Abstract
Background:
Ampullary carcinoma is a rare gastrointestinal cancer. Pathogenic germline alterations (PGA) in BRCA2 and potentially targetable somatic alterations (SA) in ERBB2 and ELF3 have been previously described in ampullary carcinoma. Memorial Sloan Kettering has implemented an opt-in strategy for germline (GT) and somatic genetic testing (ST) for patients with ampullary carcinoma to further evaluate the spectrum of PGA and SA.
Methods:
N= 45 patients with pathologically confirmed ampullary carcinoma were consented prospectively for ST using MSK-IMPACT (410–468 genes). A subset of the cohort, N=23 (of 45) patients also consented for GT using MSK-IMPACT (76–88 genes). Germline data for 21 of the remaining 22 patients not consented for GT was obtained in a de-identified fashion without clinical correlation. Clinicopathologic features, treatment history and survival data for consented patients were collected and analyzed.
Results:
Pancreaticobiliary, intestinal and mixed features of the two were the primary pathologic subtypes of ampullary carcinoma identified in this cohort. No difference in median overall survival was found between pathologic subtypes. We identified 8 of 44 (18%) patients to harbor pathogenic mutations in BRCA2, ATM, RAD50, and MUTYH. Additionally, we found a wide spectrum of SAs in genes such as KRAS, MDM2, ERBB2, ELF3, PIK3CA. Two patients in our cohort underwent SA-targeted therapy, one with a partial radiographic response.
Conclusions:
Mutations in multiple somatic and germline genes were identified in this cohort. Of significance, actionable targets were identified in the tumor and broader testing for PGAs and SAs should be considered for all patients with ampullary carcinoma.
Keywords: Ampullary, Genomic, Germline, Somatic, Mutations
Precis:
Ampullary cancer is a rare malignancy for which actionable somatic findings are relatively common. Germline alterations also occur with some frequency and both somatic and germline testing should be considered for all patients with this disease.
INTRODUCTION
Ampullary carcinoma (AC) is a rare cancer, accounting for less than 1% of all GI malignancies.1 AC arises from the ampulla of Vater and can be of several distinct pathologic subtypes, the most common being intestinal, pancreaticobiliary or a combination of the two.2–4 Compared to pancreas and biliary cancers, patients with AC generally have a higher surgical resectability rate and a more favorable prognosis. Current adjuvant therapy and treatment of advanced disease for AC have not been fully defined and most treatment protocols are extrapolated from established treatment protocols for pancreatic, biliary, and intestinal cancers.5 These points underpin the need for better characterization of this disease through genomic evaluations to further explore therapeutic targets and facilitate improvement in outcomes.
Targeted therapies based on genetic profiling have been gaining traction in other gastrointestinal malignancies such as colon, pancreas and biliary malignancies.6–11 For example, pathogenic germline alterations (PGA) in DNA repair genes such as BRCA1/2, ATM, PALB2, etc. have been implicated in pancreas cancer as targets for therapy, as a way to prognosticate patients and as a way to identify risk family members. 8, 9, 12 Specifically, the use of platinum agents and poly-ADP ribose polymerase (PARP) inhibitors are thought to be synthetically lethal to tumor cells in patients with BRCA mutations and perhaps other PGAs present in the DNA repair pathway.13 Moreover, in pancreas and colorectal cancer, deficiencies in mismatch repair, either sporadic or in Lynch syndrome, leading to hyper-mutated tumors and microsatellite instability benefit from PDL-1 inhibition. 8, 9, 12, 14–16
Notably in AC, Pinto et al. (2016) have elucidated BRCA2 as a PGA with a frequency of 14.3% in 15 patients.17 Similarly, Mandelker et al. (2017) also found one of three patients tested to have BRCA2 PGA.18 And most recently, Cloyd et al (2018) also identified a patient with known Lynch syndrome and mismatch repair deficiency to harbor AC.19 These findings indicate that there are patients with AC with therapeutically actionable PGAs and associated clinical implications that have thus far not been fully elucidated and optimally therapeutically exploited in AC. Similar to PGAs, SAs in multiple oncogenes such as BRAF, ELF3, ERBB2, etc. have been described but their clinical applicability has not been established in AC. 20−23 Though, the discovery of SAs, such as ERBB2 amplification, and sporadic SAs in mismatch genes like MSH2, MSH6 and MLH1 has prompted the possibility of targeted treatments for AC with agents such as trastuzumab or PDL-1 inhibition, respectively.11, 14, 15, 24
Thus, there is a clear imperative, given the potential for new therapeutic options, risk stratification and prognostication, for further genomic study of this disease. There is likely a broader undiscovered spectrum of targetable mutations that exist in AC and suggests that more routine testing of patients with AC for PGAs and SAs is a way to help refine current therapy, screen at risk family and prognosticate patients with AC. To further evaluate these hypotheses, we undertook a prospective study of the somatic and germline profile of N= 45 patients with AC using the MSK-IMPACT next generation sequencing (NGS), who had their care at Memorial Sloan Kettering (MSK).
