1%), gastrointestinal (8.0%), gynecological (7.1%), Head & Neck (7.1%) and other (16.9%). Summary statistics for age could not be determined due to a lack of available age data, although age range and/or mean per study are reported in Appendix 2.
Among all study participants, a median of 54.5% reported TCAM use for their cancer care. Of the studies providing data on the concomitant use of TCAM and conventional cancer treatment (n = 4,872 cancer patients), a median of 26.7% reported combining the two systems of medicine. Summary statistics for prevalence of TCAM use for cancer and concomitant TCAM in conjunction with conventional treatment are represented in Tables 2 and 3, respectively.
Table 2.
Median and Range of Overall TCAM Use- Total, Per Region, Per Income Category
| Category | Subcategory | Number of Studies Included, N (%) | Total Survey Sample, N (%) | Median TCAM Use for Cancer, % (Range) |
|---|---|---|---|---|
| Total | 25 (100) | 6,878 (100) | 54.5 (4.0 – 92.0) | |
| Income Class | ||||
| Low Income Countries | 5 (20.0) | 757 (11.0) | 44.5 (20.4 – 79.0) | |
| Lower-Middle Income Countries | 20 (80.0) | 6,121 (89.0) | 54.8 (4.0 – 92.0) | |
| World Region | ||||
| Sub-Saharan Africa | 11 (44.0) | 2,091 (30.4) | 49.4 (20.0 – 79.0) | |
| Middle East & North Africa | 3 (12.0) | 587 (8.5) | 68.0 (46.0 – 71.9) | |
| South Asia | 8 (32.0) | 3,511 (51.0) | 51.3 (4.0 – 84.0) | |
| Latin America & Caribbean | 1 (4.0) | 100 (1.5) | 92.0 | |
| East Asia & Pacific | 2 (8.0) | 589 (8.6) | 53.9 (47.9 – 59.8) |
Table 3.
Median and Range of Concomitant TCAM + Conventional Treatment Use- Total, Per Region, Per Income Category
| Category | Subcategory | Number of Studies Included, N (%) | Total Survey Sample, N (%) | Median Concomitant TCAM + Conventional Treatment, % (Range) |
|---|---|---|---|---|
| Total | 17 (100) | 4,872 (100) | 26.7 (4.0 – 84.0) | |
| Income Class | ||||
| Low Income Countries | 3 (17.6) | 383 (7.9) | 18.8 (11.8 – 79.0) | |
| Lower-Middle Income Countries | 14 (82.4) | 4,489 (92.1) | 32.7 (4.0 – 84.0) | |
| World Region | ||||
| Sub-Saharan Africa | 6 (35.3) | 730 (15.0) | 41.9 (6.7 – 79.0) | |
| Middle East & North Africa | 1 (5.9) | 100 (2.1) | 13.0 | |
| South Asia | 7 (41.2) | 3,353 (68.8) | 26.7 (4.0 – 84.0) | |
| Latin America & Caribbean | 1 (5.9) | 100 (2.1) | 63.0 | |
| East Asia & Pacific | 2 (11.8) | 589 (12.1) | 35.4 (24.0 – 46.7) |
Country Income and World Region Analysis
Eligible study data was available for 3/31 (9.7%) low income countries, and 11/52 (21.2%) lower-middle income countries. The number and percent of included study-countries for the LMI and LI stratifications per world region includes 2/16 (12.5%) in East Asia & Pacific, 0/7 (0.0%) in Europe and Central Asia, 1/6 (16.7%) in Latin America and Caribbean, 2/7 (28.6%) in Middle East and North Africa, 0/0 (0.0%) in North America, 3/7 (42.9%) in South Asia, and 6/40 (15.0%) in Sub-Saharan Africa. There were no noted differences in the overall prevalence of TCAM stratified by country income (p = 0.42) and by world region (p = 0.4). Concomitant TCAM and conventional medicine use also did not differ by country income (p = 0.95) nor the world region (p = 0.8).
Type of TCAM Modalities Used
A descriptive summary of reported TCAM modalities are described in Table 4 from 20 of the 25 included studies, stratified by world region. When possible, individual products were grouped into broad categories, such as specific herbs into a general herbal medicine category. There is no weight provided to these modalities/categories. Herbal medicines and spiritual/religious healing were the only 2 modalities documented in each world region.
Table 4.
