Abstract
Group 2 innate lymphoid cells (ILC2s) play an important role in the initiation of type-2 immune responses. Numerous targets have been identified that may activate or repress ILC2 function, though few negative regulatory feedback pathways induced upon activation have been shown to be operative in ILC2s. Here we demonstrate that loss of ADAM17 from ILC2s results in a selective defect in IL-33 responsiveness, but not IL-25 responsiveness. We find that IL1R2 is significantly upregulated at both the transcript and protein level in IL-33 activated ILC2s. We are also able to demonstrate that ADAM17 regulates IL1R2 levels on ILC2s in both a constitutive and activation induced manner. Additionally, IL1R2+ ILC2s, a unique subset of ILC2s, have decreased Il5 and Il13 transcripts following IL-33 stimulation. Overall, these data suggest that the expression of IL1R2 may act as an activation-induced negative regulatory feedback mechanism to decrease ILC2 responsiveness to IL-33.
Loss of ADAM17 on ILC2s results in a selective defect in IL-33 responsiveness.
ADAM17 regulates IL1R2 levels on ILC2s after IL-33 stimulation.
IL1R2+ ILC2s, a unique subset of ILC2s, have decreased cytokine production in response to IL-33.
Keywords: ADAM17, IL1R2, IL-33, ILC2
Introduction
Group 2 innate lymphoid cells (ILC2s) were initially characterized as being a non-B/non-T cell that produced large quantities of type 2 cytokines, primarily IL-5 and IL-13. This is released in response to alarmins and helminth infection [1,2]. ILC2s initiate type 2 responses to helminth as well as allergic airway disease [3,4].
Recently, ILC2s have been divided into two subpopulations. While both subpopulations are lineage negative, and require IL-7 and IL-2, they differ in anatomical location, tissue residency, and cytokine responsiveness [5]. Natural ILC2s (nILC2s) are seeded primarily in lung, are ST2+ and Thy1hi, and respond primarily to IL-33. Inflammatory ILC2s (iILC2s) are ST2−, Thy1lo, and KLRG1hi, are primarily found in the intestines, and respond mainly to IL-25 with migration to the spleen, liver, and lungs in an S1P-dependent manner [6].
A Disintegrin and Metalloproteinase 17 (ADAM17) is classically known for its role in inflammation due to its ability to shed TNFα, ultimately leading to its early name TNFα converting enzyme (TACE) [7]. Additionally, ADAM17 is involved in the shedding of TNF receptors, IL6Rα, and many other cytokine and chemokine receptors [8]. With this knowledge, we sought to examine the role of ADAM17 in ILC2 function.
IL-1 was the first interleukin to be described and sparked a revolution in scientific discovery regarding soluble mediators of inflammation [9]. The receptor for IL-1, IL1R1 was also shown to be essential for response to microbes through its Toll-IL-1 (TIR) domain and subsequent MyD88 activation [10]. A second receptor for IL-1, IL1R2 was discovered shortly after and was attributed with having an inhibitory function on IL-1 signaling [11]. Since then, it has been appreciated that the IL1R family is composed of many cytokine receptor chains responsible for binding cytokines including IL-1α, IL-1β, IL-18, IL-33, and others.
IL-1 signaling requires two receptor components to be functional, IL1R1 and IL1RAP. It has been demonstrated that IL1RAP alone is not able to bind IL-1, and that IL1R1 that is not complexed with IL1RAP does not recruit the proper adaptor proteins necessary for signaling propagation [12]. In addition to being utilized by IL1R1 for IL-1 signaling, IL1RAP also complexes with ST2 to form the heterodimeric IL-33R [13]. Loss of either IL1RAP or ST2 completely abrogates IL-33 signaling, indicating that both proteins are essential for proper signaling.
IL1R2 has a truncated intracellular domain and lacks the TIR domain necessary for signaling through IL1R1 and is considered an inhibitory receptor [14]. IL1R2 can block IL-1 signaling in three different ways. First, IL1R2 is shed from the cell surface into a soluble form in a metalloproteinase dependent pathway allowing it to act as a decoy receptor for circulating IL-1α and IL-1β [15]. Several groups have shown that ADAM17 is responsible for IL1R2 shedding in various cell types [16,17]. Second, because of the lack of a cytoplasmic domain, IL-1 that binds to membrane IL1R2 is not able to propagate a signal. Third, studies regarding the inhibitory role of IL1R2 also found that it is able to form complexes with IL1RAP, thus sequestering IL1RAP away from IL1R1. Without available IL1RAP, IL1R1 is not able to form the signaling complex necessary for proper propagation of IL-1 downstream signaling [18,19].
