Abstract
B cell activation and differentiation are associated with marked changes in proliferative and effector functions. Each stage of B cell differentiation thus has unique metabolic demands. New studies have provided insight on how nutrient uptake and usage by B cells are regulated by B cell receptor signals, autophagy, mammalian target of rapamycin, and transcriptional control of transporters and rate-limiting enzymes. A recurring theme is that these pathways play distinct roles ranging from survival to antibody production, depending on the B cell fate. We review recently published data that define how these pathways control metabolic flux in B cells, with a particular emphasis on genetic and in vivo evidence. We further discuss how lessons from T cells can guide future directions.
Introduction:
The primary role of B cells is to produce antibodies against infectious pathogens. To accomplish this seemingly simplistic goal, a highly regulated process is initiated to clonally expand relevant B cells, followed by a massive contraction to narrow the response to the minimal set of cells required to maintain protection against re-infection. In a conventional T cell-dependent (TD) response, naïve B cells are initially activated through cognate interactions between antigen and the B cell receptor (BCR). This triggers activation signals, internalization and processing of antigen to display to cognate CD4+ T cells, and co-stimulation through CD40:CD40L interactions. After a period of limited clonal expansion, B cells then differentiate either into plasma cells (PCs), germinal center (GC) B cells, or directly into IgM+ memory B cells [1]. PCs formed early in the response are localized to the extrafollicular regions and mostly live for a period of a few days [2]. GC B cells engage multiple rounds of rapid proliferation coupled with isotype class switching, somatic hypermutation (SHM) of their immunoglobulin genes, and selection by CD4+ follicular helper T cells for high affinity variants [3]. Rare memory B cells and PCs emerge from the GC through selection processes that remain incompletely understood. Together, these cell types maintain long-term immunity against the offending pathogen and its relatives, sometimes for life [4]. The metabolic requirements of each of these stages differ [5,6]. We discuss below a number of recent studies that help explain how stage-specific metabolic requirements are communicated to B cells, and then how the resultant nutrients are used to support function.
Metabolic demands of initial B cell activation
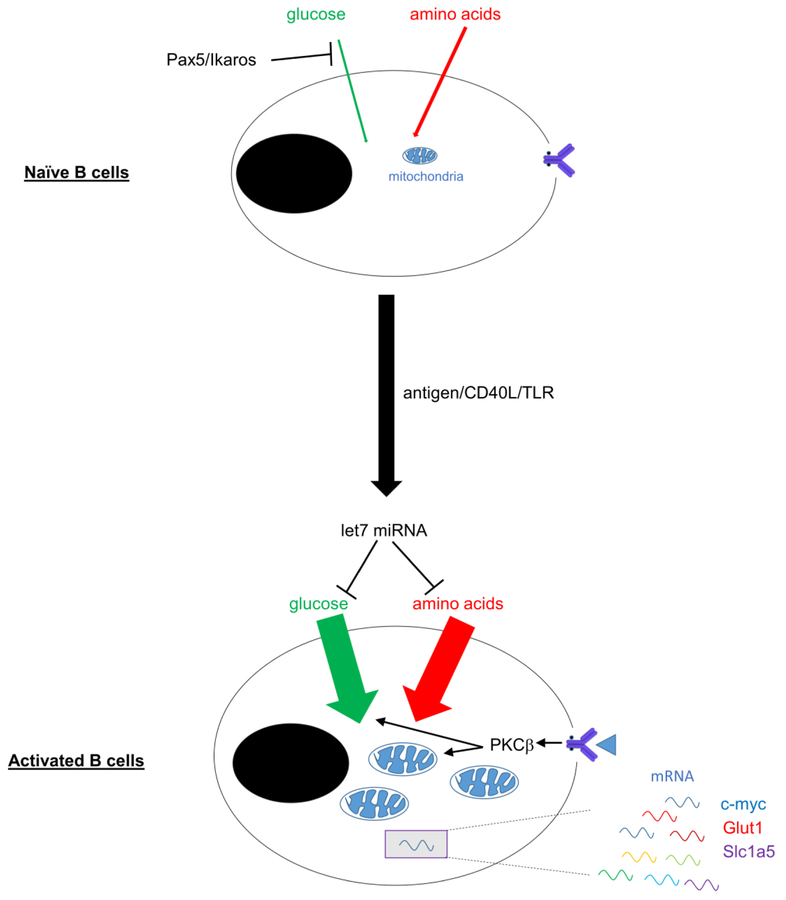
Because of the difficulties in following the very early stages of B cell activation in vivo, most work in this area has come from in vitro studies. As much of the historical work has been reviewed recently [5], we focus instead on studies published within the past few years. Naïve B cells minimally proliferate and thus have relatively few bioenergetic requirements [7], such that lineage-determining transcription factors such as Pax5 and Ikaros suppress glucose uptake and ribonucleotide synthesis [8••,9]. BCR-activated B cells must thus markedly change their metabolic profiles to expand, proliferate, and generate ATP, much of which is driven by enhanced glucose uptake through the transporter GLUT1 [10–12]. BCR-induced metabolic changes are dependent on PKCβ, which is required for the metabolic switch to glycolysis and for downstream mTOR activation, mitochondrial function, and heme biosynthesis [11,13].
