Abstract
Background
An estimated 22 million adults use marijuana in the USA. The role of marijuana in the progression of hepatic fibrosis remains unclear.
Aims
We carried out a systematic review and meta-analysis to evaluate the impact of marijuana on prevalence and progression of hepatic fibrosis in chronic liver disease.
Patients and methods
We searched several databases from inception through 10 November 2017 to identify studies evaluating the role of marijuana in chronic liver disease. Our main outcome of interest was prevalence/progression of hepatic fibrosis. Adjusted odds ratios (ORs) and hazards ratios (HRs) were pooled and analyzed using random-effects model.
Results
Nine studies with 5 976 026 patients were included in this meta-analysis. Prevalence of hepatic fibrosis was evaluated in nonalcoholic fatty liver disease (NAFLD), hepatitis C virus (HCV), and hepatitis C and HIV coinfection by two, four, and one studies. Progression of hepatic fibrosis was evaluated by two studies. Pooled OR for prevalence of fibrosis was 0.91 (0.72–1.15), I2 = 75%. On subgroup analysis, pooled OR among NAFLD patients was 0.80 (0.75–0.86), I2 = 0% and pooled OR among HCV patients was 1.96 (0.78–4.92), I2 = 77%. Among studies evaluating HR, pooled HR for progression of fibrosis in HCV–HIV coinfected patients was 1.03 (0.96–1.11), I2 = 0%.
Conclusion
Marijuana use did not increase the prevalence or progression of hepatic fibrosis in HCV and HCV–HIV-coinfected patients. On the contrary, we noted a reduction in the prevalence of NAFLD in marijuana users. Future studies are needed to further understand the therapeutic impact of cannabidiol-based formulations in the management of NAFLD.
Keywords: cannabis, cirrhosis, hepatic fibrosis, marijuana
Introduction
Marijuana use remains illegal in most countries around the world. However, based on an estimate from United Nations Office on Drugs and Crime report, a staggering 220 million people worldwide use marijuana for recreational purposes [1]. In the USA, a survey from 2015 reported ~ 22 million people as current or past marijuana users with about 19% adults between ages 18 and 25 years [2]. With legalization of marijuana use across several states in the USA this trend is expected to show an incremental growth. In epidemiological studies and systematic reviews, marijuana use has been associated with an increased prevalence of cyclical hyperemesis syndrome [3], schizophrenia [4] and obstructive lung diseases like emphysema [5]. However, it is also linked with decreased prevalence of obesity [6] and diabetes along with improved insulin resistance profiles [7,8].
Chronic liver disease is a major public health concern in the USA and globally, cirrhosis-related deaths increased ~ 46% from 838 000 lives in 1990 to 1 221 100 lives in 2013 making it the eight leading cause of death [9]. In the USA, annual aggregate costs for managing chronic liver disease is over $4 billion [10]. Hepatitis C (HCV), alco-holic liver disease, and nonalcoholic fatty liver disease (NAFLD) continue to be the top three etiologies of chronic liver disease in the USA [10] and it is estimated that by the year 2020, NAFLD would become the leading cause of advanced liver disease requiring liver transplantation [11]. Conflicting evidence exists with regard to the effect of marijuana on progression of liver disease. In-vitro and animal studies suggest a beneficial role in preventing liver injury and reducing fibrosis [12–15], while some cross-sectional studies [16,17] in HCV patients report increased prevalence of steatosis as a surrogate marker for worsening liver disease. The cannabinoid receptors 1 and 2 (CB1 and CB2) are G-protein coupled receptors that interact with endocannabinoid ligands and have a high affinity for tetrahydrocannabinol (THC) [18]. Expression of CB1 is seen primarily in brain, while that of CB2 is in immune tissues, and under normal physiologic conditions both these receptors are very weakly expressed by the liver [19]. However, in the setting of chronic liver disease, there is an upregulation of these receptors in the liver that can then influence steatosis and hepatic fibrosis [20]. In clinical practice, the number of patients with chronic liver disease patients who use marijuana has been growing because of legalization of marijuana by several states in the USA. We performed a comprehensive systematic review and meta-analysis to evaluate the association between marijuana use and worsening hepatic fibrosis.
Patients and methods
This systematic review was carried out in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [21] and meta-analysis of observational studies in epidemiology (MOOSE) [22].
