Abstract
Caffeine and its derivatives have been used, alone and in combination with other phytochemicals, as weight-loss supplements. Caffeine affects several physiological and behavioral aspects of energy balance, including increasing locomotor activity. Here, we investigate the potential for caffeine to enhance activity thermogenesis and energy expenditure (EE) even when activity level is held constant. To do this, EE and muscle thermogenesis were measured in rats during treadmill walking regimens, with and without caffeine (25 mg/kg, i.p.). Activity-related EE was significantly increased throughout the treadmill walking protocol. Muscle heat dissipation, on the other hand, was significantly increased by caffeine only at the end of the 25-min treadmill test. This study demonstrates that caffeine increases the caloric cost of physical activity, compared to the caloric cost of that same physical activity without caffeine, implicating decreased muscle work efficiency. Combined with the known ability of caffeine to increase locomotor activity, the decreased locomotor efficiency imparted by caffeine may further augment the potential for caffeine to enhance caloric expenditure.
Keywords: Physical activity, energy expenditure, skeletal muscle, thermogenesis, non-exercise activity thermogenesis, NEAT
Introduction
As US obesity rates rise, new prevention and treatment options are considered. Intervention programs include not only medical options like weight-loss surgery or medications, but also public-health initiatives such as those designed to curtail consumption of sugar-sweetened beverages.1 Sugar-sweetened beverages are a major contributor to caloric intake, particularly in children and adolescents.2 Ironically, components in some of these same beverages—caffeine and its derivatives—have been used as dietary supplements, alone and in combination with other ingredients, in attempts to control obesity or weight gain.3,4 Caffeine-induced changes in thermogenesis and activity levels can promote negative energy balance,3 and caffeine may also affect metabolic efficiency.5
Caffeine affects not only energy balance but also exercise endurance and performance.6–8 Caffeine can increase muscle performance, energy expenditure (EE), thermogenesis, and fuel utilization through several potential mechanisms.4,9 These include direct effects on behaviors including physical activity, where caffeine reliably increases locomotor activity in rats.10–12 Caffeine affects peripheral metabolism through alterations in sympathetic nervous system (SNS) activity13 and by influencing peripheral metabolic targets directly through inhibition of cAMP phosphodiesterase or adenosine receptors or activation of AMP-kinase.14–17 Lastly, caffeine activates ryanodine receptors (RYR), including RYR 1 and 3 found in skeletal muscle.9 Caffeine also has direct effects on skeletal muscle physiology;8 this could potentially occur through RYR-induced changes in the activity of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). Changes in SERCA activity, like uncoupling induced by sarcolipin, can stimulate muscle non-shivering thermogenesis.18 The potential thermogenic effects of caffeine on skeletal muscle have not been explicitly tested, however.
Elevated non-exercise activity thermogenesis (NEAT) is associated with leanness in both laboratory models and humans,19,20 suggesting that enhancing NEAT can counter weight gain. We have also found that enhanced EE during physical activity is associated with elevated skeletal muscle thermogenesis,21 where decreased fuel economy of activity serves to increase activity-associated EE, with caloric energy dissipated as heat from muscle. We have successfully measured economy of activity (also called locomotor efficiency, or skeletal muscle work efficiency22) in rats using an approach where rats walk on a treadmill at constant speed while EE is measured, and the effect of a treatment is measured in a counterbalanced manner.23–26 We have also examined muscle temperature using implanted transponders23,25 which allows for direct measurement of muscle heat dissipation during increasing workloads, also assessed using treadmill walking.24 Whereas the ability of caffeine to enhance NEAT through elevating locomotor activity is well-known,10–12 the potential to also enhance activity-related EE through engaging thermogenic or other mechanisms that impact locomotor efficiency has not been addressed. Here, we test the hypothesis that caffeine enhances EE partly through increasing skeletal muscle thermogenesis. To rule out the known effect of caffeine on physical activity levels,10 EE and muscle thermogenesis were assessed in rats while activity levels were controlled using treadmill walking protocols;23–26 this isolates the effect of muscle work efficiency at low and moderate intensities of physical activity.
