Abstract
CMYA5 is a candidate gene for schizophrenia because of the genetic association of variant rs10043986 (C>T) to this severe mental disorder. Studies of CMYA5 and its gene product, myospryn, in the brain and neuronal cells have not been previously reported. The SNP rs10043986 changes the 4,063rd amino acid from Pro to Leu, which is likely to alter protein function. To understand its potential role in the brain, we examined the neuronal expression of myospryn and its binding partner, desmin, an intermediate filament (IF) protein, and investigated how the two alleles of myospryn affect its binding to desmin. Myospryn and desmin are shown to be expressed in the brain and myospryn is shown to localize to the cytoplasm and nucleus of myoblast, neuroblastoma, and glioblastoma cell lines. Peripherin and vimentin, known brain IF proteins, have high protein similarity to desmin but were found not to interact with myospryn using yeast two-hybrid (Y2H). Using a quantitative Y2H assay and surface plasmon resonance, the T allele (Leu) of rs10043986 was found to have stronger binding to desmin than the C allele (Pro). Based on findings described in this report, we hypothesize that the interaction between myospryn to IF provides structural support and efficient rearrangement of the cytoskeleton network during early neuritogenesis.
Keywords: schizophrenia, CMYA5, intermediate filament, desmin, differential binding, single-nucleotide polymorphism (SNP)
Introduction
Schizophrenia (OMIM 181500) is a severe mental disorder with a prevalence of ~1% worldwide. The clinical features are characterized by positive symptoms (delusions, hallucination, and disorganized thinking), negative symptoms (flat affect, loss of pleasure, interest, and speech), and cognitive symptoms (poor executive functioning, trouble focusing, and memory problems) (Owen et al., 2016). The age of onset typically begins in adolescence or early adulthood in both men and women (Kirkbride et al., 2012). The incidence for men developing schizophrenia is higher than that for women before the age of 45 and peaks for both sexes in their early 20s with rates for men at almost double the rate for women (Aleman et al., 2003; Kirkbride et al., 2012). There seems to be a second incidence peak at 45 years old for women, possibly involved with the presence/absence of estrogen (van der Werf et al., 2014). Some genome-wide association studies (GWAS) have found female-specific association; however, more studies are needed to replicate the findings (Riecher-Rössler et al., 2018; Shifman et al., 2008; Wang et al., 2011; Zhang et al., 2011).
Using GWAS, more than 100 loci have been found to be associated with schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), but many risk loci have no known function (Chen et al., 2015). The cardiomyopathy associated 5 (CMYA5) gene has two single nucleotide polymorphisms (SNPs), rs10043986 (odds ratio (OR) = 1.11, 95% confidence interval (CI) = 1.04–1.18, P = 8.2 × 10−4) and rs4704591 (OR = 1.07, 95% CI = 1.03–1.11, P = 3.0 × 10−4), that were significantly and independently associated with schizophrenia in Caucasian subjects (Chen et al., 2011). The SNP rs10043986 (C>T; Minor Allele Frequency (MAF): T=0.0467/234 (1000 Genomes)) changes the 4063rd amino acid of the CMYA5 protein product, myospryn, from proline (Pro) to leucine (Leu), whereas rs4704591 (G>C; MAF: C=0.2388/1196 (1000 Genomes)) is located approximately 7kb downstream of the CMYA5 gene (Chen et al., 2011). The SNPs identified via association studies have a weak effect on schizophrenia risk individually with ORs generally <1.2 but overall account for up to half the variation in schizophrenia susceptibility (Rees et al., 2015). The association of CMYA5 has been replicated in East Asian populations (Han et al., 2018; Li et al., 2011; Wang et al., 2014; Watanabe et al., 2014; Zhang et al., 2013). Conflicting results have been observed in two studies (Furukawa et al., 2013; Hoya et al., 2017). The SNP rs10043986 was monomorphic in Han Chinese (Li et al., 2011) but not in the Caucasian population (Chen et al., 2015), which may indicate genetic heterogeneity across populations.
Myospryn, a muscle-specific, tripartite motif (TRIM)-related protein (Benson et al., 2004), is found to be downregulated in Duchenne muscular dystrophy (Tkatchenko et al., 2001) and associated with hypertension (Nakagami et al., 2007). Studies of CMYA5 have focused on its role in skeletal and cardiac muscle, but its precise function remains unknown. All known protein interactions of myospryn occur at the C-terminus, including self-association (Benson et al., 2004), binding to α-actinin (Durham et al., 2006), desmin (Kouloumenta et al., 2007), dystrophin (Reynolds et al., 2008), M-band titin (Sarparanta et al., 2010), calcineurin (Kielbasa et al., 2011), protein kinase A (Reynolds et al., 2007), calpain 3 protease (Sarparanta et al., 2010), minispryn (Fsd2) (Benson et al., 2017), cardiac ryanodine receptor (Benson et al., 2017), and dysbindin (Benson et al., 2004), another schizophrenia susceptibility gene (Straub et al., 2002). These interactions suggest that myospryn is involved in vesicular trafficking and intracellular signaling (Sarparanta, 2008; Tsoupri and Capetanaki, 2013). No studies of CMYA5 in the brain and neuronal cells have been reported, thus determining its expression in these tissues is important for understanding its role in schizophrenia.
Schizophrenia is a neurodevelopmental disorder, and abnormalities in the neuronal cytoskeleton, which plays roles in neurite outgrowth and axonal transportation in the developing nervous system, can have long-term effects on learning and memory (Birnbaum and Weinberger, 2017; Lewis and Levitt, 2002). A review of proteomics research in schizophrenia has revealed changes in cytoskeletal components (Davalieva et al., 2016). Myospryn has been shown to interact with components of the cytoskeleton such as desmin, an intermediate filament (IF) protein. The importance of this interaction was revealed by the absence of myospryn’s perinuclear localization in desmin-null neonatal cardiomyocytes (Kouloumenta et al., 2007).
The second objective for this study is to determine if the missense change of rs10043986 has functional consequences. This SNP changes a highly conserved amino acid Pro4063Leu with Pro being the risk allele and Leu the protective allele (Chen et al., 2011). Of the many binding partners of myospryn available to study, desmin was chosen because of its importance in the cytoskeleton and the fact that the desmin binding region in myospryn has been precisely mapped to the last 24 aa of the protein where rs10043986 is located. (Kouloumenta et al., 2007). Thus, a change in this region is likely to affect this interaction. Myospryn binding regions to other partners, did not include the C-terminal region in some interactions such as with dysbindin and others which did contain the region, the binding sites also contained other domains of the protein.