In this study we examine the role of ADAM17 in ILC2 biology. Initially, we created mice which lack ADAM17 on ILC2s (ADAM17ILC2−/−). To our surprise we observed a defect in IL-33 responsiveness in ADAM17ILC2−/− compared to WT mice. Interestingly, this defect in ILC2 activation was not seen in response to IL-25 stimulation. Transcriptomic approaches, paired with known ADAM17 substrates identified IL1R2 as a potential target to explain this IL-33 response deficiency. We additionally show that ADAM17 directly regulates IL1R2 on ILC2s.
Materials and Methods
Mice
Mice with Exon 2 of Adam17 floxed were purchased from The Jackson Laboratory (009597) and bred to mice expressing Cre-recombinase under linked to the Rora gene for dual expression with an IRES sequence [20]. These mice were then backcrossed to the C57Bl6/J strain for 8 generations. The resultant mice are referred to as ADAM17ILC2-/−. These mice were also crossed to Gt(ROSA)26Sortm1(EYFP)Cos/J mice (The Jackson Laboratory; 006148) to enable EYFP expression under the control of Cre-recombinase. Mice were genotyped using high resolution melting assays as described [21].
Cell Isolation and Sorting, and Culture
For isolation of WT ILC2s for expansion, WT mice were administered with 0.1μg of IL-33 i.n. for three consecutive days to expand ILC2s in vivo. ILC2s were then isolated from the lungs and sorted using a BD FACSAria Fusion SORP High-Speed Cell Sorter (BD Biosciences). Sorted ILC2s were expanded in IL-2 (10ng/mL), IL-7 (10ng/mL), and IL-33 (10ng/mL) for 7 days. Cells were then rested in IL-2 and IL-7 for 24 hours prior to stimulation. All culturing was conducted in cRPMI 1640 as in [21].
Gene Expression Analyses
Detailed methods for qRT-PCR and RNA-sequencing can be found in the Supplementary Methods.
In vivo Cytokine Models
For IL-25 challenge, 200ng of recombinant mIL-25 (R&D) in 100μL of saline was administered i.p. for 3 consecutive days. Mice were euthanized on day 4 and organs were harvested. For IL-33 challenge, 200ng of recombinant mIL-33 (BioLegend) in 20uL of saline was administered i.n. for 3 consecutive days. Mice were euthanized on day 4 and analyzed.
Organ Isolation and Flow Cytometry
A detailed description of organ digestion and flow cytometry can be found in the Supplementary Methods.
Histology
Histology was conducted as in [22]. Full slides were scanned at 40X using a Vectra Polaris (PerkinElmer) imaging system. Images were captured using Phenochart v1.0.9 (PerkinElmer).
IL1R2 Shedding Assays
A detailed description of shedding assays can be found in the Supplementary Methods.
Statistical Analyses
All statistical analyses used GraphPad Prism 7.3 and 8.0. Individual figure legends indicate statistical tests used for different experiments. Overall, Bartlett’s test for equal variance was used and if variances were not equal, equivalent non-parametric tests as indicated in figure legends were used.