Results
Muscle Thermogenesis:
1. Main effects.
All data are presented as mean ± SEM. Body weight of rats did not significantly differ between the two conditions (caffeine, 599.8g ± 15.4g; vehicle, 596.2g ± 12.9g; p = 0.306). In the vehicle condition, all 8 rats completed 15 min of treadmill walking at increasing intensity, with 7 completing 20 min, and 6 completing 25 min. After caffeine treatment, all rats completed at least 20 min of treadmill walking, with 6 completing 25 min and 1 completing 30 min. ANOVA showed a significant main effect of treadmill intensity on muscle temperature in both the right and left legs (see Figure 1; p < 0.001), where greater treadmill walking intensity induced higher muscle temperature. There was no main effect of caffeine on either the left or right leg muscle temperatures (see Figure 1A; p = 0.212 for left leg, and 0.192 for right leg).
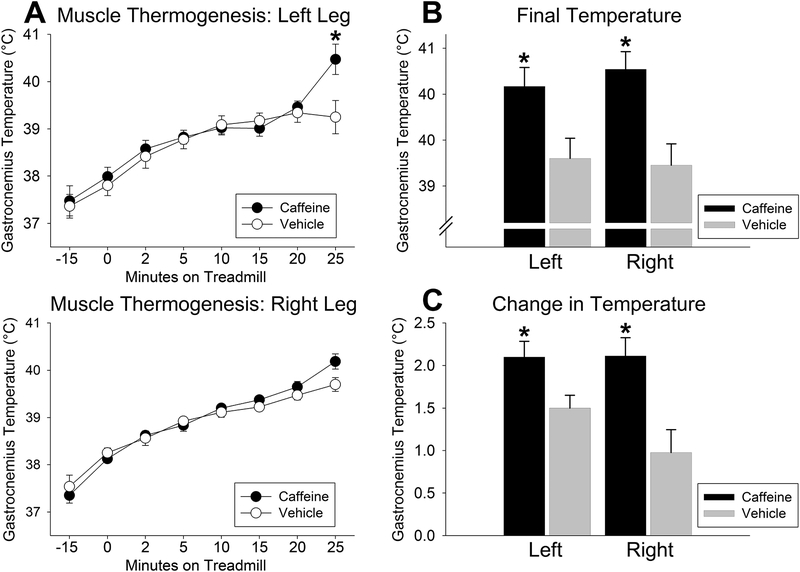
Figure 1.
Caffeine increased skeletal muscle (gastrocnemius) activity thermogenesis. (A) Gastrocnemius temperature was significantly higher at 25 minutes of treadmill walking at increasing intensity in the left leg. The final temperature immediately upon completion of walking (B) and the change in temperature from 0 min of treadmill walking to completion of walking (C) were significantly enhanced by caffeine in both the left and right hind limbs. *significantly greater than vehicle, p < 0.05 (Mean ± SEM; N = 8 through 15 min, 7 at 20 min, and 6 at 25 min).
2. Interaction between caffeine and walking intensity.
As shown in Figure 1, there was a significant interaction between caffeine treatment and treadmill-walking intensity in the left leg (p = 0.006) but not in the right leg (p = 0.221); this discordance may be due to minor differences in transponder placement. As shown in Figure 1, in the 5 rats which walked at least 25 min, temperatures in both the left and right gastrocnemius were significantly higher at 25 min compared to 0 min (immediately before treadmill walking, 15 min after caffeine injection; right leg, p = 0.017; right leg, p = 0.010). There was no significant difference in either the left or right gastrocnemius temperatures at any other time point, including before injection or 15 min after injection (i.e., immediately before treadmill walking; Figure 1A). As shown in Figure 1B, when final temperature was measured immediately after walking ceased, temperatures were significantly higher in both the left (p < 0.001) and right (p = 0.005) gastrocnemius.
3. Time spent walking.
The time spent walking (in min) was not significantly longer after caffeine treatment, though there was a trend in this direction (vehicle, 25.2 ± 1.4 min; caffeine, 27.4 ± 0.9 min; p = 0.079). Of the 8 rats, 5 walked longer after caffeine than after vehicle treatment; in all 3 rats that walked longer after vehicle treatment, the final temperature was still significantly higher in the caffeine condition in both the left (39.8 vs. 39.0°C, p = 0.021) and right (40.1 vs. 39.3°C, p = 0.039) gastrocnemius. Similarly, the change in temperature in both left and right gastrocnemius muscles between 0 min and after completing the walking protocol was significantly higher after caffeine (Figure 1C). This effect remained significant when examining the 3 rats that walked longer with vehicle compared to caffeine (left gastrocnemius, 1.6°C vs. 2.3°C, p = 0.034; right gastrocnemius, 1.2°C vs. 1.8°C, p = 0.038). Overall, muscle heat dissipation upon completion of treadmill walking was elevated after caffeine treatment relative to vehicle.