TCAM Modalities/Systems Used per World Region
| World Region | Types of Modalities/Treatments | Systems of Medicine |
|---|---|---|
| Sub-Saharan Africa | Herbal medicine, spiritual/religious healing including prayer, divinations, and incantations, dietary supplements, mind/body techniques, massage/therapeutic touch, nutrition therapy/special diets and foods, mineral therapy, music therapy, meditation/relaxation, visualization, reflexology, electromagnetic therapy, support groups, removal of mystical objects from mass, incense/vapor, skin burns with heat or herbs, drills with hot nails, cuts with local applications, holy water, holy mud, holy soil, python fat, ritual sacrifice, black stone, bloodletting, local surgery/scarification, urine therapy, magnetic water | Traditional African Medicine, Chinese Medicine/Acupuncture, Ayurveda, Osteopathy, Chiropractic, Homeopathy |
| Middle East & North Africa | Herbal medicine, spiritual/religious healing, nutrition therapy/special diets and foods, water of ZamZam (holy water from Mecca) | * |
| South Asia | Herbal medicine, spiritual/religious healing including prayer, nutrition therapy/special diets and foods, dietary supplements, yoga, meditation/relaxation, laughter therapy, physiotherapy, psychological therapy, exercise, folk remedies, mental imagery, pranic healing/energy medicine, physical abuse/torture | Unani (Perso-Arabic Traditional Medicine), Ayurveda, Naturopathy, Homeopathy, Chinese Medicine/Acupuncture, Siddha Traditional Medicine, Traditional Greek Medicine, Chiropractic, Eastern Medicine |
| Latin America & Caribbean | Herbal medicine, spiritual/religious healing including prayer, nutrition therapy/special diets and foods, dietary supplements, ointments | * |
| East Asia & Pacific | Herbal medicine, spiritual/religious healing including prayer, dietary supplements, tripe (goat, sheep, etc.), milk bath, dried foam from camel’s mouth, mare’s milk, mantra, meditation/relaxation, yoga, massage, bone setting, blessed/anointed water, charms | Mongolian Traditional Medicine, Chinese Medicine/Acupuncture |
none reported
Quality Assessment and Risk of Bias
The quality assessment and risk of bias was completed on 24 publications that include 25 studies. Of those publications, 14 (58.3%) were rated as good quality with low risk of bias, and 10 (41.7%) were rated as low quality with high risk of bias. The quality assessment reviewers agreed the following inconsistencies were common among low quality studies: lack of survey information and/or validation, lack of assessment for confounding variables such as age or gender, missing demographic data, lack of disclosure of attrition rate, limited statistical analysis or disclosure of analysis methods, and lack of a conflict of interest statement.
Discussion
Available Global Data on TCAM Prevalence for Cancer
Little is known regarding the use of TCAM for cancer care in LI and LMI countries. Of the 83 LI and LMI countries/regions of the world, we only identified prevalence data on TCAM use for cancer in 14, or 16.9%, of the countries. The largest collective sample size from our studies was from the South Asia world region, although the greatest number of studies were from the Sub-Saharan Africa region. Only 3 of 31 (9.7%) of low income countries had available data on the prevalence of TCAM for cancer, and 11 of 52 (21.2%) lower-middle income countries. Hematological cancers and breast cancer were the most commonly surveyed cancer types, which is expected due to their high prevalence. Roughly 1/2 of the surveyed cancer patients report using TCAM, or seeking a TCAM provider. Approximetly 1/4 report combining TCAM use with conventional treatment. This indicates a substantial proportion of cancer patients in LI and LMI countries seek TCAM for care, which can have significant clinical implications. However, the available data collected in this review are a very limited representation of TCAM use for cancer care in low resource countries.
It is also important to consider the quality of the data collected. Only 58.3% of the included studies were deemed good quality with low risk of bias. With the complexity and variety of traditional medicine, high quality prevalence studies are needed to provide a clear picture of the TCAM use among cancer patients. Thus, there is potential for misrepresentation of TCAM use in cancer care, which could have safety repercussions for the delivery of conventional cancer treatment and limit the management of TCAM practices.
Implications for the Delivery of Cancer Treatment
As conventional cancer treatment expands into low and lower-middle income countries, there is a responsibility to assess and address the use of local traditional, complementary, and alternative medicine strategies for cancer. Patient safety is a top priority of concern regarding the combination of traditional and conventional medicine, but respect for local tradition, a patient’s autonomy for treatment choice, and potential benefits of integration are also critically important. In regards to safety, herb/drug interactions may potentially be concerning, as well as alternative or traditional practices with a high potential for harm (i.e. non-standardized or unsanitary surgical practices, and/or toxic or contaminated herbal products).