Data sources and search strategy
The initial search strategies were developed in Ovid MEDLINE and translated to match keywords and subject headings for Ovid EMBASE, Cochrane databases, and Web of Science from inception through 9 January 2018. This search was performed by an experienced medical librarian (L.W.). The following MeSH, Emtree, and search keywords were used in various combinations: marijuana, cannabis, tetrahydrocannabinol, THC, chronic liver disease, cirrhosis, hepatic fibrosis, nonalcoholic fatty liver disease, NAFLD, hepatitis C, hepatitis B and fatty liver. Our search strategy accounted for plurals and variations in spellings with the use of appropriate wildcards. There was no restriction of language. Articles were selected for full-text review based on their title and abstract. A manual search through the bibliographies of the retrieved publications was carried out to increase the yield of potentially relevant articles. All results were downloaded into EndNote (Thompson ISI Research Soft, Philadelphia, Pennsylvania, USA), a bibliographic database manager, and duplicate citations were identified and removed.
Study selection
Eligibility criteria were determined a priori by four study authors (M.A.K., G.C., D.K., and A.A.). The included studies were required to be observational in nature or evaluated prevalence and/or progression of hepatic fibrosis in patients with chronic liver disease who smoked or did not smoke marijuana. All etiologies of chronic liver disease were included in the study. We restricted the inclusion criteria to studies with patients greater than 16 years of age. We included only fully published and peer-reviewed studies, unpublished data were excluded as there may be discrepancies between published and unpublished data [23,24]. Four reviewers (M.A.K., S.S. A.A.L., and Z.K.) screened citations and retrieved full-text publications of all potentially eligible articles. Study eligibility was assessed by these reviewers independently on the basis of the inclusion criteria, and any disagreement between reviewers was to be discussed with a senior reviewer (A.A.).
Data extraction and quality assessment
Two reviewers (M.T.F. and M.A.K) independently extracted data from eligible studies using prespecified instructions and by utilizing data extraction excel sheets. Extracted data included study design, year of publication, country, individual inclusion and exclusion criteria for these studies, modalities of outcome assessment, variables adjusted for and demographic data of included patients namely age, sex, and a total number of users and nonusers of marijuana. Quality assessment was done by two reviewers (Z.K. and S.S.) using Newcastle Ottawa scale (NOS) for observational studies [25]. This tool measures quality in the three parameters of selection, comparability, and exposure/outcome allocating a maximum of 4, 2, and 3 points, respectively. High-quality studies are scored greater than 7 on this scale, moderatequality studies, between 5 and 7 while low-quality studies score less than 5. Any disagreements in quality assessment between reviewers were discussed with a third reviewer (M.A.K) and agreement reached by consensus.
Data synthesis and statistical analysis
Our primary outcomes of interest were an association between use of marijuana and prevalence or progression of hepatic fibrosis expressed as odds ratios (ORs) and hazard ratios (HRs), respectively. We also separately evaluated the association between marijuana use and prevalence of hepatic steatosis. Adjusted outcomes from individual studies were pooled into our analysis using DerSimonian and Laird random-effects model [26]. Cochrane χ2 and I2 statistics were used to estimate statistical heterogeneity. Presence of heterogeneity was defined as a P value less than 0.1 and I2 values of greater than 50% were reflective of significant heterogeneity. Heterogeneity was expected in our estimate as we had included various etiologies of chronic liver disease. Therefore, predetermined subgroup analysis was planned on the basis of etiology of liver disease. We also carried out a second subgroup analysis on the basis of method of detection of steatosis. Publication bias was assessed through funnel plots and Egger’s test for asymmetry. If asymmetry was detected, we evaluated the potential effect of publication bias using the Duval and Tweedie nonparametric ‘trim-and-fill’ test to recalculate the effect size. All analyses were carried out using Comprehensive Meta-analysis (version 3.0; Biostat, Englewood, New Jersey, USA).