4. Brown adipose tissue.
Lastly, interscapular brown adipose tissue temperature change did not significantly differ between vehicle and caffeine treatments between injection and 0 min (respectively, 0.7°C ± 0.1°C and 0.8°C ± 0.1°C, p > 0.05) or between 0 min and the completion of treadmill walking (1.0°C ± 0.1°C and 1.4°C ± 0.1°C, p > 0.05).
Treadmill Energy Expenditure:
1. Caffeine elevated activity EE.
Body weight of rats did not significantly differ between the two conditions (caffeine, 473.9 ± 9.3g; vehicle, 473.8 ± 11.8; p = 0.495), and all rats completed the 30 min of walking. As illustrated in Figure 2A, caffeine significantly increased the calories (kcal) used to walk at a constant speed in rats. The increase in the 30-min EE above vehicle levels was significantly elevated after caffeine treatment (Figure 2B). As shown in Figure 2A, for EE, ANOVA revealed a significant main effect of caffeine (p = 0.003), and a significant main effect over the three 10-min measurement periods (p = 0.001), but no significant interaction between treatment and time on treadmill (p = 0.336). The caffeine-induced increase in caloric cost of activity ranged from 6.5% in the first 10 min to 10.0% in the final 10 min, with an average 8.3% increase for the 30-min duration of the walk. During the 5-min period before the treadmill was started, EE was also higher in the rats after caffeine treatment compared to vehicle treatment (vehicle, 2.65 ± 0.22 kcal/hr; caffeine, 3.41 ± 0.06; p = 0.020), with the caffeine-induced increase over vehicle EE ranging from < 1% to 70%. The caffeine-induced increase in EE above vehicle levels in the 5 min preceding treadmill walking was not significantly correlated with the caffeine-induced increase in EE during the 30 min of treadmill walking (r = −0.005). This discordance suggests that the factors driving variance in pre-treadmill EE did not significantly contribute to inter-rat differences in treadmill-walking EE; pre-walking EE was likely affected by differences in the rats’ locomotion27 on the static treadmill before treadmill movement started.
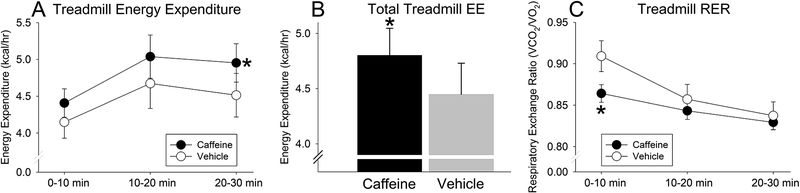
Figure 2.
Caffeine amplified activity-associated energy expenditure. Compared to vehicle treatment, caffeine increased energy expenditure (EE) throughout the 30-min duration of treadmill walking (A) as well as the overall average EE over 0–30 min (B). Caffeine also significantly decreased respiratory exchange ratio (RER; VCO2/VO2) in the first 10 min of treadmill walking only; RER during the 5 min preceding the initiation of activity was not significantly affected by caffeine. *significantly different from vehicle treatment, p < 0.05 (Mean ± SEM; N=5)
2. Respiratory exchange ratio.
As shown in Figure 2C, RER showed a significant interaction between treatment and time (p = 0.003), and a significant main effect of time spent walking (p < 0.001), but no main effect of caffeine on RER (p = 0.301). Specifically, treadmill-walking RER started high and decreased over the 30-min walking test, but RER was significantly lower after caffeine treatment in the initial 10-min period. When considering the 5-min period immediately before the rats walked on the treadmill, RER did not differ between treatments (vehicle, 0.83 ± 0.02; caffeine, 0.82 ± 0.01; p = 0.408).
