Chronic hepatitis C virus (HCV) infection has remained the leading indication for liver transplantation in the United States for approximately the last 2 decades. However, the approval of second-generation direct-acting antiviral (DAA) agents in late 2013 has already had a positive impact by improving the short-term liver transplant (LT) recipient and graft survival rates.1 Furthermore, data through 2015 demonstrated a decline in the number of HCV-related LT waitlist registrations and LT surgeries following the introduction of DAA-based regimens.2,3 Despite the downtrend, primary diagnosis of HCV remained the most common cause for liver transplantation in the United States until the end of 2015.3 In this updated report, we evaluated the current impact of DAA era on the rate of HCV-related liver transplantation in the United States.
Methods
Using the United Network for Organ Sharing database, we performed primary analysis on annual waitlist additions and LT surgery trends in patients for the 3 leading indications of chronic liver disease (CLD): (1) HCV, (2) alcoholic liver disease (ALD), and (3) nonalcoholic steatohepatitis (NASH) from January 1, 2012, to December 31, 2016. Etiology of CLD in the United Network for Organ Sharing database was obtained from primary and secondary diagnoses code entries and by using HCV serology. Each cohort was mutually exclusive and patients with a secondary diagnosis code of HCV or HCV seropositivity (2012–2016) were included only in the HCV cohort. Patients listed as Status 1A, needing retransplantation, or with concomitant hepatocellular carcinoma were excluded. Recent trends restricted to 2015–2016 were analyzed to assess etiology-based differences in demographic and clinical characteristics among LT waitlist additions and LT surgeries using chi-square test for categorical variables and Kruskal-Wallis for quantitative variables. Finally, to further assess consistency in the trends for LT waitlist additions and LT surgeries, we performed a subanalysis on limited data provided by United Network for Organ Sharing from January 1, 2017, to October 31, 2017, with only the primary indication for LT waitlist additions and LT surgeries available.
Results
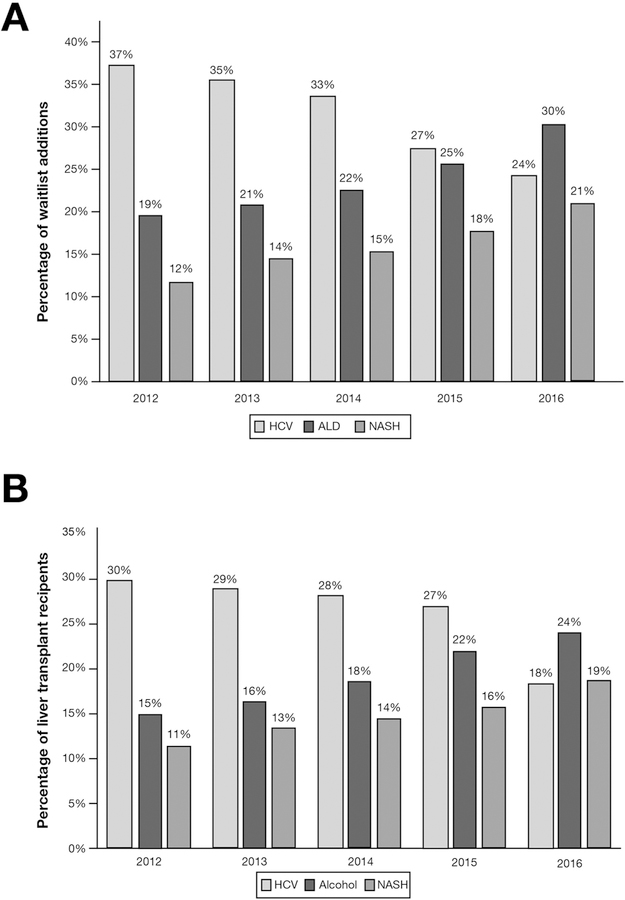
From 2012–2016, the annual increase in LT waitlist additions and LT surgeries because of CLD was 2.1% and 7.6%, respectively. From 2012–2015, chronic HCV remained the leading cause for LT waitlist additions and LT surgeries, 33% and 28%, respectively. In 2016, ALD became the leading indication for LT waitlist additions in the United States (Figure 1A) by surpassing HCV. ALD (30%) and NASH (21%) accounted for more than half of CLD LT waitlist additions in 2016. In terms of LT surgery trends, in 2016 ALD and NASH outpaced and overtook HCV as the first and second leading CLD indications for liver transplantation, respectively (Figure 1B). Furthermore, even after including concomitant ALD and HCV exclusively to the HCV LT recipient cohort, ALD remained the leading indication for liver transplantation until the end of 2016. During 2016, there was a notable decline of 18% in the annual percentage of LT surgeries for HCV. Compared with HCV and NASH, ALD-related LT waitlist additions during 2015–2016 were significantly younger in age (ALD: 54; interquartile range [IQR], 47–51; HCV: 59; IQR, 55–63; NASH: 60, IQR, 54–65; P < .001) but with a significantly higher severity of hepatic decompensation as reflected by the median Model for End-Stage Liver Disease score at listing (ALD: 21; IQR, 15–30; HCV: 14; IQR, 10–21; NASH: 17; IQR, 13–24; P < .001) and at the time of LT surgery. The limited subanalysis in 2017 continued to demonstrate ALD as the leading indication for LT waitlist additions and LT surgeries. Overall, the total number of LT surgeries performed on an annual basis is on a trajectory to reach an all-time high at the end of 2017.
Figure 1.
(A, B) Annual trends in the 3 leading indications for liver transplantation in the United States.
Discussion
ALD has now become the leading indication for liver transplantation in the United States. The rate of liver transplantation in patients with HCV is plummeting in the United States following the widespread application of DAA-based regimens in the pretransplant setting.4 Interestingly, the total number of liver transplants per year in the United States continues to rise, despite the decline HCV-related LT waitlist additions and LT surgeries.
More importantly, ALD is a well-established CLD that has been increasing in prevalence without any well-coordinated effort to tackle it. Data from the National Epidemiology Survey on Alcohol indicated that the 12-month (ending in June 2013) prevalence of alcohol use, high-risk drinking, and alcohol use disorder were on the rise across all sociodemographic groups in the United States.5 These data forewarned an increase in alcohol-related chronic comorbidities, including ALD. Similarly, NASH continues to grow as an indication for liver transplantation.2 Measures are needed to aggressively address these ominous trends in the rising rates of ALD and NASH-related liver transplantation.
Acknowledgments
Funding
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Cholankeril G, Li AA, March KL, et al. Improved outcomes in HCV patients following liver transplantation during the era of direct acting antiviral agents. Clin Gastroenterol Hepatol 2018; 16:452–453. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barua S, Greenwald R, Grebely J, et al. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–223. [DOI] [PubMed] [Google Scholar]
- 5.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017; 74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]