Even though the potential for harm exists, traditional medicine practices play an important role in the culture and lifestyle of many communities. Traditional medicine far outdates the use of modern conventional medicine approaches. Patients often have a greater sense of trust in traditional medicine, and will frequently patronize a traditional healer before seeking western conventional treatment (Afungchwi, Hesseling, & Ladas, 2017). When considering a disease such as cancer, a delay of presentation for effective treatment can have detrimental effects on outcomes (Malik & Gopalan, 2003). Accessing, managing, and integrating traditional practices with conventional cancer care may increase patient satisfaction, or potentially build trust and improve delivery of highly researched and effective medical treatment to locations in need.
Several modern cancer treatments were derived from traditional medicines, such as herbs, and increasing research over the past few decades indicates a potential for substantial benefit of TCAM for cancer care, when used appropriately (Blaes et al., 2011). Additional systematic research on TCAM products and therapies for cancer care is important, especially in low resource areas with a high use of traditional medicine. Identifying local, low cost, non-invasive traditional practices with benefit to cancer patients could wield significant benefit to the cancer populations of low and lower-middle income countries.
It is difficult to determine the best approach to addressing the use of traditional medicine in cancer care. Most conventionally trained oncologists are unfamiliar with the mechanisms or practices of traditional medicine. The training of traditional healers can also vary dramatically, as can their practices and the modalities which they use for treatment. More research is needed to assess who is using traditional medicine for cancer, what they are using, its effects on the efficacy of conventional therapy, and how it fits into the cancer care process.
Limitations
It is important to note all studies included in this review were conducted in a hospital setting, which may reduce generalizability. Due to limited resources, cancer patients in LI or LMI countries may not present, or be able to attend a hospital for cancer treatment. In addition, patients who chose to completely forgo conventional cancer treatment are not well represented in these studies.
Conclusion
To fight the global burden of cancer it is essential to consider the variables that impact patient safety and treatment delivery and efficacy. The use of traditional, complementary, and alternative medicine for cancer in LI and LMI countries is one of many important variables to consider. The current lack of available data on TCAM use in LDCs creates the potential for numerous avenues of future research opportunities. High quality research on the prevalence of TCAM use in cancer is needed for a greater percentage of LDCs. Additional research possibilities include examining the influence of TCAM on timing of cancer diagnosis and treatment, identifying beneficial and harmful TCAM practices for the support and treatment of cancer patients, or ways to incease communication between traditional healers and conventional doctors to improve cancer care delivery in LDCs. Our results indicate a large proportion of cancer patients in LI and LMI countries use TCAM for cancer care, which requires additional research for the safety and wellbeing of cancer patients in less developed countries.
Table 1:
Gender and Cancer Type of Survey Participants
| Category | Subcategory | Number of Studies Included N (%) | Number of Cancer Patients Included N (%) | Gender, N (%)* | Cancer Types Surveyed N (%)**,*** |
|---|---|---|---|---|---|
| Total | 25 (100) | 6,878 (100) | M: 2,676 (42.3) F: 3,651 (57.7) |
Br: 1,140 (24.1), HN: 333 (7.1), L: 74 (1.6), GI: 378 (8.0), Gy: 335 (7.1), GU: 218 (4.6), He: 1447 (30.6) O: 798 (16.9) | |
| Income Class | |||||
| Low Income Countries | 5 (20.0) | 757 (11.0) | M: 281 (37.1) F: 476 (62.9) |
Br: 191 (44.7), HN: 22 (5.2), GI: 31 (7.3), Gy: 53 (12.4), GU: 9 (2.1), He: 64 (15.0), O: 57 (13.3) | |
| Lower-Middle Income Countries | 20 (80.0) | 6,121 (89.0) | M: 2395 (43) F: 3,175 (57) |
Br: 949 (22.1), HN: 311 (7.2), L: 74 (1.7), GI: 347 (8.1), Gy: 282 (6.6), GU: 209 (4.9), He: 1383 (32.2), O: 741 (17.2) | |
| World Region | |||||
| Sub-Saharan Africa | 11 (44.0) | 2,091 (30.4) | M: 803 (38) F: 1287 (62) |
Br: 545 (30.9), HN 137 (7.8), L: 4 (0.2), GI: 67 (3.8), Gy: 214 (12.2), GU: 64 (3.6), He: 452 (25.7), O: 278 (15.8) | |
| Middle East and North Africa | 3 (12.0) | 587 (8.5) | M: 149 (25) F: 438 (75) |
Br: 236 (40.2), HN: 53 (9.0), L: 20 (3.4), GI: 62 (10.6), Gy: 25 (4.3), He: 184 (31.3), O: 7 (1.2) | |
| South Asia | 8 (32.0) | 3,511 (51.0) | M: 1,423 (48.1) F: 1,538 (51.9) |
Br: 284 (16.8), HN: 143 (8.5), L: 50 (3.0), GI: 75 (4.4), Gy: 96 (5.7), GU: 14 (0.8), He: 662 (39.3), O: 362 (21.5) | |
| Latin America and Caribbean | 1 (4.0) | 100 (1.5) | M: 63 (63.0) F: 37 (37.0) |
He: 77 (77.0), O: 23 (23.0) | |
| East Asia and Pacific | 2 (8.0) | 589 (8.6) | M: 238 (40.4) F: 351 (59.6) |
Br: 75 (12.7), GI: 174 (29.5), GU: 140 (23.8), He: 72 (12.2), O: 128 (21.7) |
Denominator for gender analysis is sum of male (n) + female (n) from studies that provided gender information
Denominator for cancer type analysis is sum of all cancer types (n) from studies that provided cancer type information
Br = Breast, HN = Head & Neck, L = Lung and Respiratory, GI = Gastrointestinal and Abdominal, Gy = Gynecological, He = Hematological, O: Others
Acknowledgments
Funding:
The Research Fellowship in Complementary and Integrative Health (5T32AT003378–12) from the National Center for Complementary and Integrative Health provides salary support for Dr. Hill. This work was also supported by the University of North Carolina Lineberger Comprehensive Cancer Center under a tier 1 pilot development grant.