Discussion
Caffeine induces negative energy balance by altering multiple aspects of behavior and metabolism, including physical activity, thermogenesis, and EE.10,13,14,28–30 Here, we demonstrate that caffeine enhances activity-related EE, akin to NEAT in humans, in rats even when physical activity is not changed (Figure 1). Specifically, caffeine significantly increased the caloric cost of treadmill walking by 8%. The activity demands were the same between conditions, minimizing differences in muscle performance. Therefore, the increased EE associated with this activity implicates decreased skeletal muscle work efficiency (Figure 2). Walking at gradually greater intensities increased leg muscle temperatures after both vehicle and caffeine treatments. Caffeine treatment significantly enhanced muscle heat dissipation after 25 min of walking, suggesting that some of the additional caloric expenditure induced by caffeine was dissipated as heat energy. Altogether, these studies suggest that caloric expenditure during physical activity, at least moderate-level activity, is enhanced by caffeine.
As shown in Figure 1A, after caffeine, rats had higher EE during activity, even with no change in workload (i.e., the same speed and distance). While locomotor efficiency is also influenced by other muscle contraction-related factors, the controlled speed, distance, and load in this study minimized the contribution of these factors. Altogether, this implicates lower muscle work efficiency after caffeine treatment. The standardized treadmill walking also rules out other dominant sources of EE. First, caffeine increases locomotion,10 which was equalized during (but not before) treadmill walking. Second, caffeine and vehicle did not significantly differ in their effects on interscapular brown fat temperature. The effect of caffeine on muscle work efficiency and thermogenesis seen here is consistent with the known ability of caffeine to enhance muscle power and exercise endurance.6–9 and reduce perceived exertion.31 The ability of caffeine to augment athletic performance is more pronounced when endurance is required, as opposed to short-term activity;6,9 in vitro, slow-twitch type I fibers are more sensitive to caffeine compared to fast-twitch type II fibers.9 We have demonstrated elevated caloric cost of activity in association with high intrinsic aerobic capacity,32 where running performance is fueled by more effective oxygen delivery and substrate oxidative capacity.33 Analogously, the data presented here suggest that caffeine’s enhancement of performance comes at a cost of decreased locomotor efficiency, resulting in additional calories burned for the same activity or workload.
With respect to performance, not all rats completed the graded treadmill walking assessment of muscle thermogenesis (Figure 1); this variance and noncompliance is observed frequently, even though the treadmill test does not require strenuous activity.21,23,26 While no effect of caffeine was detected on the duration of treadmill walking measured here, the current study was not designed to measure endurance or fatigue, and thus had inadequate statistical power and temporal resolution to adequately evaluate the effect of caffeine on endurance, performance, or fatigue in rats. The results described here present an interesting question, however, of whether the mechanisms underlying the ergogenic effects of caffeine31 overlap with those modulating fuel economy or thermogenesis. The investigation of muscle temperature during physical activity and activity-related EE in humans would be required to determine if caffeine had the ability to meaningfully affect muscle work efficiency in humans. The duration of this effect also warrants investigation, given that the data presented here comprise limited 30-min bouts of activity.
Our investigations of muscle thermogenesis have revealed similarities in the ability of metabolic challenges to affect muscle temperature and fuel economy of activity, with increased heat dissipation associated with conditions of low fuel economy.21,24,25 For example, phenotypically lean rats show elevated muscle temperature during controlled physical activity, as well as higher caloric cost of activity, compared to obesity-prone rats.21,32 Activation of brain melanocortin receptors also induces muscle thermogenesis and increases activity-related EE.25 Weight loss induced by caloric restriction has the opposite effect, decreasing muscle thermogenesis during low-intensity activity while increasing locomotor efficiency beyond what can be accounted for by the reduced body weight.24 Indeed, changes in skeletal muscle work efficiency have been implicated in adaptive thermogenesis during calorie restriction in humans.22 The ability of caffeine to increase fuel cost of activity and alter muscle thermogenesis is consistent with evidence of caffeine or caffeine derivatives altering metabolism, including sympathetic control of thermogenesis.4,9,13
The pattern of activity-associated muscle heat dissipation seen after caffeine treatment (Figure 1A) differs from previous investigations of activity-associated changes in muscle temperature, however.21,23–25 Here, muscle temperature steadily increased throughout treadmill walking, with caffeine increasing temperature to a greater extent at the higher walking intensities (Figure 1A). Other manipulations alter activity-associated muscle temperature at all walking intensities (activation of brain melanocortin receptors)25 or at lower walking intensities only (caloric restriction).24 The activity-associated increase in muscle temperature (Figure 1) consistently displays a truncated dynamic range relative to activity-related EE, with maximal temperatures reached at moderate workloads,21,23–25 while EE continues to increase as activity becomes progressively more demanding (e.g., running to exhaustion). This implies that low-to-moderate-intensity activity is subject to more flexibility in fuel economy and muscle thermogenesis, consistent with evidence from human weight-loss studies.22,34 While the ability of caffeine to enhance muscle thermogenesis only at the higher activity intensity is noteworthy (Figure 1A), it leaves open the possibility of other, non-thermogenic mechanisms of energy transfer at the lower workloads where caffeine increased caloric cost of activity (Figure 2A). Alternatively, it is possible that calorimetric measurement of gas exchange is a more sensitive measurement of the physiological processes underlying muscle work efficiency compared to temperature measurement.