Results
Search strategy yield, study characteristics, and quality assessment
The search strategy identified 8004 citations of which 905 were removed as duplicates; a further 7027 were excluded after title and abstract review. Backward snowballing of remaining 72 articles identified no additional studies. Therefore, 72 full-text articles were reviewed of which 63 were excluded because of ineligibility based on our inclusion criteria. Finally, nine observational studies [16,17,27–33] were included in the systematic review. One study [30] had two datasets; therefore, 10 datasets were included in the quantitative synthesis. Figure 1 illustrates the study selection process. Two studies [28,31] were prospective observational, whereas the remaining seven were retrospective cross-sectional [16,17,27,29,30,32,33] in nature. Three studies [16,17,32] exclusively included patients with HCV, two studies (with three datasets) included only NAFLD patients [27,30], three studies [28,31,33] included HCV, and HIV-coinfected patients, whereas the remaining one study [29] included 79% patients with HCV and 21% patients with HIV. Four studies [16,17,29,32] evaluated prevalence of fibrosis by evaluating hepatic steatosis on liver biopsy, two studies [30,33] used ultrasound as a measure of identifying hepatic steatosis, one data set [30] used elevation of liver enzymes (when other common causes of liver enzyme abnormality were ruled out), one study [27] used ICD-9 codes exclusively and the remaining two studies [28,31] evaluated progression of fibrosis by using aspartate aminotransferase to platelet ratio index and fibrosis-4 scores. Study characteristics are highlighted in Table 1. Seven studies were labeled as high quality as per NOS assessment, whereas the remaining two studies were rated as moderate quality. Detailed quality assessment is provided in Table 2.
Fig. 1.
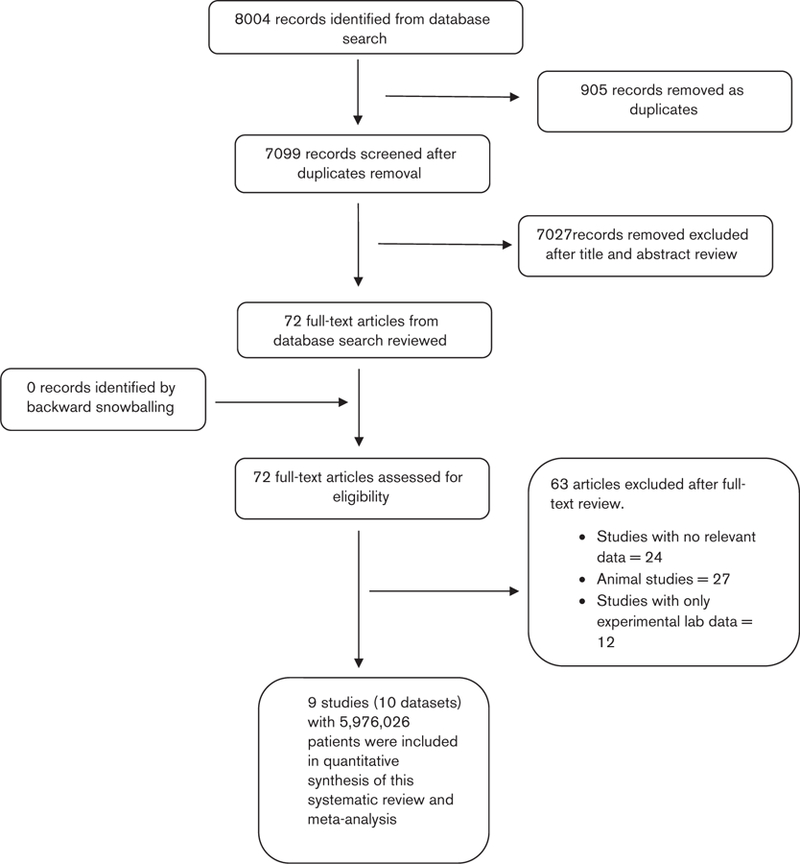
PRISMA flowchart for study selection
Table 1.