Appendix 1: Countries/Regions Included in Search Terms
Afghanistan, Armenia, Bangladesh, Benin, Bhutan, Bolivia, Burkina Faso, Burundi, Cabo Verde (Cape Verde) Cambodia, Cameroon, Central African Republic, Chad, Comoros (Comores, Comoro), Congo (Democratic Republic of Congo), Côte d’Ivoire, Djibouti, Egypt, El Salvador, Eritrea, Ethiopia, Gambia, Ghana, Guatemala, Guinea, Guinea Bissau, Haiti, Honduras, India, Indonesia, Kenya, Kiribati, Korea (Democratic People’s Republic of Korea), Kyrgyzstan (Kyrgyz, Kirghizia, Kirghiz, Kirgizstan), Lao PDR (Laos), Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Micronesia, Moldova (Republic of Moldova), Mongolia, Morocco, Mozambique, Myanmar (Burma), Nepal, Nicaragua, Niger, Nigeria, Pakistan, Papua New Guinea, Philippines (Phillippines, Philipines, Phillipines), Rwanda (Ruanda), Samoa, Sao Tome (Principe), Senegal, Sierra Leone, Solomon Islands, Somalia, South Sudan, Sri Lanka, Sudan, Swaziland, Syria (Syrian Arab Republic), Tajikistan (Tadzhikistan, Tadjikistan, Tadzhik), Tanzania (United Republic of Tanzania), Timor (Timor-Leste), Togo, Tonga, Tunisia, Uganda, Ukraine, Uzbekistan (Uzbek), Vanuatu, Vietnam, West Bank (Gaza), Yemen, Zambia, Zimbabwe
Appendix 2: TCAM Prevalence Studies Meeting Inclusion Criteria, Stratified by World Region
| Author/Year | Country | World Region - Income Level* | Total Survey Sample, N | Prevalence of Overall TCAM Use for Cancer, N (%) | Concomitant TCAM + Conventional Treatment, N (%) | Gender, N (%) | Age Range and/or Mean (SD)** | Cancer Types Surveyed N (%)*** |
|---|---|---|---|---|---|---|---|---|
| Afungchwi et al. 2017 | Cameroon | SSA- LMI | 387 | 213 (55.0) | - | M: 222 (57.5) F: 164 (42.5) |
R: 1–17 | He: 387 (100.0) |
| Yomi et al. 1995 | Cameroon | SSA- LMI | 200 | 40 (20.0) | - | M: 89 (44.5) F: 111 (55.5) |
R: 2–80 M: 41 |
Br: 53 (26.5), HN: 58 (29.0), GI: 9 (4.5), Gy: 30 (15.0), GU: 5 (2.5), O: 45 (22.5) |
| De Ver Dye at al. 2011 | Ethiopia | SSA - LI | 69 | 40 (58.0) | 13 (18.8) | M: 2 (2.9) F: 67 (97.1) |
R: <40–70 | Br: 69 (100.0) |
| Erku. 2016 | Ethiopia | SSA - LI | 195 | 154 (79.0) | 154 (79.0) | M: 89 (45.6) F: 106 (54.4) |
R: 18->60 | Br: 74 (37.9), GI: 22 (11.3), Gy: 24 (12.3), He: 61 (31.3), O: 14 (7.2) |
| Clegg-Lamptey et al. 2009 | Ghana | SSA - LMI | 89 | 44 (49.4) | 6 (6.7) | M: 0 (0.0) F: 89 (100.0) |
R: 28–86 M: 53.7 (11.7) |
Br: 89 (100) |
| Yarney et al. 2013 | Ghana | SSA - LMI | 98 | 72 (73.5) | 72 (73.5) | M: 48 (49.0) F: 50 (51.0) |
R: 18–89 | Br: 21 (21.4), HN: 15 (15.3), O: 62 (63.3) |
| Ly et al. 2002 | Mali | SSA - LI | 44 | 10 (22.7) | - | M: 1 (2.3) F: 43 (97.7) |
R: 25–80 M: 46 (19.5) |
Br: 44 (100) |
| Asuzu et al. 2015 | Nigeria | SSA - LMI | 400 | 138 (34.5) | - | M: 95 (23.7) F: 305 (76.3) |
R: 18–94 years, M: 50.9 (14.6) | Br: 133 (33.3), HN: 39 (9.8), Gy: 128 (32.0), GU: 19 (4.8), O: 81 (20.3) |
| Ezeome at al. 2007 | Nigeria | SSA - LMI | 160 | 104 (65.0) | 104 (65.0) | M: 68 (42.5) F: 92 (57.5) |
R: 13–86 M: 52.3 |
Br: 58 (36.3), HN: 3 (1.9), L: 4 (2.5), GI: 27 (16.9), Gy: 3 (1.9), GU: 31 (19.4), He: 1 (0.6), O: 33 (20.6) |
| Alexander 1985 | Tanzania | SSA - LI | 119 | 53 (44.5) | 14 (11.8) | M: 64 (53.8) F: 55 (46.2) |
M: 42.8 (13.4) treated M: 40.4 (15.6) untreated |
Br: 4 (3.4), HN: 22 (18.5), GI: 9 (7.6), Gy: 29 (24.4), GU: 9 (7.6), He: 3 (2.5), O: 43 (36.1) |