As with T cells, antigen receptor-stimulated B cells use second signals such as Toll-like receptor- or CD40-engagement to become fully activated, thereby reducing the chances of autoimmunity. In the absence of these secondary signals, B cells accumulate mitochondrial calcium and produce excessive amounts of superoxides, eventually leading to activation-induced cell death [14]. Costimulatory signals such as TLR activation enhance uptake of glucose and glutamine, which are used as carbon sources for the pentose phosphate pathway to support proliferation, de novo lipogenesis to expand the endoplasmic reticulum [15], and for mitochondrial respiration and anaplerosis [16]. Nutrient uptake and the extent of B cell activation is tempered by the expression of the let-7 microRNA cluster, which suppresses glucose and glutamine transporter expression. In the absence of let-7, excessive antibodies are produced in response to T-independent antigens in vivo. These studies collectively provide a valuable baseline that begins to explain how B cells integrate antigen-specific signals with metabolic programs to support subsequent differentiation (Figure 1).
Figure 1: Metabolic changes following initial B cell activation.
Resting naïve B cells import relatively little glucose, in part due to repression by the transcription factors Pax5 and Ikaros. After ligation of the BCR and co-ligation of either CD40 or Toll-like receptors (TLR), an influx of glucose and glutamine is triggered. Accompanying this elevated nutrient import, the size of the cell expands, mitochondrial mass increases, and transcriptional changes are enacted to support metabolic function.
Germinal center metabolism:
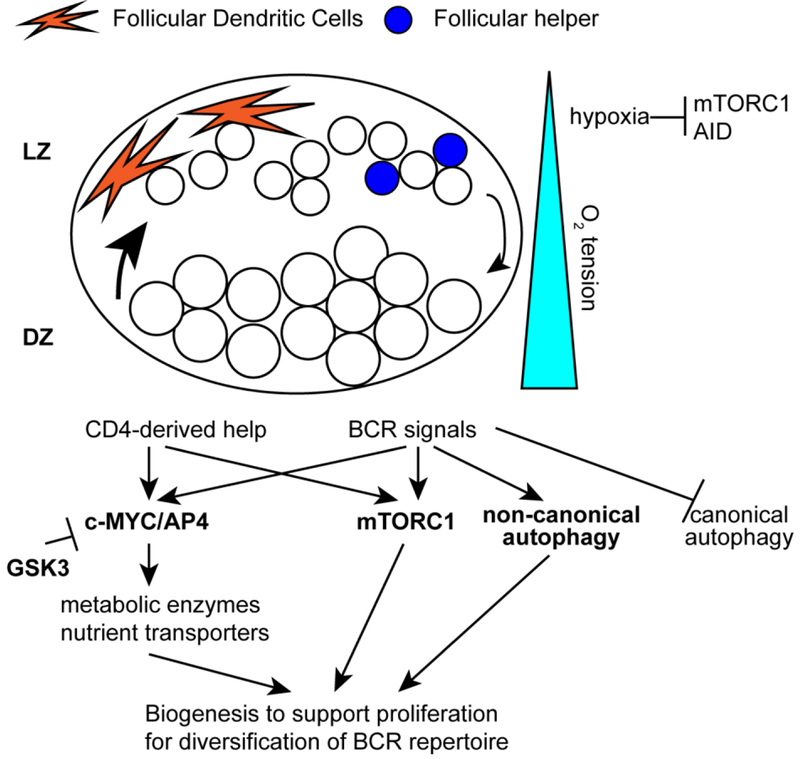
Following initial T-dependent activation, antigen-specific B cells and CD4+ T cells form GCs, which are specialized anatomical structures located within the centers of B cell follicles. GCs are divided into two distinct areas, light- and dark-zones (LZ and DZ), in which B cells exist in distinct proliferative and metabolic states [17,18]. In the LZ, B cells are mostly in a non-proliferative state where they compete with one another for CD4+ T cell help by presenting peptide on MHC class II. Only a small fraction of LZ B cells are selected by T cells to enact a proliferative program as they migrate to the DZ to divide for several days. These proliferating B cells are the templates for AID-mediated SHM, and are thus critical for the emergence of protective antibodies with affinity-enhancing mutations. This proliferative program is enabled by an anabolic response driven by the transcription factor c-MYC, which not only regulates cell cycle progression but also promotes expression of transporters of nutrients and enzymes for glycolysis and glutaminolysis [19–21]. GC B cells require both BCR and CD40 signals for c-MYC induction, thus enforcing a stringent selection checkpoint dependent on CD4+ T cell help [22]. As a counterexample, either BCR or CD40 engagement is sufficient for naive B cells to induce c-MYC and proliferation. Despite the absolute requirement for c-MYC in GC B cells, its expression is restricted to a small fraction of LZ B cells and becomes undetectable in proliferating DZ B cells [17,20,21]. This