Results and Discussion
Loss of ILC2 ADAM17 results in decreased IL-33 responsiveness
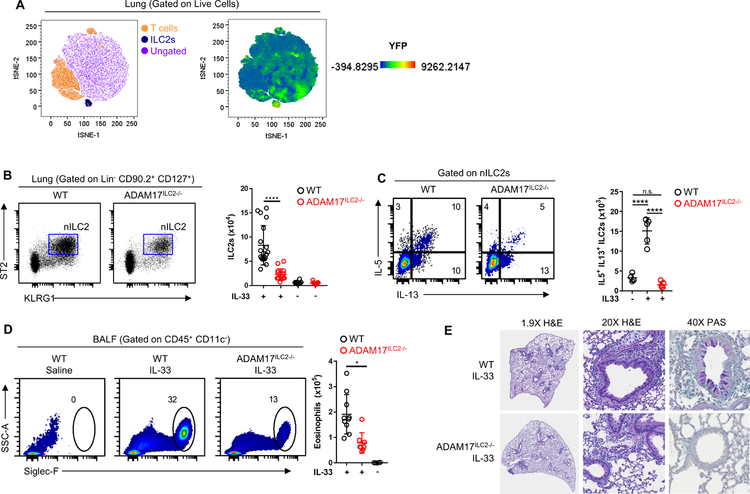
To first confirm the specificity of the Rora-Cre, we examined lungs of IL-33 stimulated mice from RoraYFP mice in which YFP is under control of the Rora Cre. Using tSNE analysis, it can be appreciated that YFP expression was limited to the ILC2 cluster (Fig. 1A). This finding confirms a previous paper which also used the Rora Cre for ILC2 specific knockouts [23]. To examine the role of ADAM17 in ILC2 function, we first assessed whether ILC2s from ADAM17ILC2−/− mice responded normally to IL-33 challenge. While we saw no difference in the number of ILC2s present in saline treated WT or ADAM17ILC2−/− mice, we observed significantly fewer nILC2s in the lungs of IL-33 treated ADAM17ILC2−/− mice compared to WT (Fig. 1B).
Figure 1: Loss of ILC2 ADAM17 decreases responsiveness to IL-33.
(A) tSNE analysis from lung of RoraYFP mice showing YFP expression limited to ILC2 cluster. (B) WT and ADAM17ILC2−/− mice were challenged i.n. with IL-33 and ILC2s were measured in the lung. (C) IL-5 and IL-13 were measured by flow cytometry on ILC2s from B. (D) Eosinophil numbers in BALF from mice in B were measured by flow cytometry. (E) Representative histological sections of lungs from IL-33 challenged mice. *P < 0.05, **P < 0.01, ****P < 0.0001, one-way ANOVA (B-D). Data are pooled from three (mean ± s.d.) independent experiments.
Upon activation with IL-33, nILC2s rapidly make IL-5 and IL-13. When we examined these ILC2s for IL-5 and IL-13 production, we saw that nILC2s from ADAM17ILC2−/− mice had significantly fewer numbers of IL-5+ IL-13+ dual producing nILC2s compared to WT nILC2s (Fig. 1C). Along with increased nILC2 accumulation, IL-33 challenge induces an ILC2-mediated eosinophilia in both the lung and bronchoalveolar lavage fluid (BALF) of mice. IL-33 challenged ADAM17ILC2−/− mice had significantly fewer relative and absolute numbers of eosinophils in the BALF than IL-33 challenged WT mice (Fig. 1D), supporting the finding of decreased nILC2 activation in ADAM17ILC2−/− mice. Additionally, the lungs of IL-33 challenged ADAM17ILC2−/− mice had reduced leukocyte infiltration and mucus compared to WT mice, by H&E and PAS staining respectfully (Fig. 1E).
Loss of ILC2 ADAM17 does not influence IL-25 responsiveness
Due to the drastic defect in IL-33 responsiveness in ILC2s from ADAM17ILC2−/− mice, we wanted to examine whether this defect was present in response to other alarmins, namely IL-25. IL-25 stimulation is known to activate iILC2s in vivo, therefore we challenged mice with 3 i.p. injections of rIL-25 daily for 3 days and analyzed the mice the following day. When challenged i.p. with IL-25, iILC2s accumulate in the lung and mesLN in an S1P dependent manner [6]. IL-25 challenged ADAM17ILC2−/− mice had no difference in lung iILC2 numbers when compared to WT mice (Supplemental Fig. 1A–B).
We next examined iILC2s in the lungs of IL-25 challenged mice for IL-5 and IL-13 production as well proliferation with Ki67 staining. We did not see a difference in IL-5 production, IL-13 production, or Ki67 staining between IL-25 challenged ADAM17ILC2−/− and WT mice (Supplemental Fig. 1C–F). These data suggest there is no defect in activation or proliferation in these cells with IL-25 challenge. Also, we saw very little Ki67+ staining in nILC2s compared to iILC2s from both IL-25 challenged ADAM17ILC2−/− mice and WT mice, as expected (Supplemental Fig. 1C–F). Taken together, these results suggest a significant defect in IL-33 ILC2 responsiveness in the absence of ADAM17 on ILC2s.