METHODS
Patients
Between July 2014 to April 2018, written informed consent for somatic testing (ST) under an IRB approved protocol (NCT 01775072) was obtained for N= 45 patients. From November 2015 onward, written informed consent for germline testing (GT) under the same IRB approved protocol was obtained for 23 of the 45 (51%) of the cohort. All tumor samples collected for ST or GT were formalin fixed and paraffin-embedded to ensure quality control and consistency. ST for 410–468 genes was performed via tumor samples obtained by biopsy or resection of the primary or metastatic site. SAs with the highest OncoKB level for each patient were reported in this study. OncoKB is a method of annotating SAs via their known or hypothesized biologic and oncogenic effects. Furthermore, OncoKB seeks to prognosticate and stratify SAs by their potential treatment implication as stratified by the level of evidence that an SA is predictive of drug response based on US Food and Drug Administration labeling.25
For GT, tumor samples from the patient were obtained via biopsy or resection at the primary tumor site or metastatic site and compared to ‘normal’ peripheral blood from the patient to test for PGAs in 76–88 genes. For all 45 patients, clinical, demographic and pathologic information was correlated and collected for each patient consented for ST. Additionally, GT was performed in an anonymized fashion on 21 patient samples consented only for ST and the germline data was extracted and reported herein as is without patient clinical data available for analysis. No germline data was available for one patient in the cohort. In total, 44 of 45 (98%) patient samples in this cohort were analyzed for PGAs. The data collection cut off was in April 2018.
Sequencing, Variant Calling, and Results Reporting
All results from ST and GT were reported to ordering physician and patient. Using patient tumor samples from biopsy or resection, DNA was extracted and analyzed using MSK-IMPACT, a hybridization capture-based NGS assay, to identify insertions and deletions, nucleotide variants, structural rearrangements and copy number variations as previously described.18, 26 The American College of Medical Genomics and Genetics (ACMG) criteria were used to classify all identified variants.
Microsatellite Analysis and Tumor Mutational Burden
Microsatellite analysis was performed via MSI-sensor analysis using uses >1000 informative microsatellite regions targeted by MSK-IMPACT. As previously described, MSI-sensor interrogated the length of all genomic microsatellite loci included in MSK-IMPACT capture region across the tumor and matched normal.27 Greater than 10% of microsatellite loci showing microsatellite instability was designated as microsatellite unstable (MSI-H).
Tumor mutation burden (TMB) was derived from MSK-IMPACT by dividing the number of sequence mutations reported by the total genomic area where the mutations were reported. Patients with TMB > 15 non-synonymous mutations/megabase (calculated as median TMB + 2 × interquartile range TMB) were considered to have a high TMB.26
Loss of Heterozygosity
Loss of heterozygosity (LOH) analysis was performed on all samples found to have PGAs. In total, eight patients with PGAs were analyzed. We determined the total and allele-specific copy number from the sequence coverage and genotypes of polymorphic single nucleotide polymorphisms across the genomes that can identify regions of LOH by using the FACETS algorithm. Tumor zygosity of each PGA was inferred in the tumor according to its variant allele fraction and the FACETS segment on which it occurred.12, 28
Statistical Analysis
All statistical analysis was two-tailed. All categorical variables were compared using Fisher’s exact test with a p <0.05 being statistically significant. Kaplan Meier (KM) curves were generated for survival analysis and compared with log-rank testing. Median overall survival (OS) was calculated from time of initial tissue collection or time of diagnosis.
RESULTS
Patients Characteristics
N= 45 patients underwent ST and 23 (51%) patients also underwent GT. Of the 22 patients who were not consented for GT, 21 of the 22 had germline data obtained in an anonymous fashion without identifying clinical information. Median age at diagnosis was 63 years. Details regarding ethnicity, surgical resection, smoking history, history of diabetes and family history of cancer are outlined in Table I. All 45 ACs were ampullary adenocarcinomas. Their histologic subtypes were as followed: 10 (22%) intestinal, 22 (49%) pancreaticobiliary, 4 (9%) mixed intestinal and pancreaticobiliary, and 9 (20%) other.