| Kazaura et al. 2007 | Tanzania | SSA - LI | 330 | 67 (20.4) | - | M: 125 (37.9) F: 205 (62.1) |
R: 21–84 M: 48 (13.5) |
- |
| Chaturvedi et al. 2002 | India | SA - LMI | 550 | 206 (37.5) | 70 (12.7) | - | - | - |
| Gupta et al. 2002 | India | SA - LMI | 533 | 302 (56.7) | 33 (6.2) | M: 313 (58.7) F: 220 (41.3) |
R: 18->50 | He: 533 (100) |
| Kumar et al. 2016 | India | SA - LMI | 1117 | 432 (38.7) | 432 (38.7) | M: 501 (44.9) F: 616 (55.1) |
- | - |
| Malik et al. (1 pg 304) 1997 | Pakistan | SA - LMI | 158 | 76 (48.1) | - | M: 66 (41.8) F: 92 (58.2) |
M 45.8 (16.7) | - |
| Malik et al. (2 pg 305) 1997 | Pakistan | SA - LMI | 100 | 4 (4.0) | 4 (4.0) | M: 22 (22.0) F: 78 (78.0) |
R: 10–84 | Br: 38 (38.0), L: 4 (4.0), Gy: 8 (8.0), He: 16 (16.0), O: 34 (34.0) |
| Malik et al. 2000 | Pakistan | SA - LMI | 191 | 104 (54.5) | 51 (26.7) | M: 74 (38.7) F: 117 (61.3) |
R: 17–85 | Br: 51 (26.7), HN: 12 (6.3), L: 9 (4.7), GI: 27 (14.1), Gy: 18 (9.4), GU: 14 (7.3), He: 14 (7.3), O: 46 (24.1) |
| Tovey et al. 2005 | Pakistan | SA - LMI | 362 | 304 (84.0) | 304 (84.0) | M: 213 (58.8) F: 149 (41.2) |
R: 0–90 | Br: 100 (27.6), HN: 35 (9.7), GI: 21 (5.8), Gy: 30 (8.3), He: 42 (11.6), O: 134 (37.0) |
| Broom et al. 2010 | Sri Lanka | SA - LMI | 500 | 337 (67.4) | 337 (67.4) | M: 234 (46.8) F: 266 (53.2) |
M: 45.6 (15.4) | Br: 95 (19.0), HN: 96 (19.2), L: 37 (7.4), GI: 27 (5.4), Gy: 40 (8.0), He: 57 (11.4), O: 148 (29.6) |
| Brahmi et al. 2011 | Morocco | MENA - LMI | 100 | 46 (46.0) | 13 (13.0) | M: 34 (34.0) F: 66 (66.0) |
R: 26–70 M: 48 (13) |
Br: 44 (44.0), HN: 4 (4.0), L: 9 (9.0), GI: 28 (28.0), Gy: 8 (8.0), O: 7 (7.0) |
| Tazi et al. 2013 | Morocco | MENA - LMI | 384 | 276 (71.9) | - | M: 115 (29.9) F: 269 (70.1) |
R: 23–72 M: 49 |
Br: 89 (23.2), HN: 49 (12.8), L: 11 (2.9), GI 34 (8.9), Gy: 17 (4.4) He: 184 (47.9) |
| Jaradat et al. 2016 | West Bank/Palestine | MENA - LMI | 103 | 70 (68.0) | - | M: 0 (0.0) F: 103 (100.0) |
R: 16->60 M: 48.84 |
Br: 103 (100) |
| Oyunchimeg et al. 2017 | Mongolia | EAP - LMI | 482 | 231 (47.9) | 116 (24.0) | M: 174 (36.1) F: 308 (63.9) |
R: >20 | Br: 75 (15.6), GI: 174
(36.1) GU: 140 (29.0), O: 93 (19.3) |
| Sander et al. 2013 | Myanmar | EAP - LMI | 107 | 64 (59.8) | 50 (46.7) | M: 64 (59.8) F: 43 (40.2) |
M: 6.25 (3.2) | He: 72 (67.3), O: 35 (32.7) |
| Ladas et al. 2014 | Guatemala | LAC - LMI | 100 | 92 (92.0) | 63 (63.0) | M: 63 (63.0) F: 37 (37.0) |
R: 0.58–19 | He: 77 (77.0), O: 23 (23.0) |
SSA = Sub-Saharan Africa, MENA = Middle East & North Africa, SA = South Asia, LAC = Latin America & Caribbean, EAP = East Asia & Pacific, LMI = Lower-Middle Income, LI = Low Income
All units are in years
Br = Breast, HN = Head & Neck, L = Lung and Respiratory, GI = Gastrointestinal and Abdominal, Gy = Gynecological, He = Hematological, O: Others
Footnotes
Disclaimer/Conflict of Interest
The authors have no conflict of interest to disclose regarding this systematic review.
Contributor Information
Jacob Hill, Email: jacob_hill@med.unc.edu, University of North Carolina at Chapel Hill, Department of Physical Medicine and Rehabilitation, Program on Integrative Medicine, P: 919.966.8586, A: 101 Manning Dr. Chapel Hill, NC 27599.
Coleman Mills, Email: coleman.mills@unc.edu, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Epidemiology, P: 919.966.8586, A: 135 Dauer Drive, Chapel Hill, NC 27599.