paradox is explained by a MYC-induced transcription factor AP4, which maintains many c-MYC target genes, including nutrient transporters and metabolic enzymes after downregulation of c-MYC [23•]. Activity of mTORC1 correlates well with engagement of the MYC-AP4 pathway. mTORC1 is activated upon selection of GC B cells in the LZ and is required for cell growth, sustained expansion, and dwelling of selected B cells in the DZ [24••]. Thus, c-MYC-dependent and mTORC1-centered metabolic programs, both of which are activated by T cell help, independently or synergistically support durable proliferation of DZ B cells.
While cell-intrinsic programs triggered in selected GC B cells by T cell-derived help enact metabolic pathways to meet the demands of DZ B cell proliferation, environmental factors specific to the GC microenvironment also modulate GC B cell differentiation. One such example is restriction of mTORC1 activation and AID expression in LZ B cells by hypoxia [25]. Hypoxic conditions in the LZ induce elevated activity of HIF transcription factors. HIFs in turn inhibit activity of mTORC1 and expression of AID, and thereby restrict proliferation of GC B cells and isotype class switching [25]. As a result, overexpression of HIFs through genetic deletion of their inhibitor VHL leads to diminished GC responses. Given that oxygen availability is normally controlled by proximity to blood vessels, it is unclear how hypoxia is preferentially induced in LZ vs. DZ cells, or whether the reciprocal deletion of HIF transcription factors would be sufficient to cause exaggerated GC responses. The enzyme glycogen synthase kinase 3 (GSK3) is also important for GC B cell responses. Through restriction of c-MYC, GSK3 protects proliferating B cells from deprivation of nutrients, such as glucose and amino acids [26]. Since GCs contain a high density of activated cells with elevated glycolytic and mTORC1 activity, GC B cells may have adapted or been desensitized to nutrient deprivation in the microenvironment through GSK3-mediated pathways.
The engagement of autophagy is another notable metabolic program in GC B cells. Compared to resting B cells, autophagy is upregulated in GC B cells, which is consistent with the demand for nutrients to support rapid proliferation [27]. Interestingly, B cells lacking WIPI2, a component in the autophagy pathway, differentiate preferentially to ASCs at the expense of GC B cells, and this altered B cell differentiation is associated with a specific increase in non-canonical autophagy and elevated mitochondrial activity. Although the detailed mechanism is unknown, a shift between distinct autophagy pathways or autophagy-independent function of WIPI2, may control the checkpoint between GC B cells and PCs. These recent findings are collectively summarized in Figure 2.
Figure 2: Metabolic and transcriptional regulation of germinal center B cell response.
B cell clones that express BCRs with higher affinity than neighboring clones are selected by follicular helper T cells. The selected clones upregulate transcription factors c-MYC and AP4 and activate mTORC1 to support their rapid proliferation, which may be modulated by local environmental factors, such as hypoxia or nutrient deprivation.