ADAM17 inhibition alters ILC2 activation and survival in vitro
Upon stimulation with IL-2 + IL-7 + IL-33, vehicle treated ILC2s increase IL-5 and IL-13 production compared to IL-2 + IL-7 treated ILC2s (Supplemental Fig. 2A). Similar to our in vivo data, we found that ILC2s treated with an ADAM17 inhibitor (INCB) made significantly less IL-5 and IL-13 when stimulated with IL-33 compared to vehicle treated ILC2s (Supplemental Fig. 2A). However, ADAM17 inhibited ILC2s no difference in the amount of IL-5 and IL-13 was observed when treated with IL-25 (Supplemental Fig. 1A). This matched our findings in vivo.
Next, we wanted to examine apoptosis in ADAM17 inhibited ILC2s following IL-33 stimulation. To do this, we used Annexin V and propidium iodide (PI) staining. Interestingly, ADAM17 inhibited ILC2s treated with only IL-33 had significantly increased levels of early apoptotic (Supplemental Fig. 2B, D) and late apoptotic (Supplemental Fig. 2C, D) cells compared to vehicle treated ILC2s. It was also evident that this increase in apoptotic cells could be observed as early as 1 day of stimulation through 3 days of stimulation. Taken with our in vivo findings, these results indicate that inhibition of ADAM17 results in defective responsiveness to IL-33.
IL1R2 is upregulated on ILC2s following IL-33 stimulation
Our results indicated a clear defect in IL-33 responsiveness in ILC2s in the absence or inhibition of ADAM17. However, from this data, we did not have a clear mechanism as to what the role of ADAM17 was in IL-33 mediated ILC2 activation. To do this, we isolated naïve ILC2s from WT mice and cultured them for 3 days in the presence of either IL-2 + IL-7 or IL-2 + IL-7 + IL-33 and conducted RNA-sequencing. We then examined the fold change in IL-2 + IL-7 + IL-33 vs. IL-2 + IL-7 treated ILC2s for known ADAM17 substrates (Fig. 3A).
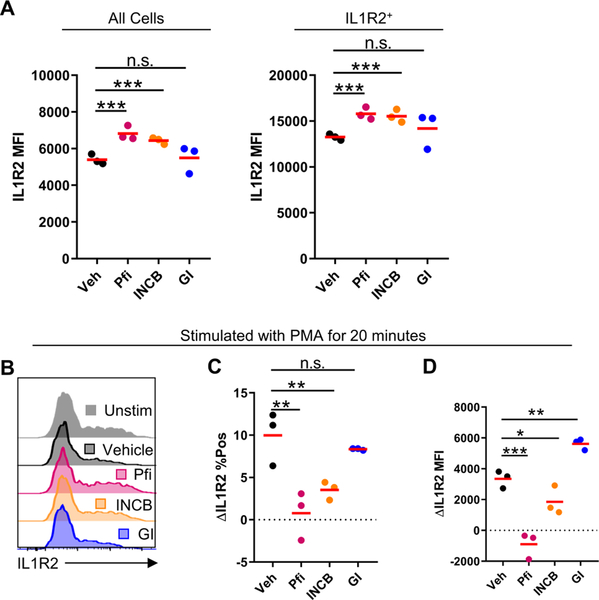
Figure 3: ADAM17 regulates IL1R2 on ILC2s.
(A) Isolated and expanded WT ILC2s were cultured in IL-2 + IL-7 + IL-33 for 72hrs with indicated inhibitors and IL1R2 levels were measured by flow cytometry. (B) Isolated and expanded ILC2s were incubated with indicated inhibitors for 30 minutes followed by PMA stimulation for 20 minutes. Statistical quantification of (C) change in % of cells IL1R2+ and (D) change in MFI following PMA stimulation. n.s. P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. one-way ANOVA (A, C, D). Data are representative of two (mean) independent experiments.