Characteristics of included studies
| References | Design | Inclusion criteria | Exclusion criteria | Outcome analyzed | Method of outcome identification | N | Males | Age (mean) | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Hezode et al. [ 16] | Retrospective cross-sectional analysis of prospective cohort | HCV, liver biopsy specimen, single HCV risk factor identifcation, | Other forms of liver disease, concomitant hepatitis B, HIV, immunosuppression, ongoing use of illicit drugs other than marijuana, previous antiviral treatment | Liver steatosis, fibrosis prevalence | METAVIR scoring system | 270 | 186 | 43.2 | Age, sex, BMI, source of contamination, alcohol, HCV genotype, duration of HCV, tobacco, methadone use |
| Hezode et al. [17] | Retrospective cross-sectional analysis of prospective cohort | HCV, liver biopsy specimen, available fasting glucose, triglycerides, cholesterol level | Concomitant hepatitis B, HIV, immunosuppression, ongoing use of illicit drugs other than marijuana, previously treated for chronic hepatitis C | Liver steatosis, fibrosis prevalence | METAVIR scoring system | 315 | 223 | 45.1 | Age, sex, BMI, source of contamination, alcohol, serum HCV RNA load, tobacco, methadone use |
| Ishida et al. [29] | Retrospective cross-sectional analysis of prospective cohort | Age> 18 years, English speaking, detectable HCV RNA | History of HCV treatment > 3 months, other chronic liver disease | Liver steatosis, fibrosis prevalence | METAVIR scoring system | 204 | 140 | 46.8 | Age, race, HCV viral load/genotype, HIV status, duration of HCV, BMI, alcohol use, cannabis prescription |
| Brunett et al. [28] | Prospective study | Age >16, HIV and HCV coinfection. | Age< 16, HIV negatrve status, HCV negative status, ESLD | Liver fibrosis progression in HIV/HCV coinfection | APRI scores | 690 | 503 | 44 | Age, sex, income, alcohol use, injection drug use, BMI, duration of infection |
| Liu et al. [32] | Retrospective case-control study | Marijuana use, liver bopsy, HCV RNA | NR | Fibrosis stage on liver biopsy | Batts Ludwig system stages F0-F4 on liver biopsy | 377 | 145 | 43.9 | Age, weight, HCV RNA/genotype, sex, ALT, HIV coinfection, race, immigrant status, i.v. drug use history, alcohol use |
| Kelly et al. [31] | Prospective study | WIHS cohort of HIV/HCV coinfection | Cirrhosis at time of enrollment | Risk of progression to advanced fibrosis | FIB-4 values and APRI outcomes | 575 | 0 | 40 | Age, ethnicity, race, diabetes, hypertension, BMI, tobacco, i.v. drug use, alcohol use, HCV genotype, CD4, HIV/HCV VL |
| Adejumo et al. [27] | Retrospective cross-sectional study | Age 18–90 years, nonalcoholic fatty liver disease | Alcohol use | Prevalence of NAFLD | HCUP-NIS database, ICD-9-CM codes | 5 950391 | NR | NR | Age, sex, diabetes, alcohol use, race, median income, BMI, HLD, HTN |
| Kim et al. [30], NHANES (2005–2014) | Retrospective cross-sectional study | NR | Significant alcohol consumption, viral hepatitis, nonavailability of BMI, serum aminotransferase, platelet count | Prevalence of NAFLD | Suspected NAFLD using sex based ALT elevations > 30 for men > 19 for women | 14 080 | 7040 | 39.6 | Age, sex, BMI, HTN, Diabetes, ethnicity, tobacco, education, economic status, cholesterol |
| Kim et al. [30], NHANES III | Retrospective cross-sectional study | NR | Pregnancy, viral hepatitis, iron overload, significant alcohol consumption, missing hepatic UU or data (ALT, AST, BMI) | Prevalence of NAFLD | US diagnosed steatosis | 8286 | 4143 | 37.6 | Age, sex, BMI, KTN, diabetes, ethnicity, tobacco, education, economic status, cholesterol, cocaine use |
| Nordmann et al. [33] | Retrospective cross-sectional analysis of prospective cohort | Age > 18 years, chronic HIV-HCV coinfection, written informed consent. | No self-administered questionnaires data on can nabis use | Prevalence of steatosis | US | 838 | NR | 44.9 | BMI, alcohol, current or lifetime use of lamivudina/zidovusine, tobacco use |
ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ESLD, end-stage liver disease; HCV, hepatitis C virus; HTN, hypertension; i.v., intravenous; NAFLD, nonalcoholic fatty liver disease; NR, not reported; ULT, Ultrasonography; US, ultrasound; VL, viral load.
Table 2.
Quality assessment of included studies using Newcastle Ottawa Scale
| References | Selection |
Comparability |
Outcome |
Quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | Outcome not present at start | Adjustment for primary and secondary factors | Assessment by record linkage | Long enough follow up for outcome to occur | Adequacy of follow-up | ||
| Hezode et al. [16] | X | X | X | − | XX | X | X | X | High quality |
| Hezode et al. [17] | X | X | X | − | XX | X | X | X | High quality |
| Ishida et al. [29] | X | X | X | − | XX | X | X | X | High quality |
| Brunett et al. [28] | X | X | X | X | XX | X | X | X | High quality |
| Liu et al. [32] | X | X | X | − | XX | X | X | X | High quality |
| Kelly et al. [31] | X | X | X | X | XX | X | X | X | High quality |
| Adejumo et al. [27] | X | X | X | − | XX | − | X | − | Moderate quality |
| Kim et al. [30] | X | X | X | − | XX | − | X | − | Moderate quality |
| Nordmann et al. [33] | X | X | X | − | XX | X | X | X | High quality |
X, a point given in scale; XX, no point given in scale.