Table I:
Clinical Characteristics of Total Patient Cohort
| Clinical Characteristics | N= 45 |
|---|---|
| Median Age at Diagnosis (years) | 64 |
| Stage at Diagnosis | |
| I | - |
| IIA | 3 (7%) |
| IIB | 28 (62%) |
| III | 5 (11%) |
| IV | 9 (20%) |
| Sex | |
| Male | 26 (58%) |
| Female | 19 (42%) |
| Ethnicity | |
| White | 35 (78%) |
| Non-Ashkenazi Jewish | 29 (83%) |
| Ashkenazi Jewish | 6 (17%) |
| Asian | 4 (9%) |
| Black | 1 (2%) |
| Latino/Hispanic | 2 (4%) |
| Other | 3 (7%) |
| Surgical Resection | |
| Yes | 25 (55%) |
| No | 20 (45%) |
| Smoker | |
| Yes (Current or Former) | 19 (42%) |
| No | 26 (58%) |
| Diabetes | |
| Yes | 31 (69%) |
| No | 14 (31%) |
| Family History of Cancer | |
| First or second degree relative with any cancer | 35 (78%) |
| First or second degree relative with a GI/Biliary Cancer | 14 (31%) |
Pathogenic Germline Alterations (PGAs) and Somatic Alterations (SAs) found
Eight of 44 patients with available germline data were found to have PGAs. PGAs in BRCA2 (3), ATM (3), APC (1), MUTYH (1), and RAD50 (1) were identified in these eight patients. These mutations were all deleterious. Table II demonstrates further details regarding demographics, treatment and survival for the five patients with PGA and was consented to GT. Notably, all of these consented patients with PGA had a family history of cancer. One consented patient had the same BRCA2 PGA as his father, who had BRCA2 related male breast cancer. Of the two consented patients with ATM PGAs, one patient had a strong first-degree family history cancer consisting of pancreas, breast, lung, and colon. Only these two patients of five consented patients (40%) with PGAs met guidelines for germline testing on the basis of a strong family history.
Table II:
Germline Alterations Found and Corresponding Clinical Characteristics
| Germline Alteration | LOH | Pathology | Ethnicity | Age @ Diagnosis | Stage at Initial Diagnosis | Resection (Y/N) | Metastatic Disease (Y/N) | Metastatic Chemotherapy | Survival from Diagnosis (Months) |
|---|---|---|---|---|---|---|---|---|---|
| ATM- c.8266A>T(p.Lys2756Ter) | Yes | Pancreaticobiliary | White | 67 | IIB | Y | Y | mFOLFIRINOX* | 16 |
| ATM– c.9023G>A (p.Arg3008His) | Yes | Pancreaticobiliary | White | 69 | IIB | Y | N | NA | 2 |
| BRCA2– c.3420dupT (p.Thr1141Tyrfs*3) | Yes | Intestinal | Ashkenazi Jewish | 45 | IIB | Y | N | Gemcitabine & Cisplatin | 2 |
| BRCA2- c.9285C>A (p.Asp3095Glu) | Yes | Pancreaticobiliary | Top Eastern | 35 | IV | N | Y | Gemcitabine & Cisplatin* | 18 |
| BRCA2- c.5946delT (p.Ser1982Argfs) | No | Pancreaticobiliary | Ashkenazi Jewish | 73 | IIB | Y | Y | Gemcitabine & Cisplatin | 84 |
No longer on this therapy at the time of data cutoff
Three patients that did not consent to GT were found to have PGAs. One patient was found to have a PGA in ATM-c.3576G>A (p.Lys1192Lys) and one had a PGA in APC-c.3920T>A (p.Ile1307Lys). The last patient had two PGAs: one in RAD50-c.2801dupA (p.Asn934LysfsTer10) and one in MUTYH-c.1187G>A (p.Gly396Asp).