In this study, we provide evidence that myospryn and desmin are expressed in various regions of the brain, localized primarily in the cytoplasm, and weakly to moderately co-localized in brain derived cell lines when desmin is transfected. Peripherin and vimentin, which share high protein homology with desmin, do not interact with myospryn. The CMYA5 SNP rs10043986 altered binding between myospryn and desmin, which provides a direct functional link between a common genetic variant to schizophrenia.
Material and Methods
Western Blotting
Mouse brain and heart tissues were dissected from a mix of young adult male and female wild-type C57BL/6 mice under the approval of the IACUC of Virginia Commonwealth University (protocol number AM10025). Tissues were homogenized in HENTS buffer (250mM HEPES-NaOH pH7.7, 1mM EDTA, 1X protease inhibitor cocktail (Sigma-Aldrich), 1% Triton X-100, 0.1% SDS), incubated on ice for 20 minutes, and centrifuged at 14,000 rpm for 20 minutes at 4°C to extract soluble proteins. The insoluble pellet was further denatured with Buffer 1 (Leung and Liem, 2006). Lysates were run on SDS-PAGE gels and electroblotted onto a PVDF membrane (BioRad), which were then blocked for 4 hours at room temperature in 5% milk in TBST and incubated with primary antibody overnight at 4°C. Immunoblotting with rabbit anti-myospryn UT266 antibody (1:2000) has been previously described (Durham et al., 2006). Other primary antibodies include mouse anti-α-Tubulin (1:1000, Sigma-Aldrich), rabbit anti-desmin (1:1000, Abcam), and rabbit Pan-Actin Antibody (1:1000; Cell Signaling Technology). The appropriate HRP secondary antibody (1:10,000) was added for 30–45 minutes at room temperature. Visualization of the immunoreactive proteins was detected using Western Lightning Plus-ECL, Enhanced Chemiluminescence Substrate (PerkinElmer) and Blue Basic Autorad Film (GeneMate) for autoradiography. These experiments were repeated at least three times.
Reverse Transcription (RT)-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol. cDNA was generated with 1μg of total RNA and 100ng of both oligo dT and random hexamers heated at 70°C for 10 minutes. A mix of 1X M-MLV RT Buffer, 10mM DTT, 1mM dNTPs, 10 units RNasin (Promega) was added, followed by 200 units of M-MLV Reverse Transcriptase (Promega) or 1μL DEPC ddH2O (-RT) at 37°C for one hour and 95°C for 5 minutes. PCR was performed in 6.25μL reactions with 1.25μL cDNA, 1X Phire Reaction Buffer, 0.2mM dNTPs, 0.5pmol/μL primers, and 0.05μL of Phire DNA polymerase (Finnzymes). MJ Research PTC-200 PCR Thermal Cycler was used. Cycling conditions were 94°C 1.5 min, (94°C 30s, annealing temperature 55°C (Cmya5), 50°C (Desmin), and 60°C (mGapdh) 30s, and 72°C 30s) for 18 (mGapdh) or 30 cycles, and 72°C 7 min. The following primers were used, Cmya5: F: 5’-TGTACTGGAGCGTGAACAAGG-3’, R: 5’-CATTGGTGGCTCTCACATAG-3’, Desmin: F: 5’-ATGGCCTTGGATGTGGAGATC-3’, R: 5’-TGTTGTTGCTGTGTAGCCTC-3’, and mGapdh: F: 5’- TGGCAACAATCTCCACTTTGC-3’, R: 5’- AGCCTCGTCCCGTAGACAAAA-3’.
Cell Culture
N18TG2 (mouse neuroblastoma), U87 (human glioblastoma), and T98G (human glioblastoma) were maintained in DMEM/F-12 with GlutaMAX™ with 10% fetal bovine serum (FBS) (Life Technologies). N18TG2 was a gift from Dr. Dana Selly, and U87 and T98G were gifts from Dr. Paul Fisher. C2C12 (mouse myoblast), obtained from ATCC, was maintained in DMEM (high glucose, pyruvate) (Life Technologies) with 10% FBS. All cell lines were maintained in 5% CO2 at 37°C. 1×106 cells were grown on a Poly-L-Lysine coated coverslip overnight then transfected with mEmerald-Desmin-N-18 (Addgene #54060) using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol.
Immunocytochemistry
Cells were fixed in cold 4% paraformaldehyde in PBS for 10 minutes, permeabilized with 0.25% Triton X-100 in PBS for 10 minutes, blocked with 5% donkey serum, 0.3% Triton X-100 in PBS for 1 hour, and incubated with anti-myospryn UT266 antibody (1:200) and mouse Desmin Antibody D33 (1:100; Thermo Scientific) diluted in blocking solution for 1 hour. After washing in PBST, cells were incubated with Alexa Fluor 568 donkey anti-rabbit and Alexa Fluor 488 donkey anti-mouse (1:250, Molecular Probes) for 1 hour and counterstained with 0.1μg/mL DAPI for 30 minutes. Coverslips were mounted on microscope slides using SlowFade Diamond Antifade Mountant. All steps were performed at room temperature. Images were obtained using Zeiss LSM 700 Confocal Laser Scanning Microscopy with Plan-Apochromat 63x/1.40 Oil DIC M27 objective. Zen Software (Black Edition) was used to calculate the colocalization coefficients, and the degree of colocalization was categorized according to Zinchuk and Grossenbacher-Zinchuk, 2014. The experiments were repeated at least twice. Five images with different cells were captured for each cell line. For C2C12, the analysis was performed on the whole image, whereas the analysis for N18TG2 and T98G was performed only on cells that expressed transfected desmin. The mean and 95% confidence interval of each coefficient were calculated.