Meta-analysis
Evaluation of hepatic fibrosis in hepatitis C patients using liver biopsy
Four studies [16,17,29,32] with 1166 patients evaluated prevalence of hepatic fibrosis in HCV patients between marijuana users and nonusers and presented results as adjusted OR. Pooled OR with 95% confidence intervals (CIs) for prevalence of fibrosis in the setting of marijuana use was 1.96 (0.78–4.92), Cochran’s Q-test, P < 0.003, I2 = 75% (Fig. 2). Funnel plot appeared symmetric and no publication bias was detected with Egger’s test of asymmetry (intercept 1.16, P = 0.12, two tailed). Sensitivity analysis was performed after excluding Ishida et al. [29] as this study was a comparison between daily versus nondaily marijuana smokers, and there were no nonusers. Pooled OR was 1.44 (0.58–3.57), Cochran’s Q-test, P < 0.01, I2 = 71%. Therefore, we did not find any evidence of increased prevalence of fibrosis among marijuana users. Our analysis remained robust even after sensitivity analysis. Therefore, marijuana use was not associated with increased prevalence of hepatic fibrosis among HCV patients.
Fig. 2.
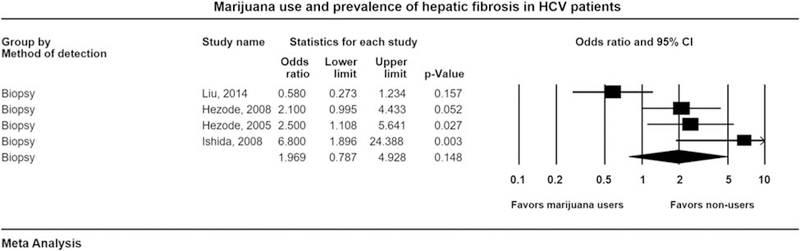
Forest plot for evaluating association of marijuana use with the prevalence of hepatic fibrosis in hepatitis C (HCV) patients by liver biopsy. CI, confidence interval.
Evaluation of hepatic steatosis
Three studies [27,30,33] (including four datasets) with 5 973 595 patients, evaluated the prevalence of hepatic steatosis among marijuana users and nonusers. Among these, two studies evaluated the prevalence in NAFLD patients, whereas one study evaluated prevalence in HCV–HIV patients. Pooled OR with 95% CI for prevalence of steatosis was 0.80 (0.75–0.85), Cochran’s Q-test, P = 0.48, I2 = 0% (Fig. 3). Sensitivity analysis was done after excluding Adejumo and colleagues, as this cross-sectional study provided 5 950 391 patients in our cohort and we wanted to make sure that this study was not skewing the effect size. Pooled OR was 0.73 (0.63–0.84), Cochran’s Q-test, P = 0.72. Subgroup analysis was done on the basis of etiology of liver disease. Pooled OR for prevalence of NAFLD was 0.80 (0.75–0.86), Cochran’s Q-test, P = 0.51, I2 = 0% in favor of marijuana users (Fig. 3). Finally, among HIV and HCV-coinfected patients, pooled OR was 0.64 (0.41–0.98), I2 = 0% (Fig. 3). Therefore, the prevalence of hepatic steatosis and NAFLD was lower in marijuana users. Our second subgroup analysis was on the basis of the method of detection of hepatic steatosis. Pooled OR for patients undergoing ultrasound-based assessment was 0.73 (0.58–0.91), Cochran’s Q-test, P = 0.47, I2 = 0%.
Fig. 3.
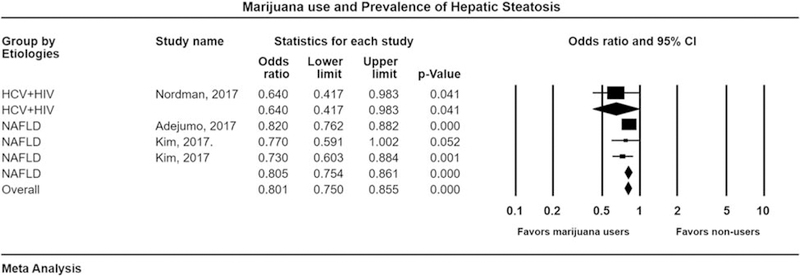
Forest plot for evaluating association of marijuana use with the prevalence of hepatic steatosis with subgroups based on etiology. CI, confidence interval; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Progression of hepatic fibrosis
Two studies [28,31] with 1265 patients (HCV and HIV coinfection) evaluated progression of fibrosis among marijuana users and nonusers. Pooled HR was 1.00 (0.93–1.07), Cochran’s Q-test, P = 0.79, I2 = 0% (Fig. 4). Therefore, the progression of hepatic fibrosis was not influenced by marijuana use in HCV–HIV-coinfected patients.