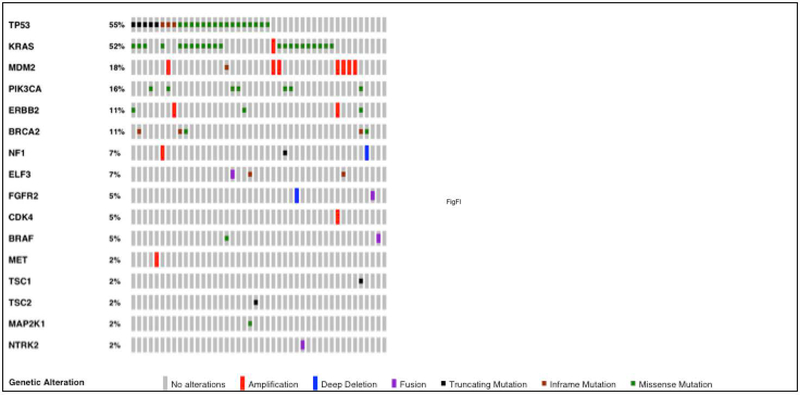
Sixteen clinically relevant SAs, based on the highest OncoKB for each patient, were found in this cohort.25 KRAS mutations comprised the largest majority of the SAs with PIK3CA, MDM2 amplification, and ERBB2 amplification also identified in a significant proportion of patients. In addition, inactivating mutations in ELF3, which are previously in AC as oncogenic, was also found in our cohort.21, 22 Figure 1 shows for an Oncoprint of SAs with the highest OncoKB found for the total cohort.25
Figure 1:
Oncoprint of Somatic Alterations with Highest OncoKb Found
Loss of Heterozygosity
All patient samples found to have PGAs underwent LOH analysis. Four of the 8 (50%) patients with PGAs were found to have LOH. These 4 patients were consented for GT and clinical data was available. Table II demonstrates the LOH status associated with the PGA and clinical details.
Targeted therapy and Survival
Median overall survival (OS) stratified for each consented patient with a PGA is listed in Table II. Patients with ATM had OS of 2 and 16 months and both were alive and/or censored at the end of the data cut-off point. For two of the three patients with BRCA2 mutations, their OS was 84 and two months. Both were censored and/or alive at the end of data cutoff. The third BRCA2 patient is deceased with an OS of 18 months. All BRCA2 patients received platinum-based therapy and two had a documented partial radiographic response as reported by treating physician. The third patient was lost to follow up and censored at 2 months.
There were a total of 18 deaths in the entire cohort at the time of data cutoff; all had metastatic disease at the time of their death. There was a median follow-up time of 14.3 months amongst survivors. The overall survival two years after initial diagnosis was 65.5% [95%CI: 9%−50%]. Refer to Figure II for the Kaplan Meir curve (KM). We found no significant difference between pathologic subtypes in terms of OS (p=0.338). Refer to Figure III for the KM between pathologic subtypes. Furthermore, there was no significant difference in OS between pathologic subtypes when the analysis was restricted to advanced disease (p=0.423).
Figure II:
Median OS for Entire Cohort
Figure III:
Median OS for Patients by Pathologic Subtype
Two patients had matched therapy based on their SAs. One patient with ERBB2 amplification received ado-trastuzumab emtansine as part of a Phase II clinical trial with a partial response sustained for approximately 6 months before developing progression of disease. He was being evaluated for additional ERBB2 targeted therapies at the time of data-cutoff. Another patient had an MDM2 amplification and participated in a clinical trial with an MDM2 inhibitor. This patient had progression of disease at the time of initial tumor re-staging at eight weeks. Of note, this patient had KRAS amplification but was wild type for both p53 and RB.
Microsatellite Analysis and Tumor Mutation Burden
Two patients were found to have an MSI-sensor score greater than 10 indicating microsatellite instability. Those two patients were also found to have an elevated TMB. The remainder was not found to have microsatellite instability or a high tumor mutation burden.
Both patients found to have high MSI-sensor scores and elevated TMB were found to lack germline alterations in the mismatch repair genes but rather they possessed somatic alterations in mismatch repair genes. We found one patient to have a SA in MSH3, absence of PMS2, and by PCR-based assay testing, to have hyper-methylation of the MLH1 promoter while the other patient had SAs in MSH2, MSH3 and MSH6. Both patients underwent resection —one after neo-adjuvant chemotherapy — and are currently, at the data cutoff time, with no evidence of recurrent disease.
DISCUSSION
Ampullary cancers harbor PGAs and SAs that may have clinically relevant therapeutic implications. 17, 18, 20–23 Current therapy and PGA screening for AC is extrapolated primarily from pancreas, biliary and colorectal cancer treatment and management in part referencing the primary pathologic subtypes of AC being intestinal and pancreaticobiliary type. Furthermore, there is growing literature demonstrating a multitude of solid tumors harboring PGAs.12, 17, 18, 29 These findings suggest that PGAs are not limited to a specific type of cancer but perhaps a pan-cancer observation. There is precedence therefore, to further explore the genetic landscape of AC.