Yeast Two-Hybrid Assay (Y2H)
CMYA5 short (NM_153610, 12185 to 12279), CMYA5 long (NM_153610, 11648 to 12279), and desmin (NM_001927, 174 to 395) were cloned in pBluescript SK+ via PCR and TA cloning. Both alleles of CMYA5 were then subcloned into pACT2, and desmin was subcloned into pAS2–1. Peripherin and vimentin were subcloned from mEmerald-Peripherin-N-18 (Addgene #54227) and mCherry-Vimentin-N-18 (Addgene #55158) into pAS2–1. The Matchmaker Two-Hybrid System 2 (Clontech Laboratories) was used according to the manufacturer’s protocols. For the Liquid Culture Assay using ONPG (ortho-Nitrophenyl-β-galactoside) as a substrate, four or five transformant colonies were assayed in triplicate, and the average of triplicate is n = 1. A two-tailed t-test was performed using JMP software.
Surface Plasmon Resonance (SPR)
Both alleles of CMYA5-HIS and BCCP-Desmin proteins were expressed in BL21(DE3) and isolated from inclusion bodies (Burgess, 2009). The CMYA5-HIS proteins were further purified using HisPur Ni-NTA Resin according to the manufacturer’s Batch Method (Thermo Scientific). SPR was performed using BiaCore T200 (GE Healthcare) with a Series S sensor chip CM5. The BCCP-Desmin ligand was immobilized using standard amine-coupling chemistry with a flow rate of 10 μL/min in HBS-EP+ Buffer (GE Healthcare). The surface was activated with a 7-minute pulse of EDC/NHS. The BCCP-Desmin protein, 15μg/ml in 10mM sodium acetate pH 4.0, was injected for 420 seconds in a flow cell (Fc); Fc1 had buffer injected to serve as a reference surface. A pulse of 1M ethanolamine, pH8.0, was injected to quench the reaction and remove non-specifically bound protein. The analytes (CMYA5 C, CMYA5 T, and 6X-His) were injected at concentrations of 0, 0.25, 0.5, 1, 2, 4, 4, and 8μM (in random order) at a flow rate of 30μL/min and at 25°C. The complex was allowed to associate and dissociate for 700 and 500 seconds, respectively. The surfaces were regenerated with two 40-second injections of 10mM glycine-HCl pH1.5. Data were obtained from Biacore T200 Evaluation Software v.1.0. The response is subtracted from the Fc1 and blank injection (0μM), normalized to 0 based on the injection start time. The response for every 0.1 second was exported to Microsoft Excel and graphed together as a sensorgram. The 8μM injection was graphed separately, and the response for CMYA5 C and CMYA5 T was subtracted from 6X-HIS. The fold-change was determined by dividing the response of CMYA5 C over the response of CMYA5 T.
Results
Expression and subcellular localization of myospryn and desmin
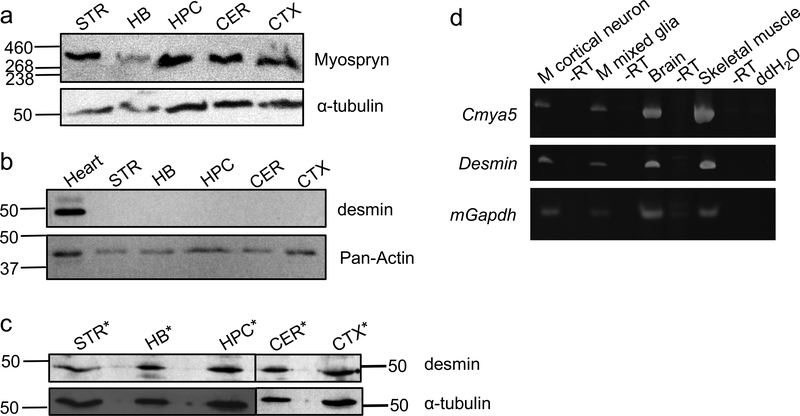
Myospryn expression has been observed in skeletal and cardiac muscle (Benson et al., 2004; Kouloumenta et al., 2007; Reynolds et al., 2008), but the expression has not been reported in the brain despite the genetic association with schizophrenia. Several brain regions, including striatum (Simpson et al., 2010), hindbrain (Crespo-Facorro et al., 2007), hippocampus (Harrison, 2004), cerebellum (Andreasen and Pierson, 2008), and cortex (Zhou et al., 2015), were selected based on histological, molecular biology, structural neuropsychological and functional imaging that suggest their involvement in the pathophysiology of schizophrenia. As shown in Fig. 1a, protein expression of myospryn was detected in those regions of mouse brain. Protein expression of desmin, a binding partner of myospryn, was not detected in the soluble fraction of similar brain regions (Fig. 1b) but was detected in the insoluble fraction (Fig. 1c). To validate the anti-desmin antibody, the U87 cell line that has no desmin expression was transfected with full-length human desmin plasmid (mEmerald-Desmin-N-18). Expression of desmin was clearly detected by the anti-desmin antibody in extracts from U87 cells transfected with desmin expression plasmid but not in untransfected cells and mEmerald vector only-transfected cells. (Fig. S1). Furthermore, Cmya5 and Desmin transcripts are both expressed in mouse primary cortical neurons, mixed glia, brain, and skeletal muscle (Fig. 1d).
Fig. 1. Expression of myospryn and desmin.
a) Myospryn protein was expressed in mouse striatum (STR), hindbrain (HB), hippocampus (HPC), cerebellum (CER), and cortex (CTX). b) Desmin protein was expressed in the soluble fraction of the mouse heart but not in brain regions. c) Desmin protein was expressed in the insoluble fraction of mouse STR, HB, HPC, CER, and CTX. *lysates were extracted under denaturing condition. α-Tubulin and Pan-Actin were used as loading controls. HiMark Pre-stained Protein Standard (Invitrogen) was used for the myospryn blot; Precision Plus Protein Dual Color Standards (Bio-rad) was used for all other blots. d) Cmya5 and desmin transcripts were expressed in mouse cortical neurons, mixed glia, brain, and skeletal muscle. Gapdh was used as the positive control for cDNA synthesis, and -RT was the negative control for cDNA synthesis to ensure no genomic DNA contamination.