Metabolic programs to support plasma cells and memory B cells:
The major goal of the GC reaction is to output affinity-matured memory B cells and antibody-secreting PCs, the principal effectors within the B cell lineage. A model of LPS-induced differentiation in vivo has shown that PCs are formed by a series of transcriptional changes downstream of the canonical PC transcription factor BLIMP1 and EZH2 [28–30]. These transcriptional changes are division-dependent and related to ER stress genes, oxidative metabolism enzymes, and amino acid transporters, thereby leading to mTORC1 activation and enhanced antibody secretion [31,32•]. Other studies have shown that autophagy is also activated and required to regulate ER stress responses, antibody secretion, and energy metabolism [33].
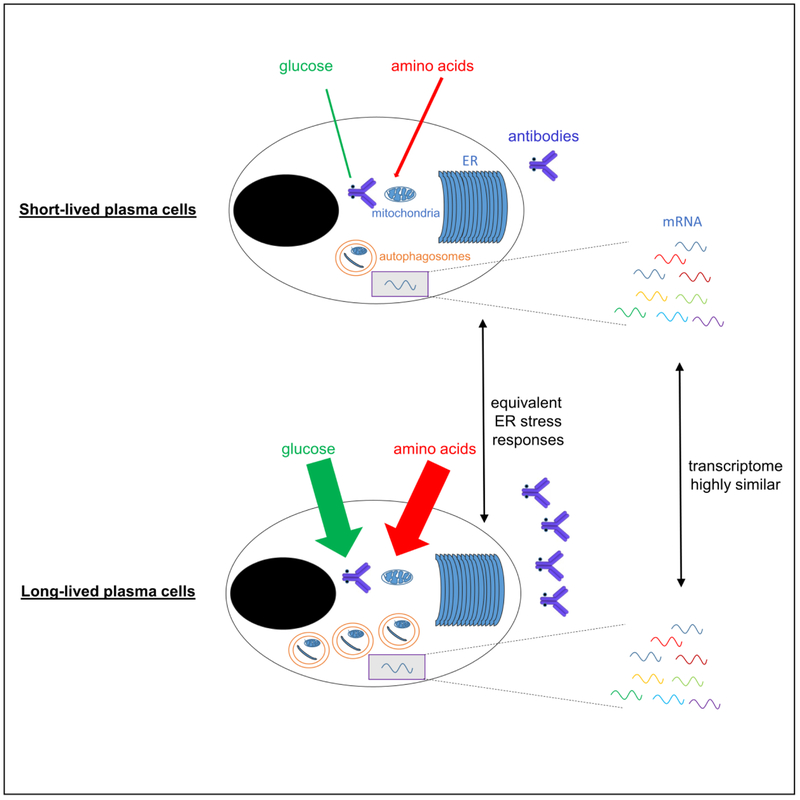
In general, PCs formed early in the response tend to be relatively short-lived, while those formed later from the GC reaction are long-lived [34•]. Yet throughout the immune response, PCs of varying lifespans are generated, demonstrating a far greater complexity belied by the simple short- vs. long-lived designation [35]. Indeed, different vaccines and infections lead to a wide range of durations of antibody production [4], though what distinguishes the underlying PCs remains a mystery. Moreover, while it has generally been assumed that T cell-independent responses fail to generate long-term immunity, many studies have conclusively demonstrated that this is not the case. In fact, through activation of B-1 cells, T-independent responses robustly yield persistent PCs that constitutively secrete antigen-specific IgM [36–39]. Recent studies have demonstrated that PC subsets of varying lifespans and antibody secretion rates can be distinguished based on uptake of a fluorescent glucose analog, surface expression of amino acid transporters, and autophagic markers [35,40•]. PCs with relatively long lifespans import more glucose and predominantly use it to glycosylate antibodies [40•], consistent with their single-minded focus on antibody production. Upon bioenergetic shortages, this glucose can be diverted away from glycosylation to glycolysis and the resultant pyruvate transported into the mitochondria to restore homeostasis [40•]. Similarly, long-lived PCs use glutamine both as a building block for antibodies and for mitochondrial respiration and anaplerosis [35]. These data suggest a link between immunoglobulin production and bioenergetic metabolism, and indeed, long-lived PCs secrete antibodies at a greater rate than do their short-lived counterparts [35]. Yet surprisingly, very few ER stress responses or transcriptional changes are associated with these profound metabolic differences [35,41]. These data demonstrate that there is no obligatory link between transcriptional regulation and metabolism, and some caution is warranted when such assumptions are made (Figure 3).
Figure 3: Metabolic, but not transcriptional changes distinguish short-from long-lived plasma cells.