Our study identified eight (18%) of 44 patients with AC with germline data to have PGAs including patients with ATM, RAD50, MUTYH mutations, not previously reported in this disease. In addition, our study also found PGAs in BRCA2 in three patients consistent with previously described findings. 17, 18 The finding of ATM, RAD50, and MUTYH, in addition to BRCA2, in AC suggests that there are possibly more PGAs in the DNA repair pathway that exist in a clinically relevant percentage of patients with AC. Further study in a larger cohort of population is warranted to elucidate the entire spectrum of PGAs in AC. The therapeutic applicability of PGAs in BRCA2 and possibly other PGAs in the DNA repair pathway like ATM primarily relates to the use of platinum agents to create DNA damage in conjunction with PARP inhibitors to inhibit DNA repair in an already repair deficient tumor via the concept of synthetic lethality.8, 9, 12 All five patients consented to GT in our cohort may benefit from such strategies however only two of the five patients (40%) would have met family history criteria for screening. In addition, four of these five (80%) consented patients were found to have LOH in either BRCA2 or ATM mutations further supporting the use of platinum and PARP inhibitors in these patients. These findings suggests that a broader unselected manner of screening for PGAs as suggested in other cancers like pancreas may be warranted in AC.12
Another possible target of therapy in AC is patients who have PGAs or SAs causing mismatch repair deficiency and subsequent microsatellite instability and or a high TMB. Identifying these patients is of increasing relevance and importance given the potential benefit from immunotherapy approaches such as PDL-1 inhibition.8, 9, 12, 14–16 In our cohort, two patients were found to have a high tumor mutational burden with an associated high MSI-sensor score indicating mismatch repair deficiency. Both patients are without recurrent disease after resection at the data cutoff time however, should they have recurrent disease they would be strong candidates for PDL-1 directed immunotherapy approaches. Additionally, these two patients were found to have SAs, not PGAs, in mismatch repair genes, which certainly implicates the importance of testing for SAs, TMB, and microsatellite instability as a way to identify all mismatch repair deficient patients. Our finding supports prior studies that have shown these alterations exist in AC.19, 24 Interestingly, the two patient in our study with microsatellite instability and associated high TMB had the intestinal pathologic subtype of AC suggesting that testing for mismatch repair is especially important in patients who has AC with the intestinal pathologic subtype.
Our patient cohort demonstrated a wide spectrum of somatic alterations, of which several are therapeutically actionable. For example, one patient in our cohort with ERBB2 amplification experienced a partial response to a clinical trial drug that was based on his ST and is participating in further clinical trials targeting this alteration. Additionally, we had one patient who received an MDM2 inhibitor as a result of ST. This patient notably had wild type p53 and RB genes, which in past studies, have been suggested as positive determinants for response to MDM2 inhibition.30, 31 While this patient did not benefit, despite his lack of p53 and RB mutations, further study is warranted to fully evaluated MDM2 inhibition as a possible treatment in MDM2 amplified ampullary tumors.
Regarding pathologic subtypes in this cohort, pancreaticobiliary (49%) made up the majority of the cohort. In the study reported herein, we did not find a difference in overall median OS between the pathologic subtypes. This finding is contrary to current research suggesting pancreaticobiliary subtypes having a poorer prognosis likely due to several limitations of the study. Firstly, the patient cohort is small due to the rarity of this disease and a percentage of patients were censored or lost to follow up, or both. Furthermore, there are fewer patients in this cohort with the intestinal subtype as compared to the pancreaticobiliary subtype. Lastly, a larger proportion of patients who were found to have PGAs, four out of five patients, were of the pancreaticobiliary subtype conferring possible sensitivity to platinum agents and improving survival.
Other limitations of the study include the inability to thoroughly evaluate the full genetic spectrum of AC due to the small cohort size. This is especially true for PGAs as the frequency of PGAs in our study, 18%, suggests that PGAs may be more prevalent than previously suspected. A larger cohort size may further distinguish any significant clinical or genetic differences between the two common pathologic subtypes of AC. Finally, not every SA found in this cohort may have therapeutic applicability or known clinical significance and the utility of these findings need further study to elucidate.