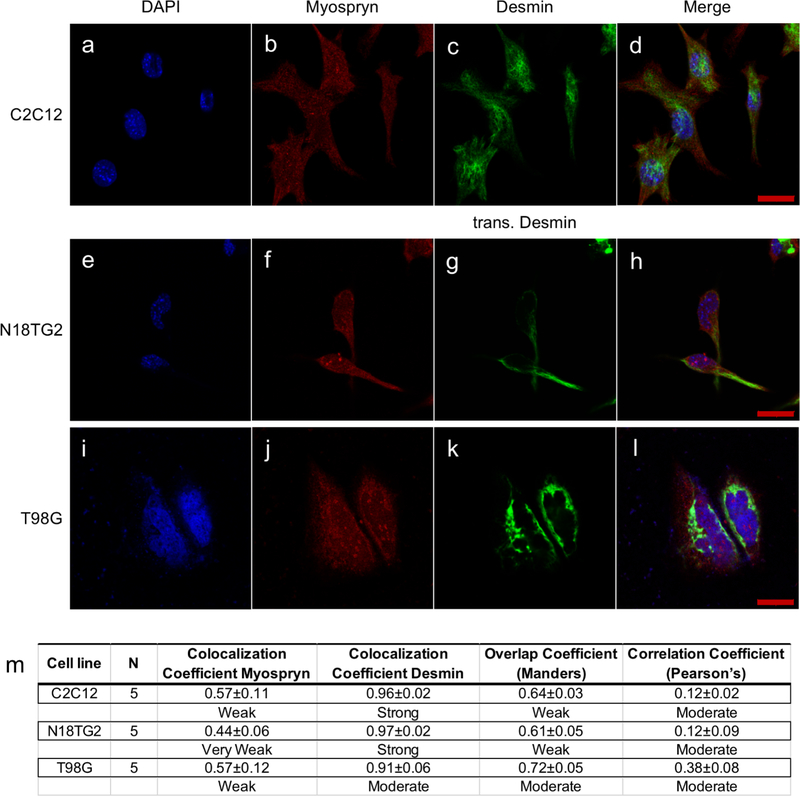
Myospryn is known to localize in the cytoplasm and perinuclear region of muscle cells (Durham et al., 2006; Kouloumenta et al., 2007). Since the RT-PCR result showed that Cmya5 and Desmin were expressed in both neurons and glia, N18TG2 mouse neuroblastoma and T98G human glioblastoma cell lines were used to determine the subcellular localization of myospryn and desmin. Initially, C2C12 mouse myoblast cells were used to demonstrate the localization of myospryn in the cytoplasm and nucleus (Fig. 2a-d). A similar localization pattern was observed in N18TG2 (Fig. 2e-h) and T98G cells (Fig. 2i-l). The localization of desmin was also determined, which revealed a filamentous network in the cytoplasm of C2C12 cells (Fig. 2c). However, endogenous desmin expression in N18TG2 and T98G was too weak to visualize; therefore, a full-length human desmin was transfected. The transfected desmin in N18TG2 and T98G cells were also localized in the cytoplasm (Fig. 2g&k). The degree of colocalization between myospryn and desmin was calculated and categorized based on Mander’s overlap coefficient (MOC) and Pearson’s correlation coefficient (PCC) (Zinchuk and Grossenbacher-Zinchuk, 2014). Myospryn and transfected desmin in N18TG2 were weakly (MOC = 0.61) to moderately (PCC = 0.12) colocalized, similar to the degree of colocalization in C2C12 (MOC = 0.64; PCC = 0.12), while T98G had a stronger degree of colocalization (MOC = 0.72; PCC = 0.38) (Fig. 3m). Greater than 90% of desmin colocalized with myospryn and only about 50% of myospryn colocalized with desmin. The immunofluorescence analysis showed weak to moderate colocalization of myospryn and desmin in these neuronal cell lines. Immunohistochemistry was subsequently performed to determine whether myospryn and desmin are expressed in the brain. Analysis of mouse brain cryosections showed that myospryn and desmin were weakly or extremely weakly expressed throughout this tissue (Fig. S2).
Fig. 2. Subcellular localization of myospryn and desmin.
Images of DAPI, myospryn, desmin (or transfected desmin), and merged in C2C12 (a-d), N18TG2 (e-h), and T98G (i-l) showed myospryn was localized in both cytoplasm and nucleus and desmin was localized in the cytoplasm. Scale bar = 20μm. m) Merged images were analyzed for colocalization, and colocalization coefficients, overlap coefficient, and correlation coefficient for myospryn and desmin were calculated. Number of images (N) was obtained using Zeiss LSM 700 Confocal Laser Scanning Microscopy with the same setting within a cell line. Colocalization analysis was done using Zeiss Zen Software (Black Edition). All coefficients numbers are mean ± 95% confidence interval.
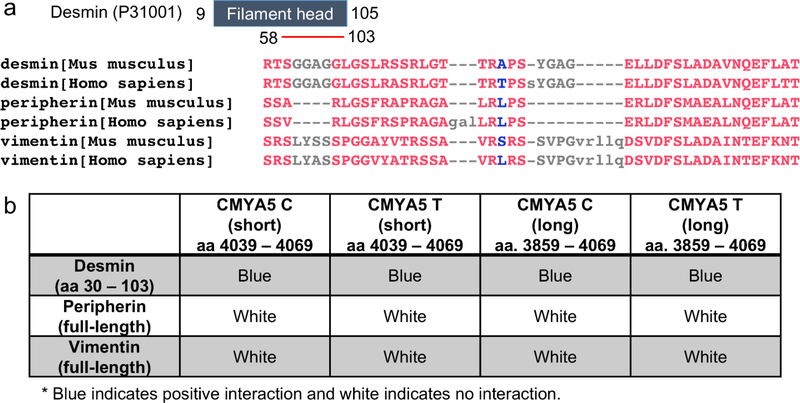
Fig. 3. Interaction between myospryn and IF proteins, desmin, vimentin, and peripherin.
a) Mouse and human desmin, peripherin, and vimentin alignment using COBALT Constraint-based Multiple Protein Alignment Tool. The minimum binding region of desmin (GenBank™ accession number P31001) to myospryn is aa 58–103 and located within the filament head domain (aa 9–105). The red color in the protein alignment indicates highly conserved residues and blue indicates less conserved residues determined using 2 Bits Conservation Setting. b) Summary of Y2H Colony-Lift Filter Assay, where the blue indicates positive interaction, and white indicates no interaction. Desmin interacts with both alleles of myospryn and with both short (aa 4039–4069) and long (aa 3859–4069) myospryn clones, whereas vimentin and peripherin did not show any interaction.