Short-lived plasma cells import less of a fluorescent glucose analog and amino acids than do long-lived plasma cells. However, these cell types are highly similar in ER stress responses and transcriptional profiles. Adapted from [35].
Metabolic support of memory B cells and recall responses:
Unlike their memory T cell counterparts, which have been the subject of many metabolic studies, only a few reports have described properties of memory B cell metabolism. An elegant set of studies have shown that memory B cells lacking the autophagy gene ATG7 form normally, but fail to persist after immunizations with haptens or infections with influenza [42,43]. As a result, recall responses are severely diminished. ATG7 protects memory B cells against an unusual form of death related to oxidative stress rather than conventional apoptosis or RIPK1-driven necrosis. Another recent study showed that upon stimulation with TLR ligands and IFNα, human IgD+ memory B cells rapidly become glycolytic, activate mTORC1, and differentiate into plasmablasts to a greater extent than do naïve B cells [44]. Whether these differences between memory and naïve B cells reflect an intrinsically distinct metabolic wiring or simply the magnitude of TLR signaling remains to be determined.
Future directions: Lessons from T cells
Studies of B cell metabolism lag behind those of their T cell cousins. Yet this provides opportunities for analogous concepts to be adopted and tested while avoiding some of the pitfalls already encountered by other immunometabolism studies. Some of these issues are technical. Inhibitors such as etomoxir, which antagonizes long-chain fatty acid oxidation, are plagued by off-target effects [45], resulting in inconsistencies between genetic and pharmacological studies [46,47]. Yet relying on genetic studies alone can also be problematic, as deletion of genes such as Cpt1a can be compensated by the paralogs Cpt1b and Cpt1c, leading to incomplete ablation of fatty acid oxidation [46]. Choosing non-redundant genes such as Cpt2 that are essential to a pathway to perform genetic studies [48], and carefully titrating pharmacological inhibitors are the best ways to avoid such issues as the B cell immunometabolism field moves forward.
A key biological question that has emerged in the T cell field is whether metabolic pathways are supportive of a given lineage, as opposed to instructive, which implies the suppression of alternate fates. In most contexts, the evidence suggests that metabolic programs play supportive roles. For example, memory T cells normally depend on fatty acid oxidation and spare respiratory capacity for formation and function [47], but functional cells can also be generated by maintaining glycolysis after the effector stage [49]. Thus, a specific metabolic pathway is required for neither enforcing memory T cell fate nor for extinguishing the effector lineage. In most cases we suspect a supportive role will be the dominant metabolic mechanism in B cells as well. For example, although mTORC1 activation assists in the expression of the germinal center-determining transcription factor BCL6 [50], this pathway is activated in plasma cells as well [32]. Nonetheless, if anything is to be gleaned from recent years of immunometabolism research, it is to expect the unexpected.
Highlights:
-Nutrient uptake by naïve B cells is limited by Pax5 and Ikaros
-Antigen and second signals promote B cell nutrient uptake and metabolic rewiring
-Germinal center B cells use c-myc and mTOR for their proliferative burst
-Plasma cell longevity and antibody secretion are defined by metabolic pathways
Acknowledgements:
This work was supported by National Institutes of Health grants R01AI129945 (D.B.) and R01AI130152 (T.E.) and by the Leukemia and Lymphoma Society (T.E.). The funding sources had no role in data collection, interpretation, or writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests:
D.B. is a co-founder of Sana Biotechnology, Inc., and owns significant stock in Forty Seven Inc. T.E. declares no competing financial interests.
References
- 1.Taylor JJ, Pape KA, Steach HR, Jenkins MK: Humoral immunity. Apoptosis and antigen affinity limit effector cell differentiation of a single naive B cell. Science 2015, 347:784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC: Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med 2000, 192: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora GD, Nussenzweig MC: Germinal centers. Annu Rev Immunol 2012, 30:429–457. [DOI] [PubMed] [Google Scholar]
- 4.Amanna IJ, Carlson NE, Slifka MK: Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007, 357:1903–1915. [DOI] [PubMed] [Google Scholar]
- 5.Boothby M, Rickert RC: Metabolic Regulation of the Immune Humoral Response. Immunity 2017, 46:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam WY, Bhattacharya D: Metabolic Links between Plasma Cell Survival, Secretion, and Stress. Trends Immunol 2018, 39:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milasta S, Dillon CP, Sturm OE, Verbist KC, Brewer TL, Quarato G, Brown SA, Frase S, Janke LJ, Perry SS, et al. : Apoptosis-Inducing-Factor-Dependent Mitochondrial Function Is Required for T Cell but Not B Cell Function. Immunity 2016, 44:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan LN, Chen Z, Braas D, Lee JW, Xiao G, Geng H, Cosgun KN, Hurtz C, Shojaee S, Cazzaniga V, et al. : Metabolic gatekeeper function of B-lymphoid transcription factors. Nature 2017, 542:479–483.••Mutations in the B lineage-defining transcription factors Pax5 and Ikaros have been associated with malignancies. Chan and colleagues provide an explanation by demonstrating that these factors limit glucose uptake and Warburg metabolism.