Quefeng Li, Email: quefeng@email.unc.edu, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Biostatistics, P: 919.962.6450, A: 135 Dauer Drive, Chapel Hill, NC 27599.
Jennifer S. Smith, Email: jsssmith@email.unc.edu, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Epidemiology, P: 919.966.7450, A: 135 Dauer Drive, Chapel Hill, NC 27599.
References:
- Abdullahi AA (2011). Trends and challenges of traditional medicine in Africa. African Journal of Traditional, Complementary, and Alternative Medicines : AJTCAM, 8(5 Suppl), 115–23. 10.4314/ajtcam.v8i5S.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afungchwi GM, Hesseling PB, & Ladas EJ (2017). The role of traditional healers in the diagnosis and management of Burkitt lymphoma in Cameroon: understanding the challenges and moving forward. BMC Complementary and Alternative Medicine, 17(1), 209 10.1186/s12906-017-1719-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GA (1985). A survey of traditional medical practices used for the treatment of malignant tumors in an East African population. Social Science & Medicine (1982), 20(1), 53–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3975670 [DOI] [PubMed] [Google Scholar]
- American Cancer Society. (2015). Global Cancer Facts & Figures 3rd Edition. American Cancer Society, (800), 1–64. 10.1002/ijc.27711 [DOI] [Google Scholar]
- Asuzu CC, Elumelu-Kupoluyi T, Asuzu MC, Campbell OB, Akin-Odanye EO, & Lounsbury D (2017). A pilot study of cancer patients’ use of traditional healers in the Radiotherapy Department, University College Hospital, Ibadan, Nigeria. Psycho-Oncology, 26(3), 369–376. 10.1002/pon.4033 [DOI] [PubMed] [Google Scholar]
- Blaes AH, Kreitzer MJ, Torkelson C, & Haddad T (2011). Nonpharmacologic Complementary Therapies in Symptom Management for Breast Cancer Survivors. Seminars in Oncology, 38(3), 394–402. 10.1053/j.seminoncol.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Block KI, & Gyllenhaal C (2002). Clinical corner: herb-drug interactions in cancer chemotherapy: theoretical concerns regarding drug metabolizing enzymes. Integr Cancer Ther, 1(1), 83–89. 10.1177/1534735402001001007 [DOI] [PubMed] [Google Scholar]
- Bodeker G, & Kronenberg F (2002). A public health agenda for traditional, complementary, and alternative medicine. American Journal of Public Health, 92(10), 1582–91. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12356597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmi SA, El M’rabet FZ, Benbrahim Z, Akesbi Y, Amine B, Nejjari C, & El Mesbahi O (2011). Complementary medicine use among Moroccan patients with cancer: a descriptive study. The Pan African Medical Journal, 10, 36 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22187618 [PMC free article] [PubMed] [Google Scholar]
- Broom A, Wijewardena K, Sibbritt D, Adams J, & Nayar KR (2010). The use of traditional, complementary and alternative medicine in Sri Lankan cancer care: Results from a survey of 500 cancer patients. Public Health, 124(4), 232–237. 10.1016/j.puhe.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Chaturvedi U, & Sanyal B (2002). Alternative medicine and cancer patients in less developed countries. The Lancet Oncology. 10.1016/S1470-2045(01)00615-5 [DOI] [PubMed] [Google Scholar]
- Clegg-Lamptey JNA, Dakuboandy JCB, & Attobra YN (2009). Psychosocial aspects of breast cancer treatment in Accra, Ghana. East African Medical Journal, 86(7). 10.4314/eamj.v86i7.54152 [DOI] [PubMed] [Google Scholar]