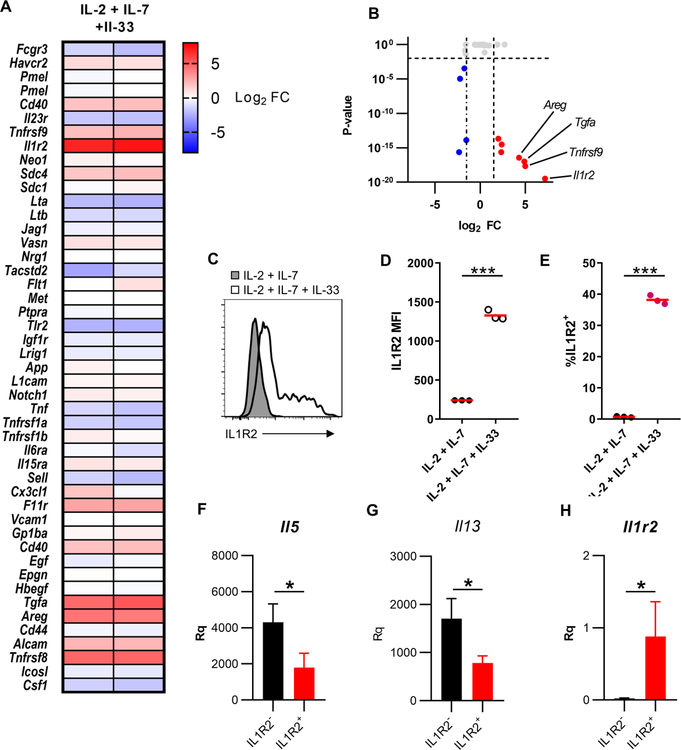
One of the more striking differentially expressed genes was Il1r2 (Fig. 2A, B). Il1r2 belongs to the IL-1 receptor family which consists of the machinery necessary for IL-33 signaling. To validate this target, we showed that IL1R2 protein was increased on ILC2s following stimulation with IL-2 + IL-7 + IL-33 compared to IL-2 + IL-7 stimulation by flow cytometry (Fig. 2C–E). To our knowledge, this is the first demonstration of protein expression of IL1R2 on ILC2s.
Figure 2: Activated ILC2s express multiple ADAM17 substrates.
(A) Heat map of fold changes from IL-2 + IL-7 + IL-33 stimulated ILC2s vs. IL-2 + IL-7. (B) Volcano plot of genes known to code for ADAM17 substrates. (C) Isolated and expanded ILC2s were cultured with the indicated cytokines and IL1R2 levels were measured by flow cytometry. (D) Statistical quantification of IL1R2 MFI from C. (E) Statistical quantification of % of cells IL1R2+ from C. qRTPCR analysis of (F) Il5 (G) Il13 (H) Il1r2 from IL1R2− and IL1R2+ ILC2s following 3 days of stimulation with IL-2 + IL-7 + IL-33 relative to Tbp and Rrp8. *P < 0.05, ***P < 0.001, Unpaired Student’s t-test (D-E), Mann-Whitney U test (F-H). Data are representative of two (D-E; mean ± s.d.) and one (F-H; mean ± s.d., n=4 per group) independent experiments.
To determine the role of IL1R2 expression on ILC2s, we sorted IL1R2− and IL1R2+ ILC2s that had been stimulated with IL-2 + IL-7 + IL-33 for 72hrs. To our surprise, both Il5 and Il13 transcript levels were significantly decreased in IL1R2+ ILC2s compared to IL1R2− ILC2s (Fig. 2F–G). The bimodality of IL1R2 expression on activated ILC2s and the functionally differences between the populations suggest that IL1R2 expression on ILC2s may define a unique subset of ILC2s. To confirm this, a more global transcriptomics approach will be needed to further investigate these populations. Additionally, these findings suggest that IL1R2 expression may be negatively regulating ILC2 responsiveness. IL1R2+ ILC2s had significantly higher Il1r2 expression than IL1R2− ILC2s (Fig. 2H), further suggesting that this population is functionally distinct from IL1R2− ILC2s.