- 9.Xiao G, Chan LN, Klemm L, Braas D, Chen Z, Geng H, Zhang QC, Aghajanirefah A, Cosgun KN, Sadras T, et al. : B-Cell-Specific Diversion of Glucose Carbon Utilization Reveals a Unique Vulnerability in B Cell Malignancies. Cell 2018, 173:470–484 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, Chiles TC: Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 2006, 107:4458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair D, Dufort FJ, Chiles TC: Protein kinase Cbeta is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem J 2012, 448:165–169. [DOI] [PubMed] [Google Scholar]
- 12.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, et al. : Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 2014, 192:3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsui C, Martinez-Martin N, Gaya M, Maldonado P, Llorian M, Legrave NM, Rossi M, MacRae JI, Cameron AJ, Parker PJ, et al. : Protein Kinase C-beta Dictates B Cell Fate by Regulating Mitochondrial Remodeling, Metabolic Reprogramming, and Heme Biosynthesis. Immunity 2018, 48:1144–1159 e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, et al. : Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufort FJ, Gumina MR, Ta NL, Tao Y, Heyse SA, Scott DA, Richardson AD, Seyfried TN, Chiles TC: Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem 2014, 289:7011–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Manteiga JM, Mari S, Godejohann M, Spraul M, Napoli C, Cenci S, Musco G, Sitia R: Metabolomics of B to plasma cell differentiation. J Proteome Res 2011, 10:4165–4176. [DOI] [PubMed] [Google Scholar]
- 17.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC: Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 2010, 143:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen CD, Okada T, Tang HL, Cyster JG: Imaging of germinal center selection events during affinity maturation. Science 2007, 315:528–531. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. : The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calado DP, Sasaki Y, Godinho SA, Pellerin A, Kochert K, Sleckman BP, de Alboran IM, Janz M, Rodig S, Rajewsky K: The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol 2012, 13:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R: The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012, 13:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo W, Weisel F, Shlomchik MJ: B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity 2018, 48:313–326 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou C, Verbaro DJ, Tonc E, Holmgren M, Cella M, Colonna M, Bhattacharya D, Egawa T: The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity 2016, 45:570–582.•C-myc is genetically required for the proliferative burst of DZ B cells, but its expression is only observed in LZ B cells. Chou et al. show that expression of the transcription factor AP4 maintains expression of c-myc targets through a handoff mechanism.
- 24.Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, Victora GD: Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 2017, 46:1045–1058 e1046.••Using a genetic and pharmacologically tunabe system, Ersching and colleagues demonstrate that mTORC1 is required to prepare LZ B cells for DZ proliferation.
- 25.Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, Thomas JW, Hiebert S, Haase VH, Boothby MR: Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 2016, 537:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, Richardson AD, Conner EM, Benschop RJ, Woodgett JR, et al. : Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol 2017, 18:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Martin N, Maldonado P, Gasparrini F, Frederico B, Aggarwal S, Gaya M, Tsui C, Burbage M, Keppler SJ, Montaner B, et al. : A switch from canonical to noncanonical autophagy shapes B cell responses. Science 2017, 355:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo M, Price MJ, Patterson DG, Barwick BG, Haines RR, Kania AK, Bradley JE, Randall TD, Boss JM, Scharer CD: EZH2 Represses the B Cell Transcriptional Program and Regulates Antibody-Secreting Cell Metabolism and Antibody Production. J Immunol 2018, 200:1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MJ, Patterson DG, Scharer CD, Boss JM: Progressive Upregulation of Oxidative Metabolism Facilitates Plasmablast Differentiation to a T-Independent Antigen. Cell Rep 2018, 23:3152–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharer CD, Barwick BG, Guo M, Bally APR, Boss JM: Plasma cell differentiation is controlled by multiple cell division-coupled epigenetic programs. Nat Commun 2018, 9:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL: Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol 2016, 17:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones DD, Gaudette BT, Wilmore JR, Chernova I, Bortnick A, Weiss BM, Allman D: mTOR has distinct functions in generating versus sustaining humoral immunity. J Clin Invest 2016, 126:4250–4261.•Jones et al. demonstrate stage-specific requirements for mTORC1. At early stages, mTORC1 loss prevents survival, expansion and B cell differentiation. Once plasma cells are formed, mTORC1 impacts antibody secretion rates but not survival.