Activity-related fuel utilization was affected by caffeine as well. RER typically ranges between 0.7 and 1.0, decreasing in conditions of fat utilization (e.g., cold exposure, illness, high-fat diet32,35). RER immediately before the treadmill test was not significantly affected by caffeine, despite the caffeine-induced difference in EE at this time. As shown in Figure 2C, during the first 10 min of treadmill walking, RER was lower after caffeine treatment compared to the vehicle condition, suggesting that fat oxidation was relatively enhanced by caffeine at this time. This is consistent with caffeine’s lipolytic effects on white adipose tissue, attributed to its actions on adenosine receptors,36 as well as its direct effect on fuel uptake and metabolism.15
The ability of caffeine to increase activity thermogenesis and energy expenditure through alterations in muscle work efficiency may stem from one or more of its known actions on metabolism. First, caffeine may affect peripheral metabolism through changing autonomic outflow. For example, increased SNS outflow to skeletal muscle increases muscle glucose uptake.37,38 Caffeine’s thermogenic actions on brown adipose tissue have been attributed to SNS outflow as they are prevented by chemical sympathetic denervation.13 Caffeine may also act directly on skeletal muscle through a multitude of possible routes,9 including inhibiting phosphodiesterase activity,14 inhibition of adenosine receptors,39 activating AMPK,15–17 or inhibition of glycogen phosphorylase.9 Caffeine’s thermogenic actions may also be a result of its potentiation of RYR-induced Ca2+ release from muscle sarcoplasmic reticulum.9 Skeletal muscle is the primary expression site of RYR1, mutations of which are associated with malignant hyperthermia.40 Enhanced sarcoplasmic Ca2+ release may also increase the sarco/endoplasmic reticulum Ca2+ ATP-ase (SERCA) activity. SERCA and an associated protein, sarcolipin, have been implicated in skeletal muscle thermogenesis, generating heat at the expense of ATP.41–43 The potential role of Ca2+ cycling in economy of physical activity is supported by the association between SERCA and enhanced muscle work efficiency seen during weight loss-induced adaptive thermogenesis in humans.44
Altogether, this study demonstrates that caffeine decreases fuel economy of physical activity, increasing the caloric cost of activity even when activity level or workload is held constant. This is accompanied by an amplification of muscle thermogenesis, but only after 25 min of treadmill walking. This increase in calorie use may partly underlie the known ability of caffeine to induce weight loss or prevent weight gain in laboratory animals36,45,46 and promote negative energy balance in humans.4,29,30 Similar to the ability of caffeine to enhance athletic performance,9 the metabolic effects reported here may occur through multiple mechanisms, including elevated SERCA activity secondary to caffeine’s increase of RYR-induced sarcoplasmic reticulum Ca2+ release. This demonstration of caffeine’s ability to amplify activity-associated energy expenditure and skeletal muscle heat dissipation is consistent with these mechanisms and provides new quantification of physiological effects not previously demonstrated. Overall, caffeine has the potential to increase NEAT in humans through its known ability to promote physical activity combined with greater caloric use during that activity.
Methods
Animals.
Two studies were conducted, both using adult male Sprague-Dawley rats, N=8 for muscle thermogenesis, N=6 for treadmill EE. Sample size was based on prior data showing treatment-induced changes in temperature and EE within rat.24,25 Rats were housed in a temperature-controlled room (24°C ± 1°C) on a 12:12-h light-dark cycle with lights-on at 0700 Eastern Standard Time. Measurements were completed in the middle of the light phase (≥ 3.5 hrs after lights on) to prevent contamination from residual nighttime activity thermogenesis.25 Rats were given free access to both water and standard laboratory chow (5P00 MRH 3000, T.R. Last Co. Inc.). All procedures were approved by Kent State University Institutional Animal Care and Use Committee. Briefly, rats were treated with vehicle or caffeine prior to treadmill walking protocols that enforce standardized activity during measurements of EE or muscle temperature, described below.