Interaction between myospryn rs10043986 and type III IF
Since endogenous localization of desmin could not be detected in neuroblastoma and glioblastoma cells, we reasoned that other IF proteins might bind to myospryn in those cells. Performing BLAST using Accession# P31001, aa 58–103, against mouse and human protein databases, we found that peripherin and vimentin are highly similar to desmin. This region of mouse desmin shares 91% (43/47) identity with human desmin, 61% (23/38) and 58% (23/40) identity with mouse and human peripherin, and 69% (11/16) identity with mouse and human vimentin (Fig. 3a). Y2H was used to determine if myospryn interacts with desmin, peripherin, and vimentin. Desmin (aa. 30–103) interacted with myospryn, and the amino acid change did not abolish the binding (Fig. 3b). However, full-length peripherin and vimentin did not interact with the c-terminus region of human myospryn (aa. 3859–4069) (Fig. 3b).
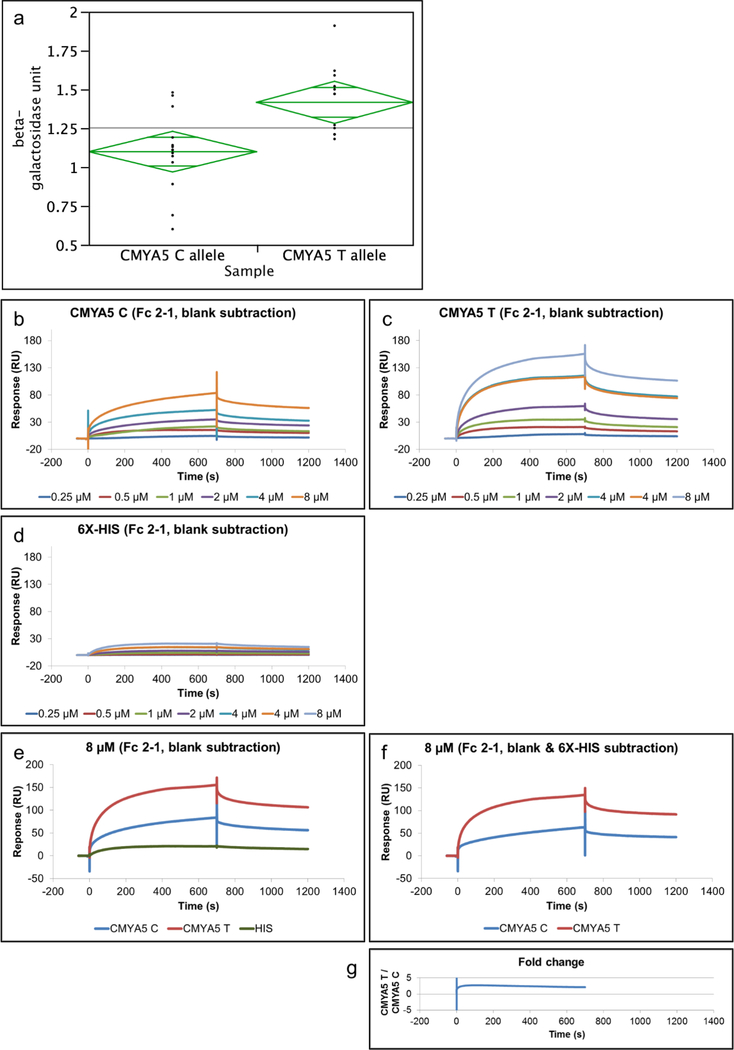
The SNP rs10043986 (Pro4063Leu) is within the binding site of myospryn and desmin, and a quantitative Y2H assay was used to determine the effect on binding. The average β-galactosidase activity for CMYA5 C allele was 1.10 units (SE = 0.063), and the average β-galactosidase activity for CMYA5 T allele was 1.42 units (SE = 0.066). One unit of β-galactosidase is defined as the amount which hydrolyzes 1 μmol of ONPG to o-nitrophenol and D-galactose per minute. The major allele (Pro or C allele) showed weaker binding compared to the minor allele (Leu or T allele) (t = 3.47, df = 25, p-value = 0.0019) (Fig. 4a). Next, SPR was used to verify the differential binding. The fusion proteins were purified from E. coli BL21(DE3) due to high protein expression and yield; however, it should be noted that one limitation of using bacterial cells is lack of post-translational modifications. Fig. 4b-d shows sensorgrams (response versus time) for injections of CMYA5C-HIS, CMYA5T-HIS, and 6X-HIS negative control at various concentrations over a CM5 sensor chip surface immobilized with desmin. Overall, CMYA5 T (Fig. 4c) was found to have a higher response than CMYA5 C (Fig. 4b). A low response from the 6X-HIS negative control (Fig. 4d) indicated the protein tag did not interfere with the interaction. The 8μM injections were graphed separately to show a better comparison (Fig. 4e). Comparative analysis of each analyte after 6X-HIS negative control subtraction (Fig. 4f) showed that CMYA5 T had on average 2.4-fold higher response than the CMYA5 C, indicating that CMYA5 T has more binding to desmin than the CMYA5 C (Fig. 4g).
Fig. 4. Binding property of rs10043986 to desmin.
a) Beta-galactosidase activity of the two alleles of rs10043986 from the Y2H liquid cultures assay using ONPG as the substrate. The average β-galactosidase activity for CMYA5 C allele was 1.10 units (SE = 0.063), and the average β-galactosidase activity for CMYA5 T allele was 1.42 units (SE = 0.066). The observed means were significantly different (t = 3.47, df = 25, p-value = 0.0019). One unit of β-galactosidase is defined as the amount which hydrolyzes 1 μmol of ONPG to o-nitrophenol and D-galactose per minute. Sensorgrams for injection of b) CMYA5 C, c) CMYA5 T, and d) 6X-His negative control over a sensor chip surface immobilized with 570 RU desmin. The proteins were allowed to associate for 700 seconds and dissociate for 500 seconds, and the concentrations used were 0.25, 0.5, 1, 2, 4, and 8 μM. e) Sensorgrams for comparing 8 μM injection of all three analytes and f) after 6X-HIS subtraction. g) Fold-change was determined by CMYA5 T RU divided by CMYA5 C RU. CMYA5 T has about 2.4-fold higher RU than the CMYA5 C after 6X-HIS subtraction.