This study illustrates the spectrum of undiscovered PGAs and SAs that exist in AC. This study has shown that indeed, AC has a broad spectrum of PGAs and SAs such as BRCA2, ATM, KRAS, MDM2, PI3CKA, and ERBB2. The utility of finding such PGAs and SAs may have therapeutic benefit that will need to be clinically validated in further studies and trials. Furthermore, there may be implications for screening and prognosticating patients and possible at risk family members if PGAs are identified. Given the possible therapeutic implications, our findings suggest that more routine somatic and germline testing should be recommended for all patients with AC
Acknowledgments
Funding sources: Cancer Center Support Grant P30 CA008748
Research Support: Celgene, MabVax Therapeutics, Genentech-Roche, Astra-Zenica, ActaBiologica, Sanofi
Footnotes
Conflict of Interest:
Eileen M. O’Reilly MD
REFERENCES
- 1.Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J. Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 2000;12: 75–79. [DOI] [PubMed] [Google Scholar]
- 2.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36: 1592–1608. [DOI] [PubMed] [Google Scholar]
- 3.Chang DK, Jamieson NB, Johns AL, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31: 1348–1356. [DOI] [PubMed] [Google Scholar]
- 4.Morini S, Perrone G, Borzomati D, et al. Carcinoma of the ampulla of Vater: morphological and immunophenotypical classification predicts overall survival. Pancreas. 2013;42: 60–66. [DOI] [PubMed] [Google Scholar]
- 5.Ghosn M, Kourie HR, El Rassy E, et al. Where does chemotherapy stands in the treatment of ampullary carcinoma? A review of literature. World J Gastrointest Oncol. 2016;8: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti A, Giuliani J, Muggia F. Targeted agents and oxaliplatin-containing regimens for the treatment of colon cancer. Anticancer Res. 2014;34: 423–434. [PubMed] [Google Scholar]
- 7.Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol. 2015;33: 1787–1796. [DOI] [PubMed] [Google Scholar]
- 8.Krantz BA, Yu KH, O’Reilly EM. Pancreas adenocarcinoma: novel therapeutics. Chin Clin Oncol. 2017;6: 30. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen ES, O’Reilly EM, Brody JR, Witkiewicz AK. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology. 2016;150: 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7: 943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeon TT, Ahn DH, Bogenberger JM, et al. Novel targeted therapy strategies for biliary tract cancers and hepatocellular carcinoma. Future Oncol. 2018;14: 553–566. [DOI] [PubMed] [Google Scholar]
- 12.Lowery MA, Wong W, Jordan EJ, et al. Prospective Evaluation of Germline Alterations in Patients With Exocrine Pancreatic Neoplasms. J Natl Cancer Inst. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Reilly EM, Lee JW, Lowery MA, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124: 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu ZI, Shia J, Stadler ZK, et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res. 2018;24: 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto P, Peixoto A, Santos C, et al. Analysis of Founder Mutations in Rare Tumors Associated With Hereditary Breast/Ovarian Cancer Reveals a Novel Association of BRCA2 Mutations with Ampulla of Vater Carcinomas. PLoS One. 2016;11: e0161438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelker D, Zhang L, Kemel Y, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA. 2017;318: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cloyd JM, Chun YS, Ikoma N, et al. Clinical and Genetic Implications of DNA Mismatch Repair Deficiency in Biliary Tract Cancers Associated with Lynch Syndrome. J Gastrointest Cancer. 2018;49: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechtman JF, Liu W, Sadowska J, et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Mod Pathol. 2015;28: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingras MC, Covington KR, Chang DK, et al. Ampullary Cancers Harbor ELF3 Tumor Suppressor Gene Mutations and Exhibit Frequent WNT Dysregulation. Cell Rep. 2016;14: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yachida S, Wood LD, Suzuki M, et al. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell. 2016;29: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumhoer D, Zlobec I, Tornillo L, et al. Immunophenotyping and oncogene amplifications in tumors of the papilla of Vater. Virchows Arch. 2008;453: 579–588. [DOI] [PubMed] [Google Scholar]
- 24.Agaram NP, Shia J, Tang LH, Klimstra DS. DNA mismatch repair deficiency in ampullary carcinoma: a morphologic and immunohistochemical study of 54 cases. Am J Clin Pathol. 2010;133: 772–780. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu B, Ye K, Zhang Q, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30: 1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiki AY, Caenepeel S, Cosgrove E, Su C, Boedigheimer M, Oliner JD. Identifying the determinants of response to MDM2 inhibition. Oncotarget. 2015;6: 7701–7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaikh MF, Morano WF, Lee J, et al. Emerging Role of MDM2 as Target for Anti-Cancer Therapy: A Review. Ann Clin Lab Sci. 2016;46: 627–634. [PubMed] [Google Scholar]