- 33.Pengo N, Scolari M, Oliva L, Milan E, Mainoldi F, Raimondi A, Fagioli C, Merlini A, Mariani E, Pasqualetto E, et al. : Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol 2013, 14:298–305. [DOI] [PubMed] [Google Scholar]
- 34.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ: A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016, 44:116–130.•Weisel and colleagues demonstrate that long-lived plasma cells emerge late from the germinal center. These findings suggest that differential cues during ontogeny direct plasma cell lifespan.
- 35.Lam WY, Jash A, Yao C, D’Souza L, Wong R, Nunley RM, Meares GP, Patti GJ, Bhattacharya D: Metabolic and Transcriptional Modules Independently Diversify Plasma Cell Lifespan and Function. Cell Rep 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bortnick A, Chernova I, Quinn WJ 3rd, Mugnier M, Cancro MP, Allman D: Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol 2012, 188:5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N: Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds AE, Kuraoka M, Kelsoe G: Natural IgM is produced by CD5-plasma cells that occupy a distinct survival niche in bone marrow. J Immunol 2015, 194:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohannon C, Powers R, Satyabhama L, Cui A, Tipton C, Michaeli M, Skountzou I, Mittler RS, Kleinstein SH, Mehr R, et al. : Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat Commun 2016, 7:11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam WY, Becker AM, Kennerly KM, Wong R, Curtis JD, Llufrio EM, McCommis KS, Fahrmann J, Pizzato HA, Nunley RM, et al. : Mitochondrial Pyruvate Import Promotes Long-Term Survival of Antibody-Secreting Plasma Cells. Immunity 2016, 45:60–73.•Lam and colleagues demonstrate that long-lived plasma cells have a greater capacity to import pyruvate into the mitochondria than do short-lived cells. Ablation of this pathway leads to a loss of long-lived plasma cells.
- 41.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, et al. : Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015, 43:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M, Hong MJ, Sun H, Wang L, Shi X, Gilbert BE, Corry DB, Kheradmand F, Wang J: Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat Med 2014, 20:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Kodali S, Jang A, Kuai L, Wang J: Requirement for autophagy in the long-term persistence but not initial formation of memory B cells. J Immunol 2015, 194:2607–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torigoe M, Iwata S, Nakayamada S, Sakata K, Zhang M, Hajime M, Miyazaki Y, Narisawa M, Ishii K, Shibata H, et al. : Metabolic Reprogramming Commits Differentiation of Human CD27(+)IgD(+) B Cells to Plasmablasts or CD27(−)IgD(−) Cells. J Immunol 2017, 199:425–434. [DOI] [PubMed] [Google Scholar]
- 45.Yao CH, Liu GY, Wang R, Moon SH, Gross RW, Patti GJ: Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of beta-oxidation. PLoS Biol 2018, 16:e2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, Samborska B, Hsieh WY, Wong AH, Stuve P, et al. : Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL: Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012, 36:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nomura M, Liu J, Rovira II, Gonzalez-Hurtado E, Lee J, Wolfgang MJ, Finkel T: Fatty acid oxidation in macrophage polarization. Nat Immunol 2016, 17:216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phan AT, Doedens AL, Palazon A, Tyrakis PA, Cheung KP, Johnson RS, Goldrath AW: Constitutive Glycolytic Metabolism Supports CD8(+) T Cell Effector Memory Differentiation during Viral Infection. Immunity 2016, 45:1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raybuck AL, Cho SH, Li J, Rogers MC, Lee K, Williams CL, Shlomchik M, Thomas JW, Chen J, Williams JV, et al. : B Cell-Intrinsic mTORC1 Promotes Germinal Center-Defining Transcription Factor Gene Expression, Somatic Hypermutation, and Memory B Cell Generation in Humoral Immunity. J Immunol 2018, 200:2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]