- Davis EL, Oh B, Butow PN, Mullan BA, & Clarke S (2012). Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. The Oncologist, 17(11), 1475–81. 10.1634/theoncologist.2012-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ver Dye T, Bogale S, Hobden C, Tilahun Y, Hechter V, Deressa T, … Reeler A. (2011). A mixed-method assessment of beliefs and practice around breast cancer in Ethiopia: implications for public health programming and cancer control. Global Public Health, 6(7), 719–31. 10.1080/17441692.2010.510479 [DOI] [PubMed] [Google Scholar]
- Erku DA (2016). Complementary and Alternative Medicine Use and Its Association with Quality of Life among Cancer Patients Receiving Chemotherapy in Ethiopia: A Cross-Sectional Study. Evidence-Based Complementary and Alternative Medicine, 2016, 1–8. 10.1155/2016/2809875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeome ER, & Anarado AN (2007). Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria. BMC Complementary and Alternative Medicine, 7(1), 28 10.1186/1472-6882-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Shafiq N, Kumari S, & Pandhi P (2002). Patterns and perceptions of complementary and alternative medicine ( CAM ) among leukaemia patients visiting haematology clinic of a north Indian tertiary care hospital. Pharmacoepidemiology and Drug Safety, 11, 671–676. [DOI] [PubMed] [Google Scholar]
- INCTR Strategies-INCTR – International Network for Cancer Treatment and Research. (2016). Retrieved December 20, 2016, from http://www.inctr.org/about-inctr/inctr-strategies/
- Jaradat NA, Shawahna R, Eid AM, Al-Ramahi R, Asma MK, & Zaid AN (2016). Herbal remedies use by breast cancer patients in the West Bank of Palestine. Journal of Ethnopharmacology, 178, 1–8. 10.1016/j.jep.2015.11.050 [DOI] [PubMed] [Google Scholar]
- Kazaura R Kombe, D. Yuma, safina. Mtiro, Hussein. Mlawa, G. (2007). Health Seeking Behaviour Among Cancer Patients attending Ocean Road Cancer Institute, Tanzania. East African Journal of Public Health, 4(1), 19–22. Retrieved from http://www.bioline.org.br/request?lp07004 [PubMed] [Google Scholar]
- Kumar D, Goel NK, & Pandey AK (2016). Complementary and alternative medicine use among the cancer patients in Northern India. South Asian Journal of Cancer, 5(1), 8–11. 10.4103/2278-330X.179689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladas E, Ricas S, Ndao D, Damoulakis D, Yuan Yuan B, Cheng B, … Antillon F. (2014). Use of Traditional and Complementary/Alternative Medicine (TCAM) in Children with Cancer in Guatelama. Pediatric Blood and Cancer, 61, 687–692. [DOI] [PubMed] [Google Scholar]
- Ly M, Diop S, Sacko M, Baby M, Diop CT, & Diallo D (2002). Cancer du sein : Facteurs influencant l’itineraire therapeurtique des usagers d’un service d’oncologie medicale a Bamako (Mali). Bulletin Du Cancer, 89(3), 323–6. [PubMed] [Google Scholar]
- Malik IA, & Gopalan S (2003). Use of CAM results in delay in seeking medical advice for breast cancer. European Journal of Epidemiology, 18(8), 817–822. 10.1023/A:1025343720564 [DOI] [PubMed] [Google Scholar]
- Malik IA, Khan NA, & Khan W (2000). Use of unconventional methods of therapy by cancer patients in Pakistan. European Journal of Epidemiology, 16(2), 155–160. 10.1023/A:1007621104789 [DOI] [PubMed] [Google Scholar]