Treatment.
Dose and timing of caffeine treatment were chosen based on optimal effects on activity in rats, where 15–30 mg/kg caffeine intraperitoneally (i.p.) was most effective in enhancing locomotor activity in adult male rats, with a 15-min delay after injection.10 The combination of i.p. injection (as opposed oral gavage) and the 15–20 min delay after injection in the studies conducted here minimized physical activity and thermogenesis related to stress. Caffeine solutions were prepared using pharmaceutical grade caffeine (Sigma-Aldrich 93784) and sterile saline; pre-made aliquots were frozen for a maximum 6 weeks. The rats were injected (i.p.) with 25 mg/kg caffeine solution or an equal volume of vehicle (1ml/kg) before the treadmill test; rats were tested in one of two treadmills, with each rat undergoing all of its treatments in the same treadmill. Treatment order was counterbalanced and separated by at least 1 day, and treatments were given at the same time of day (within 2.5 hours) within rat to eliminate potential time-of-day effects. All treadmill tests were completed in an environmental chamber kept at a temperature thermoneutral for rats (25.0 – 25.9°C).
Muscle Thermogenesis.
Skeletal muscle (gastrocnemius) thermogenesis was assessed using a graded treadmill walking test which captures the dynamic range of muscle heat dissipation during increasing intensity of physical activity.21,23–25 Temperature transponders (IPTT-300, BioMedic Data Systems) were surgically implanted in the hind legs bilaterally, against the gastrocnemius muscle group and the interscapular region of brown adipose tissue, as previously described;23–25 the sensor measures temperature with 0.1°C resolution. Muscle temperature was first measured before injection of caffeine or vehicle. After injection, rats were placed on the stationary treadmill for 15 min, then muscle temperatures were measured again (0 min) before the treadmill was started. During treadmill walking, temperatures were measured after 2 minutes (7 m/min at 0° incline), then every 5 minutes for 30 minutes of increasing speed and incline (after 5 and 10 min of 7 m/min at 0° incline, 15 min at 9 m/min and 0° incline, 20 min at 9 m/min and 10° incline, 25 min at 11 m/min and 10° incline, and 30 min at 11 m/min and 20° incline). This protocol conforms to previous studies, and is able to document the acute rise in muscle temperature with the onset of activity and thereafter due to the slower change in temperature seen after 5 minutes of treadmill walking.21,24,25 Rats walked on the treadmill for 30 min or until they would no longer walk, whichever came first. Rats vary in the amount of time they will walk on this protocol, and this protocol captures the dynamic range of temperature changes and the subsequent temperature plateau, while working within the constraints of the time needed to measure temperature.21,24,25,47 Final temperature was measured immediately upon completion of treadmill walking regardless of test duration. Interscapular brown fat temperatures were measured before injection and before and after treadmill walking.
Treadmill Energy Expenditure.
The caloric cost of activity was measured while rats walked at a constant speed on a treadmill. Immediately after injection of caffeine or vehicle, rats were placed into the enclosed treadmill apparatus, monitored by an Oxymax FAST small-animal calorimetry system (Columbus Instruments; Columbus OH) calibrated using primary gas standards. Fresh air was provided to the sealed treadmill at 3.5–3.6 LPM, and sampled at 0.5 LPM. Starting at 20 min after injection to allow time for the onset of caffeine action10 while avoiding acute arousal caused by the injection procedure, the treadmill was run at a constant low speed (7 meters per minute) at 0° incline for 30 minutes while gas exchange was measured every 10 seconds.
Statistical Analyses.