Discussion
Association studies first identified CMYA5, which is mainly expressed in muscle tissue, to be important in schizophrenia. The present study provides the first evidence that myospryn and one of its binding partners, desmin IF, are expressed in regions of the brain, suggesting that they might be relevant to schizophrenia pathophysiology. Multiple databases also provide evidence that myospryn (Cmya5) and desmin (Des) are expressed in the brain and specific cell types. The Allen Mouse Brain Atlas showed that Cmya5 is expressed in the cerebellum, whereas Des is expressed across the brain (Lein et al., 2007). The immunohistochemistry data shows protein expression across the entire mouse brain for both proteins though at very low levels. The Allen Human Brain Atlas suggested CMYA5 and DES have expression throughout the brain (Hawrylycz et al., 2012). A few regions that have high CMYA5 expression include the occipital lobe, pontine tegmentum, and myelencephalon; as for DES, the highest expression is observed in the cingulate gyrus and myelencephalon (Hawrylycz et al., 2012). The Barres Brain RNA-seq transcriptome database showed that in mouse Cmya5 has the highest expression in astrocytes and much lower expression in neurons, oligodendrocyte precursor cells (OPCs), newly formed oligodendrocytes (NFOs), myelinating oligodendrocytes (MOs), microglia, and endothelial cells, whereas Des has the highest expression in endothelial cells, followed by OPCs and much lower expression in astrocytes, neurons, NFOs, MOs, and microglia (Zhang et al., 2014). Though the expression is not high, there are several cell types where the two genes overlap in expression. In the human brain, CMYA5 has the highest expression in mature astrocytes, followed by medium expression in oligodendrocytes, fetal astrocytes, and neurons, and low expression in endothelial and microglia/macrophage (Zhang et al., 2016). However, no expression of DES in those cell types is shown (Zhang et al., 2016). It is unclear as to why the human RNA-seq data does not correlate with the Human Allen Brain Atlas data with respect to DES expression or with the mouse sequencing data. In the human RNA-seq studies, only small amounts of tissues could be obtained at directed sites in the brain as constrained by the surgical needs of the patients, so it may not be representative of genes expressed in subsets of cells within a brain region which could explain these differences. More studies are needed to determine the region-specific and cell-specific expression of myospryn and desmin. It would be particularly interesting to determine the expression and overlap of expression of these genes in the developing brain, as schizophrenia is a neurodevelopmental disorder (Rapoport et al., 2012). The developmental expression of myospryn is not completely known though its transcript is expressed in the mouse brain at E15.5 but not at E10.5 (Durham et al., 2006), specifically in the superficial stratum of dorsal pallium/isocortex (Hsiung, Ph.D. Dissertation, 2017). Additional insight into the developmental expression of CMYA5 can be gleaned from the BrainSpan Atlas of the Developing Human Brain. Little to no expression is observed throughout the human brain during development in the Developmental Transcriptome data but expression is observed in the Prenatal LMD Microarray (Miller et al., 2014). RNA-seq usually provides higher specificity and sensitivity, thus the lack of expression using RNA-seq but not microarray suggest an issue with the microarray probe such as non-specific and cross-hybridization.
A CMYA5 variant that is associated with schizophrenia, rs10043968, resulted in a two-fold higher binding response of myospryn to desmin in vitro. We also show that there is co-localization in neuronal and glial cell lines when both myospryn and desmin are expressed. It is unfortunate that the endogenous expression of desmin in the brain is so low that co-localization experiments in brain tissues are difficult to interpret and co-immunoprecipitation studies with insoluble IF proteins cannot be performed. Desmin is known as a muscle-specific type III IF protein (Paulin and Li, 2004) though it was shown to be expressed in the brain in the present study. Its subcellular localization is not readily apparent in the adult mouse brain perhaps due to its low level of expression., Alternatively, myospryn might bind to other type III IF proteins. Interactions between myospryn and peripherin or vimentin were examined given their amino acid homology to desmin within the filament head domain. The interaction between the c-terminus region of myospryn to peripherin or vimentin was not observed using Y2H. Again, the interactions could not be determined using co-immunoprecipitation due to the insolubility of IF proteins and their need to be extracted under denaturing conditions. It is still possible that myospryn forms complexes with those neuronal IF proteins but does not directly interact.
Proteomic studies have found upregulation of desmin and vimentin in schizophrenia brains, providing evidence that the disruption of cytoskeleton and its associated signal transduction proteins are involved in schizophrenia (Davalieva et al., 2016). Vimentin is widely expressed in mesenchymal cells, but it is also expressed in the nervous system during early embryonic development and is important for axonal outgrowth, whereas peripherin is expressed in neurons of the peripheral nervous system and is important for axonal guidance and regeneration (Hol and Capetanaki, 2017). In addition to desmin, myospryn also interacts with other cytoskeletal proteins, such as α-actinin (Durham et al., 2006), dystrophin (Reynolds et al., 2008), and titin (Sarparanta et al., 2010), suggesting its involvement with the cytoskeleton and that the binding of myospryn’s protective allele to IF protein might provide more efficient rearrangement of the cytoskeletal network during early neuritogenesis. Preliminary data suggest that rs10043986 affects the colocalization of myospryn and F-actin in vivo, which may be through the interaction between myospryn and α-actinin (Hsiung, Ph.D. Dissertation, 2017). It will be interesting to determine if rs10043968 also affects binding of myospryn with its wide-range of binding partners. To understand their interactions and functions in the brain will provide more understanding of the pathways involved in schizophrenia pathophysiology. Identifying functional consequences of the rs10043981 variant that leads to a protective effect will provide a direct link of a common genetic variant to its risk for schizophrenia.