ADAM17 regulates IL1R2 levels on ILC2s
We hypothesized that in ILC2s, IL1R2 occupying ILRAP, thus decreasing the amount of IL1RAP available to complex with ST2 and due to the necessity of IL1RAP for IL-33 signaling through ST2, there would be decreased IL-33 signaling, similar to inhibition of IL-1 signaling by IL1R2 [18,19].We next wanted to determine whether ADAM17 had a role in the regulation of IL1R2 on ILC2s following IL-33 stimulation. To test this, we stimulated WT ILC2s with IL-2 + IL-7 + IL-33 for 3 days in the presence of either a vehicle control, the ADAM17 inhibitor TMI-002 (Pfi), the ADAM17 inhibitor INCB3619 (INCB), or the ADAM10 inhibitor GI254023X (GI). There was a significant increase in overall IL1R2 MFI in both the Pfi and INCB inhibitor groups compared to vehicle treatment (Fig. 3A). When we examined IL1R2+ cells, IL1R2 MFI was significantly higher in Pfi and INCB treated cells as well, when compared to vehicle treated (Fig. 3A). Because of the overlap of substrates between ADAM10 and ADAM17, we also treated cells with the ADAM10 inhibitor GI and did not see a difference in IL1R2 levels in both overall cells as well as IL1R2+ cells, suggesting that ADAM10 does not play a role in IL1R2 regulation on ILC2s (Fig. 3A).
These data suggest that ADAM17 plays a role in the constitutive regulation of IL1R2 following IL-33 stimulation of ILC2s. To test whether IL1R2 was cleaved from ILC2s in response to a strong ADAM17 activation stimuli using PMA, we first cultured ILC2s in the presence IL-2 + IL-7 + IL-33 for 3 days. Following this stimulation, we washed the cells and added the indicated inhibitors or vehicle for 30 minutes prior to a 20-minute PMA stimulation. Following PMA stimulation, we detected a ~10% change in the percent of cells that were IL1R2+ in the vehicle treated group (Fig. 3B–C). This decrease was almost completely blocked in the ADAM17 inhibited groups (Fig. 3B–C). Similarly, we noted a decrease in overall MFI and IL1R2+ cell MFI following PMA stimulation in vehicle treated cells which was again almost completely blocked in ADAM17 inhibited cells (Fig. 3B, D). These results imply that IL1R2 is able to be shed from ILC2s in response to PMA and that ADAM17 is the primary sheddase responsible. Further studies will have to be conducted to determine the physiological stimuli that regulate IL1R2 constitutive regulation and activation induced shedding in vivo. Overall, we propose a model in which loss of ADAM17 on ILC2s selectively reduces activation and survival in response to IL-33, mediated through increased IL1R2 levels. This increased IL1R2 alters IL1RAP levels, reducing free IL1RAP able to interact with ST2. Thus, IL-33 signaling is altered.
Supplementary Material
Highlights:
Loss of ADAM17 on ILC2s results in a selective defect in IL-33 responsiveness.
ADAM17 regulates IL1R2 levels on ILC2s after IL-33 stimulation.
IL1R2+ ILC2s, a unique subset of ILC2s, have decreased cytokine production in response to IL-33.
Acknowledgements
We would like to thank Matthew Zellner and Amanda Leonard for help in mouse colony management as well as Jessica Wimberly for her help with tissue harvest.