- Malik I, & Qureshi A (1997). Communication with Cancer Patients. Annals of the New York Academy of Sciences, 809(1 Communication), 300–308. 10.1111/j.1749-6632.1997.tb48093.x [DOI] [PubMed] [Google Scholar]
- Obrist M, Osei-Bonsu E, Awuah B, Watanabe-Galloway S, Merajver SD, Schmid K, & Soliman AS (2014). Factors related to incomplete treatment of breast cancer in Kumasi, Ghana. The Breast, 23(6), 821–828. 10.1016/j.breast.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyunchimeg B, Hwang JH, Ahmed M, Choi S, & Han D (2017). Complementary and alternative medicine use among patients with cancer in Mongolia: a National hospital survey. BMC Complement Altern Med, 17(1), 58 10.1186/s12906-017-1576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff P, Al-Sukhun S, Blanchard C, & Shulman LN (2016). Access to Cancer Therapeutics in Low- and Middle-Income Countries. American Society of Clinical Oncology Educational Book, 36, 58–65. 10.14694/EDBK_155975 [DOI] [PubMed] [Google Scholar]
- Sandar K, Win L, Han W, Aye Aye K, Than Than L, Kyu Kyu S, … Khin M. (2013). Use of Complementary and Alternative Medicine (CAM) in Children with Cancer at Yangon Children’s Hospital. The Mayanmar Health Sciences Research Journal, 25(3), 183–188. [Google Scholar]
- Stewart B, & Wild C (2014). World Health Organization World Cancer Report 2014.
- Tazi I, Nafil H, Mahmal L, Harif M, Khouchani M, Saadi Z, … Tahri A. (2013). Les médecines alternatives et complémentaires chez les patients cancéreux en cours de traitement à Marrakech, Maroc: étude prospective. Bulletin de La Societe de Pathologie Exotique, 106(4), 278–285. 10.1007/s13149-013-0308-7 [DOI] [PubMed] [Google Scholar]
- Tovey P, Broom A, Chatwin J, Ahmad S, & Hafeez M (2005). Use of traditional, complementary and allopathic medicines in Pakistan by cancer patients. Rural and Remote Health, 5, 447. [PubMed] [Google Scholar]
- Villa S, Compagni A, & Reich MR (2009). Orphan drug legislation: lessons for neglected tropical diseases. The International Journal of Health Planning and Management, 24(1), 27–42. 10.1002/hpm.930 [DOI] [PubMed] [Google Scholar]
- World Bank list of economies (September 2016). (2016). Retrieved from http://www.ispo2017.org/wp-content/uploads/2016/11/World-Bank-List-of-Economies.pdf
- World Health Organization. (2002). WHO Traditional Medicine Strategy 2002–2005. WHO Traditional Medicine Strategy World Health Organization Geneva. Retrieved from http://www.wpro.who.int/health_technology/book_who_traditional_medicine_strategy_2002_2005.pdf
- World Health Organization. (2013). WHO Traditional Medicine Strategy 2014–2023. https://doi.org/2013
- World Health Organization. (2018). WHO Cancer Factsheet. Retrieved December 20, 2016, from http://www.who.int/mediacentre/factsheets/fs297/en/
- Yarney J, Donkor A, Opoku SY, Yarney L, Agyeman-Duah I, Abakah AC, & Asampong E (2013). Characteristics of users and implications for the use of complementary and alternative medicine in Ghanaian cancer patients undergoing radiotherapy and chemotherapy: a cross- sectional study. BMC Complementary and Alternative Medicine, 13, 16 10.1186/1472-6882-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomi J, & Gonsu F (1995). Causes sociales, economiques et educationnelles du diagnositic et du traitement tardif des cancers au Cameroun. Bulletin Du Cancer, 82, 724–727. [PubMed] [Google Scholar]