Fig. 4.
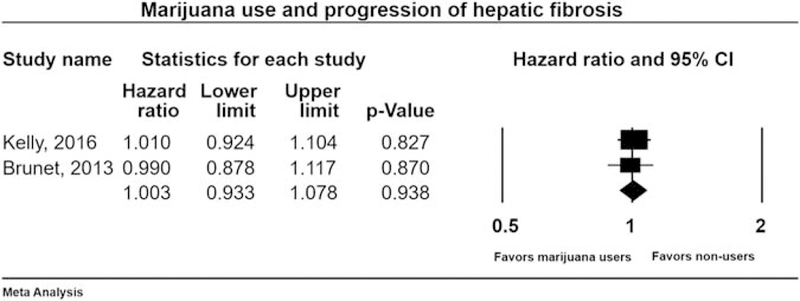
Forest plot for evaluating association of marijuana use with the progression of hepatic fibrosis in HCV–HIV-coinfected patients. CI, confidence interval; HCV, hepatitis C virus.
Discussion
Marijuana has been legalized for medicinal and/or recreational use in 23 states as well as the District of Columbia with reports of significant use of marijuana in patients with viral hepatitis monoinfection, with HCV and HIV coinfection, and increasing tendency to use marijuana in other etiologies of chronic liver disease [34,35]. Therefore, it is imperative for physicians to understand the impact of marijuana use in the setting of chronic liver disease and to educate the patient population on the limitations of available data. This systematic review and meta-analysis did not find any association between increased prevalence of hepatic fibrosis and marijuana use in patients with chronic liver disease. On the contrary, we noted a lower prevalence of hepatic steatosis in marijuana users with NAFLD and with HCV and HIV coinfection.
Biologic plausibility for the effect of marijuana in patients with chronic liver disease is on the basis of the expression of CB1 and CB2 receptors in the liver [36,37]. Two main ingredients of marijuana are THC and cannabidiol (CBD) [38]. THC preferentially acts on CB1, whereas CBD has more effect on CB2 [38]. Therefore, THC has more psychotropic effects as CB1 is mainly found in nervous tissue, whereas CBD does not have psychotropic effects. However, in patients with chronic liver disease expression of both CB1 and CB2 receptors is upregulated. CB1 receptor activation is associated with profibrogenic effects with increased expression of transforming growth factor β−1, increased stellate cell production, and decreased apoptosis leading higher rates of hepatic fibrogenesis [39]. In addition, it is also associated with decreased production of adiponectin, an adipokine with antifibrotic properties in animal models [40]. Finally, CB1 receptor also promotes hepatic fatty-acid production and participates in diet-related obesity [39]. On the contrary, activation of CB2 receptors in mice has been associated with antifibrogenic and anti-inflammatory properties such as suppression of macrophage function of antigen presentation, inhibits chemokine production by human B cells, decreases macrophage nitric-oxide production, suppresses cytotoxic T-cell proliferation, and favorably regulates tumor necrosis factor, interleukin-1, and interferon gamma production by mononuclear cells [41]. Overall, stimulation of CB2 receptor results in reduction of oxidative stress and retards cell death [12].
Overall, we did not find any association between marijuana use and increased prevalence of hepatic fibrosis when confirmed by liver biopsy. However, our primary analysis was limited by substantial heterogeneity. Our results are different from initial cross-sectional studies [16,29] which suggested increased fibrosis in patients using marijuana. Ishida and colleagues only compared daily users versus nondaily users and reported that daily users had a higher prevalence of worsening fibrosis and cirrhosis. Interestingly, their study did not find any significant association between marijuana use and mild hepatic fibrosis. The authors concluded that marijuana use may have little or no influence on initiation of fibrosis but once fibrosis sets in, it was associated with worsening liver disease. In contrast, a more recent Canadian study [32] using The Ottawa Hospital Viral Hepatitis Clinic database showed a comparable prevalence of hepatic fibrosis in marijuana users as well as nonusers that were consistent with our analysis. Likewise, we did not find any difference in the progression of hepatic fibrosis between marijuana users and nonusers among HCV–HIV-coinfected patients. These data were based on high quality prospective observational studies with 1265 patients and there was no heterogeneity in our estimate.