This work was funded by NIH/NIAID R01AI18697A1 to D.H.C. and R.K.M. Slide scanning microscopy was performed at the Cancer Mouse Models Shared Resource Core and flow cytometry utilized at the VCU Flow Cytometry Shared Resource. Both are supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL, New IL-17 Family Members Promote Th1 or Th2 Responses in the Lung: In Vivo Function of the Novel Cytokine IL-25, J. Immunol 169 (2002) 443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- [2].Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie ANJ, Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion, J. Exp. Med 203 (2006) 1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-I, Ohtani M, Fujii H, Koyasu S, Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells, Nature 463 (2010) 540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- [4].Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie ANJ, Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity, Nature 464 (2010) 1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Paul WE, IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential “inflammatory” type 2 innate lymphoid cells, Nat. Immunol 16 (2015) 161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang Y, Mao K, Chen X, Sun M-A, Kawabe T, Li W, Usher N, Zhu J, Urban JF, Paul WE, Germain RN, S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense, Science 359 (2018) 114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moss ML, Jin S-LC, Milla ME, Burkhart W, Carter HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD, Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α, Nature 385 (1997) 733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- [8].Pruessmeyer J, Ludwig A, The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer, Semin. Cell Dev. Biol 20 (2009) 164–174. doi: 10.1016/j.semcdb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [9].Dinarello CA, Immunological and Inflammatory Functions of the Interleukin-1 Family, Annu. Rev. Immunol 27 (2009) 519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- [10].Muzio M, Ni J, Feng P, Dixit VM, IRAK (Pelle) Family Member IRAK-2 and MyD88 as Proximal Mediators of IL-1 Signaling, Science 278 (1997) 1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- [11].Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A, Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4, Science 261 (1993) 472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- [12].Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G, Molecular Cloning and Characterization of a Second Subunit of the Interleukin 1 Receptor Complex, J. Biol. Chem 270 (1995) 13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- [13].Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA, IL-1 Receptor Accessory Protein and ST2 Comprise the IL-33 Receptor Complex, J. Immunol 179 (2007) 2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- [14].Colotta F, Dower SK, Sims JE, Mantovani A, The type II “decoy” receptor: A novel regulatory pathway for interleukin 1, Immunol. Today 15 (1994) 562–566. doi: 10.1016/0167-5699(94)90217-8. [DOI] [PubMed] [Google Scholar]
- [15].Peters VA, Joesting JJ, Freund GG, IL-1 receptor 2 (IL-1R2) and its role in immune regulation, Brain. Behav. Immun 32 (2013) 1–8. doi: 10.1016/j.bbi.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Orlando S, Sironi M, Bianchi G, Drummond AH, Boraschi D, Yabes D, Mantovani A, Role of Metalloproteases in the Release of the IL-1 type II Decoy Receptor, J. Biol. Chem 272 (1997) 31764–31769. doi: 10.1074/jbc.272.50.31764. [DOI] [PubMed] [Google Scholar]
- [17].Lorenzen I, Lokau J, Düsterhöft S, Trad A, Garbers C, Scheller J, Rose-John S, Grötzinger J, The membrane-proximal domain of A Disintegrin and Metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II, FEBS Lett 586 (2012) 1093–1100. doi: 10.1016/j.febslet.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [18].Neumann D, Kollewe C, Martin MU, Boraschi D, The Membrane Form of the Type II IL-1 Receptor Accounts for Inhibitory Function, J. Immunol 165 (2000) 3350–3357. doi: 10.4049/jimmunol.165.6.3350. [DOI] [PubMed] [Google Scholar]
- [19].Lang D, Knop J, Wesche H, Raffetseder U, Kurrle R, Boraschi D, Martin MU, The Type II IL-1 Receptor Interacts with the IL-1 Receptor Accessory Protein: A Novel Mechanism of Regulation of IL-1 Responsiveness, J. Immunol 161 (1998) 6871–6877. [PubMed] [Google Scholar]
- [20].Nakagawa Y, O’Leary DDM, Dynamic Patterned Expression of Orphan Nuclear Receptor Genes RORα and RORβ in Developing Mouse Forebrain, Dev. Neurosci 25 (2003) 234–244. doi: 10.1159/000072271. [DOI] [PubMed] [Google Scholar]
- [21].Lownik JC, Luker AJ, Damle SR, Cooley LF, Sayed RE, Hutloff A, Pitzalis C, Martin RK, Shikh MEME, Conrad DH, ADAM10-Mediated ICOS Ligand Shedding on B Cells Is Necessary for Proper T Cell ICOS Regulation and T Follicular Helper Responses, J. Immunol (2017) ji1700833. doi: 10.4049/jimmunol.1700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cooley LF, Martin RK, Zellner HB, Irani A-M, Uram-Tuculescu C, El Shikh ME, Conrad DH, Increased B Cell ADAM10 in Allergic Patients and Th2 Prone Mice, PLoS ONE 10 (2015) e0124331. doi: 10.1371/journal.pone.0124331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mchedlidze T, Kindermann M, Neves AT, Voehringer D, Neurath MF, Wirtz S, IL-27 suppresses type 2 immune responses in vivo via direct effects on group 2 innate lymphoid cells, Mucosal Immunol 9 (2016) 1384. doi: 10.1038/mi.2016.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.