Supplementary Material
Acknowledgements
We thank Dr. Jeffrey Dupree for the mouse cortical neurons and Dr. Babette Fuss for the mixed glial cells. We thank Dr. Qinglian Liu for the BCCP vector, Dr. Dana Selley for the N18TG2 cell line, and Dr. Paul Fisher for the U87 and T98G cell lines. We thank Dr. Daniel Conrad and Julie Farnsworth from the VCU Flow Cytometry Shared Resource Core and Dr. Michael Murphy from GE Healthcare Life Sciences for the help of Biacore T200. Services and products in support of the research project were generated by the VCU Massey Cancer Center Flow Cytometry Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. We thank Dr. Scott Henderson and Frances White for the help of confocal laser scanning microscopy and colocalization analysis. Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Role of Funding Source
This work was supported in part by the National Institutes of Health (MH101054). The funding source had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Kahn RS, Selten J-P, 2003. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch. Gen. Psychiatry 60, 565–571. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R, 2008. The role of the cerebellum in schizophrenia. Biol. Psychiatry 64, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MA, Tinsley CL, Blake DJ, 2004. Myospryn is a novel binding partner for dysbindin in muscle. J. Biol. Chem 279, 10450–10458. [DOI] [PubMed] [Google Scholar]
- Benson MA, Tinsley CL, Waite AJ, Carlisle FA, Sweet SMM, Ehler E, George CH, Lai FA, Martin-Rendon E, Blake DJ, 2017. Ryanodine receptors are part of the myospryn complex in cardiac muscle. Sci Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum R, Weinberger DR, 2017. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci 18, 727–740. [DOI] [PubMed] [Google Scholar]
- Burgess RR, 2009. Chapter 17 Refolding Solubilized Inclusion Body Proteins, in: Methods in Enzymology. Elsevier, pp. 259–282. [DOI] [PubMed] [Google Scholar]
- Chen J, Cao F, Liu L, Wang L, Chen X, 2015. Genetic studies of schizophrenia: an update. Neurosci Bull 31, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lee G, Maher BS, Fanous AH, Chen J, Zhao Z, Guo A, van den Oord E, Sullivan PF, Shi J, Levinson DF, Gejman PV, Sanders A, Duan J, Owen MJ, Craddock NJ, O’Donovan MC, Blackman J, Lewis D, Kirov GK, Qin W, Schwab S, Wildenauer D, Chowdari K, Nimgaonkar V, Straub RE, Weinberger DR, O’Neill FA, Walsh D, Bronstein M, Darvasi A, Lencz T, Malhotra AK, Rujescu D, Giegling I, Werge T, Hansen T, Ingason A, Nöethen MM, Rietschel M, Cichon S, Djurovic S, Andreassen OA, Cantor RM, Ophoff R, Corvin A, Morris DW, Gill M, Pato CN, Pato MT, Macedo A, Gurling HMD, McQuillin A, Pimm J, Hultman C, Lichtenstein P, Sklar P, Purcell SM, Scolnick E, St Clair D, Blackwood DHR, Kendler KS, GROUP investigators, International Schizophrenia Consortium, 2011. GWA study data mining and independent replication identify cardiomyopathy-associated 5 (CMYA5) as a risk gene for schizophrenia. Mol. Psychiatry 16, 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM, 2007. Neuropsychological functioning and brain structure in schizophrenia. Int Rev Psychiatry 19, 325–336. [DOI] [PubMed] [Google Scholar]
- Davalieva K, Maleva Kostovska I, Dwork AJ, 2016. Proteomics Research in Schizophrenia. Front Cell Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham JT, Brand OM, Arnold M, Reynolds JG, Muthukumar L, Weiler H, Richardson JA, Naya FJ, 2006. Myospryn Is a Direct Transcriptional Target for MEF2A That Encodes a Striated Muscle, α-Actinin-interacting, Costamere-localized Protein. J Biol Chem 281, 6841–6849. [DOI] [PubMed] [Google Scholar]
- Furukawa M, Tochigi M, Otowa T, Arinami T, Inada T, Ujike H, Watanabe Y, Iwata N, Itokawa M, Kunugi H, Hashimoto R, Ozaki N, Kakiuchi C, Kasai K, Sasaki T, 2013. An association analysis of the cardiomyopathy-associated 5 (CMYA5) gene with schizophrenia in a Japanese population. Psychiatr. Genet 23, 179–180. [DOI] [PubMed] [Google Scholar]
- Han S, An Z, Luo X, Zhang L, Zhong X, Du W, Yi Q, Shi Y, 2018. Association between CMYA5 gene polymorphisms and risk of schizophrenia in Uygur population and a meta-analysis. Early Interv Psychiatry. 12, 15–21. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, 2004. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl.) 174, 151–162. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, David Daly B, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard E, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith C, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SGN, Jones AR, 2012. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol EM, Capetanaki Y, 2017. Type III Intermediate Filaments Desmin, Glial Fibrillary Acidic Protein (GFAP), Vimentin, and Peripherin. Cold Spring Harb Perspect Biol 9, a021642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoya S, Watanabe Y, Shibuya M, Someya T, 2017. Updated meta-analysis of CMYA5 rs3828611 and rs4704591 with schizophrenia in Asian populations. Early Interv Psychiatry. doi: 10.1111/eip.12472 [DOI] [PubMed] [Google Scholar]
- Hsiung A, 2017. Functional Study of CMYA5, a Candidate Gene for Schizophrenia. Ph.D. Dissertation. Virginia Commonwealth University. [Google Scholar]
- Kielbasa OM, Reynolds JG, Wu C-L, Snyder CM, Cho MY, Weiler H, Kandarian S, Naya FJ, 2011. Myospryn is a calcineurin-interacting protein that negatively modulates slow-fiber-type transformation and skeletal muscle regeneration. The FASEB Journal 25, 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, Boydell J, Murray RM, Jones PB, 2012. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PLoS ONE 7, e31660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouloumenta A, Mavroidis M, Capetanaki Y, 2007. Proper Perinuclear Localization of the TRIM-like Protein Myospryn Requires Its Binding Partner Desmin. J Biol Chem 282, 35211–35221. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen Lin, Chen Li, Chen T-M, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong H-W, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf K-R, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR, 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Leung CL, Liem RKH, 2006. Isolation of Intermediate Filaments, in: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM (Eds.), Current Protocols in Cell Biology. John Wiley & Sons, Inc., Hoboken, NJ, USA. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P, 2002. Schizophrenia as a Disorder of Neurodevelopment. Annu Rev Neurosci 25, 409–432. [DOI] [PubMed] [Google Scholar]