We noted that the prevalence of NAFLD was lower in marijuana users as compared with nonusers. Conventionally, marijuana users have high-calorie intake with higher consumption of sodas, drink, and alcohol [42]. Therefore, a healthy lifestyle is definitely not the cause of the decreased prevalence of NAFLD. The data in the studies included in our analysis were adjusted for BMI that is a potential confounder for the prevalence of NAFLD. The National Epidemiologic Survey on Alcohol and Related Conditions reported 39% reduction in the risk of obesity among marijuana users as compared with nonusers [6]. It is hypothesized that marijuana use may affect fatty tissues in obese through omega-3 fatty acids. Batetta et al. [43] reported in laboratory studies that endogenous cannabinoids mediate the ability of omega-3 fatty acids in reducing ectopic fat deposition. Finally, a systematic review reported decreased steatosis and improved liver enzyme profile with dietary supplementation of omega-3 fatty acids [44]. Apart from fat deposition, marijuana use may have beneficial effects on insulin profile as reported by Penner et al. [45] showing lower levels of fasting insulin in marijuana users as compared to nonusers. The mechanism of action for this association is not well understood. One theory is that CBD in marijuana can act as a partial antagonist for CB1 receptors which have a role in insulin sensitivity [46,47]. Therefore, nonpsychotropic cannabinoids like CBD may have potential in the development of drugs for management of NAFLD.
This is the first meta-analysis to evaluate the association of marijuana use with the prevalence and progression of hepatic fibrosis in chronic liver disease. We carried out a comprehensive literature search and reviewed a large number of relevant studies. Our analysis may have been weakened by inherent limitations of meta-analyses and of the included studies. All included studies in our analysis were observational by design, albeit most of them were of high quality based on NOS assessment and all of them had adjusted for common variables that may influence the prevalence and progression of fibrosis. We were unable to evaluate the dose–response relationship between marijuana use versus the prevalence and progression of hepatic fibrosis as such data were not uniformly provided by the studies. Our primary analysis was limited by considerable heterogeneity and most of our study cohort was derived from one large cross-sectional study [27]. Therefore, we carried out a sensitivity analysis to assess that our estimates remained robust after exclusion of this study. Furthermore, we also carried out two subgroup analyses on the basis of etiology of chronic liver disease and method of detection of fibrosis. No heterogeneity was noted in our analyses for HCV and HIV coinfection for progression of fibrosis and prevalence of NAFLD.
Conclusion
Among marijuana users, we did not find any evidence for increased prevalence of hepatic fibrosis in HCV patients, nor did we find any progression of hepatic fibrosis in patients with HCV and HIV coinfection. On the contrary, we found a decreased prevalence of NAFLD in marijuana users. Our meta-analysis highlights the importance of evaluating the association of marijuana use and development of NALFD. Further studies may be beneficial to further understand the therapeutic impact CBD-based formulations in the management of NAFLD.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.United Nations. Office on drugs and crime. World drug report 2012 New York, NY: United Nations; 2012. [Google Scholar]
- 2.SAMHSA Adminstration. Key substance use and mental health indicators in the United States: results from the 2015 National Survey on Drug Use and Health; 2016. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf. [Accessed 16 January 2018].