Using IBM SPSS statistics, caffeine’s effect on activity-related muscle heat dissipation was analyzed using a repeated-measures ANOVA, with caffeine’s effects on temperature over 25 min of treadmill walking analyzed separately for the right and left legs. The effect of caffeine on final temperature was compared using paired t-tests (2-tailed; 1-tailed for follow-up analyses). Calorimetric variables [EE; respiratory exchange ratio (RER = VCO2/VO2)] were averaged into three 10-min blocks and analyzed using a 2×3 repeated-measures ANOVA; total EE and RER over the entire 30 min of walking were compared using 1-tailed paired t-tests. For the gas-exchange experiment, data from one rat were removed because the change in total activity-EE value fell above criterion for an outlier48 due to an unusually low vehicle-induced EE measurement, possibly secondary to the lack of an air-tight seal on the treadmill during calorimetry. Significance was determined as p<0.05. For pairwise comparisons that were not preceded by a general analysis (e.g., ANOVA), t-tests were used with correction for multiple comparison (p < 0.025 for 2 comparisons) to determine statistical significance.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grants R15-DK-097644, R15-DK-108668, and American Heart Association (AHA) Grant GIA-410805 to C. M. Novak.
Footnotes
None of the authors have any conflict of interest to declare. All authors have contributed significantly and all authors are in agreement with the content of the manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr. 2014;5(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. 2010;110(10):1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs EM, Mela DJ. Metabolically active functional food ingredients for weight control. Obes Rev. 2006;7(1):59–78. [DOI] [PubMed] [Google Scholar]
- 4.Hursel R, Westerterp-Plantenga MS. Catechin- and caffeine-rich teas for control of body weight in humans. Am J Clin Nutr. 2013;98(6 Suppl):1682S–1693S. [DOI] [PubMed] [Google Scholar]
- 5.Acheson KJ, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jequier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr. 1980;33(5):989–997. [DOI] [PubMed] [Google Scholar]
- 6.Bell DG, McLellan TM. Exercise endurance 1, 3, and 6 h after caffeine ingestion in caffeine users and nonusers. J Appl Physiol (1985). 2002;93(4):1227–1234. [DOI] [PubMed] [Google Scholar]
- 7.Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML. Effect of caffeine ingestion on muscular strength and endurance: a meta-analysis. Med Sci Sports Exerc. 2010;42(7):1375–1387. [DOI] [PubMed] [Google Scholar]
- 8.Tallis J, Duncan MJ, James RS. What can isolated skeletal muscle experiments tell us about the effects of caffeine on exercise performance? Br J Pharmacol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr. 2005;45(7–8):535–562. [DOI] [PubMed] [Google Scholar]
- 10.Marin MT, Zancheta R, Paro AH, Possi AP, Cruz FC, Planeta CS. Comparison of caffeine-induced locomotor activity between adolescent and adult rats. Eur J Pharmacol. 2011;660(2–3):363–367. [DOI] [PubMed] [Google Scholar]
- 11.Powell KR, Iuvone PM, Holtzman SG. The role of dopamine in the locomotor stimulant effects and tolerance to these effects of caffeine. Pharmacol Biochem Behav. 2001;69(1–2):59–70. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23(2):189–196. [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Seydoux J, Girardier L. Paraxanthine (metabolite of caffeine) mimics caffeine’s interaction with sympathetic control of thermogenesis. Am J Physiol. 1994;267(5 Pt 1):E801–804. [DOI] [PubMed] [Google Scholar]
- 14.Dulloo AG, Seydoux J, Girardier L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: adenosine antagonism or phosphodiesterase inhibition? Metabolism. 1992;41(11):1233–1241. [DOI] [PubMed] [Google Scholar]
- 15.Egawa T, Hamada T, Kameda N, et al. Caffeine acutely activates 5’adenosine monophosphate-activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metabolism. 2009;58(11):1609–1617. [DOI] [PubMed] [Google Scholar]
- 16.Egawa T, Hamada T, Ma X, et al. Caffeine activates preferentially alpha1-isoform of 5’AMP-activated protein kinase in rat skeletal muscle. Acta Physiol (Oxf). 2011;201(2):227–238. [DOI] [PubMed] [Google Scholar]
- 17.Egawa T, Tsuda S, Ma X, Hamada T, Hayashi T. Caffeine modulates phosphorylation of insulin receptor substrate-1 and impairs insulin signal transduction in rat skeletal muscle. J Appl Physiol (1985). 2011;111(6):1629–1636. [DOI] [PubMed] [Google Scholar]
- 18.Pant M, Bal NC, Periasamy M. Sarcolipin: A Key Thermogenic and Metabolic Regulator in Skeletal Muscle. Trends Endocrinol Metab. 2016;27(12):881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol. 2007;19(12):923–940. [DOI] [PubMed] [Google Scholar]