- Li M, Luo X, Zhang X, Yang Z, Xiang K, Xiao X, Su B, Zhao Y, Shen Y, Xu Q, Chen X, Chen J, Liu X, Yin L, Ma X, Yang S, Yu J, Diao H, Shi X, 2011. A common variant of the cardiomyopathy associated 5 gene (CMYA5) is associated with schizophrenia in Chinese population. Schizophr. Res 129, 217–219. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding S-L, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, Bennet C, Bertagnolli D, Brouner K, Butler S, Caldejon S, Carey A, Cuhaciyan C, Dalley RA, Dee N, Dolbeare TA, Facer BAC, Feng D, Fliss TP, Gee G, Goldy J, Gourley L, Gregor BW, Gu G, Howard RE, Jochim JM, Kuan CL, Lau C, Lee C-K, Lee F, Lemon TA, Lesnar P, McMurray B, Mastan N, Mosqueda N, Naluai-Cecchini T, Ngo N-K, Nyhus J, Oldre A, Olson E, Parente J, Parker PD, Parry SE, Stevens A, Pletikos M, Reding M, Roll K, Sandman D, Sarreal M, Shapouri S, Shapovalova NV, Shen EH, Sjoquist N, Slaughterbeck CR, Smith M, Sodt AJ, Williams D, Zöllei L, Fischl B, Gerstein MB, Geschwind DH, Glass IA, Hawrylycz MJ, Hevner RF, Huang H, Jones AR, Knowles JA, Levitt P, Phillips JW, Sestan N, Wohnoutka P, Dang C, Bernard A, Hohmann JG, Lein ES, 2014. Transcriptional landscape of the prenatal human brain. Nature 508, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Kikuchi Y, Katsuya T, Morishita R, Akasaka H, Saitoh S, Rakugi H, Kaneda Y, Shimamoto K, Ogihara T, 2007. Gene polymorphism of myospryn (cardiomyopathy-associated 5) is associated with left ventricular wall thickness in patients with hypertension. Hypertens. Res 30, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Sawa A, Mortensen PB, 2016. Schizophrenia. The Lancet 388, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin D, Li Z, 2004. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301, 1–7. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N, 2012. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 17, 1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, O’Donovan MC, Owen MJ.,2015. Genetics of schizophrenia. Current Opinion in Behavioral Sciences 2,8–14. [Google Scholar]
- Reynolds JG, McCalmon SA, Tomczyk T, Naya FJ, 2007. Identification and mapping of protein kinase A binding sites in the costameric protein myospryn. Biochim Biophys Acta 1773, 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JG, McCalmon SA, Donaghey JA, Naya FJ, 2008. Deregulated Protein Kinase A Signaling and Myospryn Expression in Muscular Dystrophy. J Biol Chem 283, 8070–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler A, Butler S, Kulkarni J, 2018. Sex and gender differences in schizophrenic psychoses-a critical review. Arch Womens Ment Health. doi: 10.1007/s00737-018-0847-9 [DOI] [PubMed] [Google Scholar]
- Sarparanta J, 2008. Biology of myospryn: what’s known? J Muscle Res Cell Motil 29, 177–180. [DOI] [PubMed] [Google Scholar]
- Sarparanta J, Blandin G, Charton K, Vihola A, Marchand S, Milic A, Hackman P, Ehler E, Richard I, Udd B, 2010. Interactions with M-band Titin and Calpain 3 Link Myospryn (CMYA5) to Tibial and Limb-girdle Muscular Dystrophies. J Biol Chem 285, 30304–30315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O’Donovan M, O’Neill FA, Owen MJ, Walsh D, Weinberger DR, Sun C, Flint J, Darvasi A, 2008. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 4, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E, 2010. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O’Neill FA, Walsh D, Kendler KS, 2002. Genetic Variation in the 6p22.3 Gene DTNBP1, the Human Ortholog of the Mouse Dysbindin Gene, Is Associated with Schizophrenia. Am J Hum Genet 71, 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko AV, Piétu G, Cros N, Gannoun-Zaki L, Auffray C, Léger JJ, Dechesne CA, 2001. Identification of altered gene expression in skeletal muscles from Duchenne muscular dystrophy patients. Neuromuscul. Disord 11, 269–277. [DOI] [PubMed] [Google Scholar]
- Tsoupri E, Capetanaki Y, 2013. Μyospryn: a multifunctional desmin-associated protein. Histochem Cell Biol 140, 55–63. [DOI] [PubMed] [Google Scholar]
- van der Werf M, Hanssen M, Köhler S, Verkaaik M, Verhey FR, RISE Investigators, van Winkel R, van Os J, Allardyce J, 2014. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol Med 44, 9–16. [DOI] [PubMed] [Google Scholar]
- Wang K-S, Liu X, Zhang Q, Aragam N, Pan Y, 2011. Genome-wide association analysis of age at onset in schizophrenia in a European-American sample. Am. J. Med. Genet. B Neuropsychiatr. Genet 156B, 671–680. [DOI] [PubMed] [Google Scholar]
- Wang Q, He K, Li Z, Chen J, Li W, Wen Z, Shen J, Qiang Y, Ji J, Wang Y, Shi Y, 2014. The CMYA5 gene confers risk for both schizophrenia and major depressive disorder in the Han Chinese population. World J Biol Psychiatry 15, 553–560. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Shibuya M, Someya T, 2014. The cardiomyopathy-associated 5 (CMYA5) gene and risk of schizophrenia: meta-analysis of rs3828611 and rs4704591 in East Asian populations. Asian J Psychiatr 7, 95–96. [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen Q, Ye T, Lipska BK, Straub RE, Vakkalanka R, Rujescu D, St Clair D, Hyde TM, Bigelow L, Kleinman JE, Weinberger DR, 2011. Evidence of sex-modulated association of ZNF804A with schizophrenia. Biol. Psychiatry 69, 914–917. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang H, Li M, Li H, Li Y, Valenzuela RK, Su B, Ma J, 2013. Genetic analysis of common variants in the CMYA5 (cardiomyopathy-associated 5) gene with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 64–69. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNASequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MSB, Li G, Duncan JA, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MGH, Barres BA, 2016. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fan L, Qiu C, Jiang T, 2015. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull 31, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk V, Grossenbacher-Zinchuk O., 2014. Quantitative colocalization analysis of fluorescence microscopy images. Curr Protoc Cell Biol. 62, 4.19.1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.