- 3.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller-Vahl KR, Emrich HM. Cannabis and schizophrenia: towards a cannabinoid hypothesis of schizophrenia. Expert Rev Neurother 2008; 8:1037–1048. [DOI] [PubMed] [Google Scholar]
- 5.Martinasek MP, McGrogan JB, Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respir Care 2016; 61:1543–1551. [DOI] [PubMed] [Google Scholar]
- 6.Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol 2011; 174:929–933. [DOI] [PubMed] [Google Scholar]
- 7.Alshaarawy O, Anthony JC. Cannabis smoking and diabetes mellitus: results from meta-analysis with eight independent replication samples. Epidemiology 2015; 26:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajavashisth TB, Shaheen M, Norris KC, Pan D, Sinha SK, Ortega J, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012; 2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2013 Mortality and Causes of Death Collaborators. Causes of Death C. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149:1731–1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlton M Cirrhosis and liver failure in nonalcoholic fatty liver disease: molehill or mountain? Hepatology 2008; 47:1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay P, Rajesh M, Horvath B, Batkai S, Park O, Tanchian G, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med 2011; 50:1368–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim MP, Devi LA, Rozenfeld R. Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death Dis 2011; 2:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avraham Y, Grigoriadis N, Poutahidis T, Vorobiev L, Magen I, Ilan Y, et al. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol 2011; 162:1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol 2011; 163: 1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hezode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005; 42:63–71. [DOI] [PubMed] [Google Scholar]
- 17.Hezode C, Zafrani ES, Roudot-Thoraval F, Costentin C, Hessami A, Bouvier-Alias M, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008; 134:432–439. [DOI] [PubMed] [Google Scholar]
- 18.Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol 2013; 59:891–896. [DOI] [PubMed] [Google Scholar]
- 19.Baldassarre M, Giannone FA, Napoli L, Tovoli A, Ricci CS, Tufoni M, et al. The endocannabinoid system in advanced liver cirrhosis: patho-physiological implication and future perspectives. Liver Int 2013; 33:1298–1308. [DOI] [PubMed] [Google Scholar]
- 20.Siegmund SV. Role of the endocannabinoid system in alcoholic liver disease. Dig Dis 2010; 28:751–755. [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 23.Taddio A, Pain T, Fassos FF, Boon H, Ilersich AL, Einarson TR. Quality of nonstructured and structured abstracts of original research articles in the British Medical Journal, the Canadian Medical Association Journal and the Journal of the American Medical Association. Can Med Assoc J 1994; 150:1611–1615. [PMC free article] [PubMed] [Google Scholar]
- 24.Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev 2007; 2: MR000005. [DOI] [PubMed] [Google Scholar]
- 25.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp. [Accessed 24 January 2018].
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 27.Adejumo AC, Alliu S, Ajayi TO, Adejumo KL, Adegbala OM, Onyeakusi NE, et al. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS One 2017; 12:e0176416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet L, Moodie EE, Rollet K, Cooper C, Walmsley S, Potter M, et al. Marijuana smoking does not accelerate progression of liver disease in HIV-hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis 2013; 57:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol 2008; 6:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Kim W, Kwak MS, Chung GE, Yim JY, Ahmed A. Inverse association of marijuana use with nonalcoholic fatty liver disease among adults in the United States. PLoS One 2017; 12:e0186702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly EM, Dodge JL, Sarkar M, French AL, Tien PC, Glesby MJ, et al. Marijuana use is not associated with progression to advanced liver fibrosis in HIV/Hepatitis C virus-coinfected women. Clin Infect Dis 2016; 63:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol 2014; 28:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordmann S, Vilotitch A, Roux P, Esterle L, Spire B, Marcellin F, et al. Daily cannabis and reduced risk of steatosis in human immunodeficiency virus and hepatitis C virus-co-infected patients (ANRS CO13-HEPAVIH). J Viral Hepat 2018; 25:171–179. [DOI] [PubMed] [Google Scholar]
- 34.Marijuana Policy Project. The twenty three states and one federal district with effective medical marijuana laws Available at: https://www.mpp.org/states/key-marijuana-policy-reform/. [Accessed 26 January 2018].
- 35.Ammerman S, Ryan S, Adelman WP. Committee on Substance Abuse tCoA. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics 2015; 135:e769–e785. [DOI] [PubMed] [Google Scholar]
- 36.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005; 128:742–755. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 2006; 12:671–676. [DOI] [PubMed] [Google Scholar]
- 38.Andre CM, Hausman JF, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 2016; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005; 115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 2003; 125:1796–1807. [DOI] [PubMed] [Google Scholar]
- 41.Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol 2005; 166:3–18. [DOI] [PubMed] [Google Scholar]
- 42.Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public Health Nutr 2001; 4:781–786. [DOI] [PubMed] [Google Scholar]
- 43.Batetta B, Griinari M, Carta G, Murru E, Ligresti A, Cordeddu L, et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J Nutr 2009; 139:1495–1501. [DOI] [PubMed] [Google Scholar]
- 44.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012; 56:944–951. [DOI] [PubMed] [Google Scholar]
- 45.Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 2013; 126:583–589. [DOI] [PubMed] [Google Scholar]
- 46.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology 2011; 53:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam J, Godlewski G, Earley BJ, Zhou L, Jourdan T, Szanda G, et al. Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity. Am J Physiol Endocrinol Metab 2014; 306:E457–E468. [DOI] [PMC free article] [PubMed] [Google Scholar]