- 20.Kotz CM, Levine JA. Role of nonexercise activity thermogenesis (NEAT) in obesity. Minn Med. 2005;88(9):54–57. [PubMed] [Google Scholar]
- 21.Gavini CK, Mukherjee S, Shukla C, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–192. [DOI] [PubMed] [Google Scholar]
- 23.Gavini CK, Britton SL, Koch LG, Novak CM. Inherently lean rats have enhanced activity and skeletal muscle response to central melanocortin receptors. Obesity (Silver Spring). 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almundarij TI, Gavini CK, Novak CM. Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction. Physiological reports. 2017;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavini CK, Jones WC, 2nd, Novak CM. Ventromedial hypothalamic melanocortin receptor activation: regulation of activity energy expenditure and skeletal muscle thermogenesis. J Physiol. 2016;594(18):5285–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almundarij TI, Smyers ME, Spriggs A, et al. Physical Activity, Energy Expenditure, and Defense of Body Weight in Melanocortin 4 Receptor-Deficient Male Rats. Sci Rep. 2016;6:37435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21(5):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bracale R, Petroni ML, Davinelli S, et al. Muscle uncoupling protein 3 expression is unchanged by chronic ephedrine/caffeine treatment: results of a double blind, randomised clinical trial in morbidly obese females. PLoS One. 2014;9(6):e98244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davoodi SH, Hajimiresmaiel SJ, Ajami M, et al. Caffeine treatment prevented from weight regain after calorie shifting diet induced weight loss. Iran J Pharm Res. 2014;13(2):707–718. [PMC free article] [PubMed] [Google Scholar]
- 30.Miles-Chan JL, Charriere N, Grasser EK, Montani JP, Dulloo AG. The thermic effect of sugar-free Red Bull: do the non-caffeine bioactive ingredients in energy drinks play a role? Obesity (Silver Spring). 2015;23(1):16–19. [DOI] [PubMed] [Google Scholar]
- 31.Higgins S, Straight CR, Lewis RD. The Effects of Preexercise Caffeinated Coffee Ingestion on Endurance Performance: An Evidence-Based Review. Int J Sport Nutr Exerc Metab. 2016;26(3):221–239. [DOI] [PubMed] [Google Scholar]
- 32.Novak CM, Escande C, Burghardt PR, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58(3):355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez NC, Howlett RA, Henderson KK, et al. Systemic oxygen transport in rats artificially selected for running endurance. Respir Physiol Neurobiol. 2006;151(2–3):141–150. [DOI] [PubMed] [Google Scholar]
- 34.Goldsmith R, Joanisse DR, Gallagher D, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290(2):E396–403. [DOI] [PubMed] [Google Scholar]
- 36.Panchal SK, Wong WY, Kauter K, Ward LC, Brown L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition. 2012;28(10):1055–1062. [DOI] [PubMed] [Google Scholar]
- 37.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649(1–2):343–347. [DOI] [PubMed] [Google Scholar]
- 38.Shiuchi T, Haque MS, Okamoto S, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10(6):466–480. [DOI] [PubMed] [Google Scholar]
- 39.Fredholm BB. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76(2):93–101. [DOI] [PubMed] [Google Scholar]
- 40.Lanner JT. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol. 2012;740:217–234. [DOI] [PubMed] [Google Scholar]
- 41.Bal NC, Maurya SK, Sopariwala DH, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18(10):1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamu D, Bombardier E, Smith IC, Fajardo VA, Tupling AR. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc Sport Sci Rev. 2014;42(3):136–142. [DOI] [PubMed] [Google Scholar]
- 43.Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013;288(10):6881–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldwin KM, Joanisse DR, Haddad F, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HY, Lee MY, Park HM, et al. Urine and serum metabolite profiling of rats fed a high-fat diet and the anti-obesity effects of caffeine consumption. Molecules. 2015;20(2):3107–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moy GA, McNay EC. Caffeine prevents weight gain and cognitive impairment caused by a high-fat diet while elevating hippocampal BDNF. Physiol Behav. 2013;109:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavini CK, Britton SL, Koch LG, Novak CM. Inherently Lean Rats Have Enhanced Activity and Skeletal Muscle Response to Central Melanocortin Receptors. Obesity (Silver Spring). 2018;26(5):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology. 2013;49:764–766. [Google Scholar]